Abstract
AIM: To evaluate the red cell distribution width (RDW) as an indicator of the presence of non-alcoholic steatohepatitis (NASH) and its association with fibrotic scores.
METHODS: A retrospective study was carried out that included sixty-two biopsy proven NASH, 32 simple steatosis patients and 30 healthy controls. The correlation between the clinical and histopathological features of NASH patients and RDW values was evaluated. Liver fibrosis scores were measured using a 0 to 4 point scale and were divided in to two groups; fibrosis scores 0-1 were termed mild and fibrosis scores 2-4 were termed advanced fibrosis. RDW values were compared between NASH, simple steatosis and healthy controls. Univariate and multivariate analyses were performed to evaluate the independent predicting factors for the presence of liver fibrosis caused by NASH.
RESULTS: Patients with NASH had higher RDW values compared with simple steatosis and healthy control groups [14.28% ± 0.25% vs 13.37% ± 0.12%, 12.96% ± 0.14% (P < 0.01), respectively]. Patients with advanced fibrosis had higher RDW values than the mild fibrosis group (15.86% ± 0.4% vs 13.63% ± 0.67%, P < 0.01, respectively). RDW also correlated with fibrotic scores (r = 0.579 and P < 0.01). The variables that were significant in the univariate analysis were evaluated in multivariate logistic regression analysis, and RDW was an independent predicting factor of NASH (OR = 1.75, 95%CI: 1.129-2.711, P < 0.05).
CONCLUSION: RDW a new non-invasive marker that can be used to demonstrate the presence of NASH and indicate advanced fibrotic scores.
Keywords: Non-alcoholic steatohepatitis, Liver fibrosis, Red cell distribution width, Simple steatosis, Non-invasive marker, Liver biopsy
Core tip: We evaluated the role of red cell distribution width (RDW) as an indication of nonalcoholic steatohepatitis by comparing the values of biopsy proven non-alcoholic steatohepatitis (NASH) patients with simple steatosis and healthy controls. Independent predictors of the presence of NASH and advanced liver fibrosis were evaluated by using multivariate logistic regression analyses and RDW was a statistically significant independent predictor.
INTRODUCTION
Nonalcoholic steatohepatitis (NASH) is part of the spectrum of non-alcoholic fatty liver disease (NAFLD), which comprises simple fatty liver, NASH, associated advanced fibrosis and cirrhosis[1]. NAFLD has become one of the most common forms of chronic liver diseases in the United States and worldwide, with a prevalence of 10%-24%[2-5]. NASH is characterized by ballooning, degeneration, and lobular inflammation with various stages of fibrosis. Lobular inflammation is a hallmark of NASH, which is characterized by infiltration of lymphocytes, mononuclear cells and polymorphonuclear neutrophils. Steatosis is present in all cases and affects the hepatic lobules[6]. Simple steatosis is a benign condition with minimum progression, despite a high rate of progression to cirrhosis in 15%-25% of patients with NASH[7]. Liver fibrosis is a result of chronic injury[8]. In the absence of approved treatment modalities for NASH, care should be taken on the detection of advanced fibrosis. A liver biopsy is the only method for distinguishing NASH from other diagnoses and permits the evaluation of the damage and fibrosis of the liver. However a biopsy is an invasive and expensive procedure with important complications, such as bleeding and perforation. Therefore, there has been a search for noninvasive methods of detecting the presence of NASH and liver fibrosis to avoid these procedure-related complications.
Chronic inflammation plays a significant role in disease progression to NASH[9]. In addition, some studies have shown that certain inflammatory cytokines were higher in patients with NASH[10,11].
Red cell distribution width (RDW) is a parameter of the variation of circulating red cells. This parameter demonstrates the heterogeneity of red cell volume and is a component of the complete blood count (CBC). The RDW is also a widely available, inexpensive and easily repeatable marker that measures red blood cell (RBC) volume variability. Recent reports have demonstrated that elevated RDW values were related to negative outcomes in cardiovascular and metabolic disorders, colon cancer and stroke independent of hemoglobin (HGB) values[12-16]. The association between RDW values and the severity of liver diseases has been also reported in two recent studies[17,18].
In this study, we aimed to investigate the clinical utility of RDW for indicating the presence of NASH in comparison with simple steatosis and healthy controls. We also evaluated whether there is an association between RDW values, fibrotic stages and histological features of NASH.
MATERIALS AND METHODS
Patients
This retrospective study was performed in Gazi University Department of Gastroenterology, a tertiary reference center (Ankara, Turkey), between January 2010 and May 2013. All the patients who had persistently elevated liver enzymes and hepatosteatosis on ultrasonography, in the absence of any causes of elevated aminotransferases, were evaluated in the study. Among them, patients who had histopathology consistent with NASH and simple steatosis were included in the study. The control group was created among individuals who had normal aminotransferase levels and normal abdominal ultrasonography. Patients who were diagnosed with viral hepatitis, sclerosing cholangitis, primary biliary cirrhosis, autoimmune hepatitis, hemochromatosis, Wilson’s disease, alpha-1-antitrypsin deficiency, malignancy and drug-induced liver disease were excluded. Other exclusion criteria were alcohol consumption of > 20 g/d for men and > 10 g/d for women, presence of infectious diseases on admission, chronic renal diseases, collagen vascular diseases, malignancies, hematological diseases that might impair red cell production (such as iron deficiency, B12 or folate deficiency), increased red cell destruction, hemoglobinopathies, blood transfusions, bone marrow depression, usage of anticoagulant drugs, non-steroid anti-inflammatory drugs and hepatotoxic drugs. Patients who had diabetes mellitus were on oral antidiabetics and/or insulin therapy. Patients who had hypertension were all using angiotensin-converting enzyme inhibitors and were under control in terms of hypertension.
Clinical and laboratory assessment
Demographical, clinical and laboratory data were collected and registered in a database by an uninformed clinician to prevent bias. Body mass index was calculated by dividing weight in kilograms (kg) by the square of height in meter (m) as (kg/m2).
Before the liver biopsy, venous blood samples were obtained from the antecubital vein from all patients between 8.30 and 10.00 am, after fasting for at least 8-12 h. A CBC containing RDW, hemoglobin (HB), white blood cell (WBC), neutrophils, lymphocytes, platelets and mean platelet volume (MPV) was performed with a Beckman Coulter Gen-S automated analyzer (High Wycombe, United Kingdom) within 2 h after obtaining the blood samples. RDW was calculated by the Beckman Coulter automated analyzer as RDW = SD/MCV × 100%. (The standard deviation of the volume of red blood cell (SD)/[(mean corpuscle volume) (MCV)] × 100%. RDW was calculated by dividing the SD by the MCV and then multiplying that result by 100. The SD represented the volume of erythrocytes or RBCs that were in the blood smear.
A Roche Modular System auto analyzer(Roche COBAS INTEGRA 800 (Indianapolis, United States) determined the Albumin, creatinine, blood urine nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ glutamyl aminotransferase (GGT) and alkaline phosphatase (ALP) levels. All the laboratory analyses were performed in our hospital’s hematology laboratory.
Histopathological evaluation of the liver
Percutaneous liver biopsy was performed using a 16-G disposable needle by a skilled clinician. All liver biopsy specimens included 12 or more complete portal tracts and were longer than 20-25 mm. All liver tissue samples were evaluated by the same experienced hepatopathologist who was blinded to the patients’ clinical and laboratory data. Hematoxylin and eosin (HE) and Masson trichrome stains were used for histopathological diagnoses of formalin-fixed paraffin-embedded liver tissues. The diagnosis of NASH was evaluated on Brunt’s Criteria[19]. Histological characteristics were graded according to the NAFLD scoring system recommended by the National Institute of Diabetes and Digestive and Kidney Diseases NASH Clinical Research Network[20]. Steatosis was graded as 0 ≤ 5%; 1 ≥ 33%-66%; 3 ≥ 66% lobular inflammation was graded as 0 = no foci; 1 ≤ 2 foci; 2 = 2-4 foci; 3 ≥ 4 foci; and ballooning was graded as 0 = none; 1 = few ballooning cells; 2 = many ballooning cells, respectively.
Depending on the recommendations of Brunt’s criteria steatosis (0-3), lobular inflammation (0-3) and ballooning (0-2) were then combined to establish the NAFLD activity score (0-8). Fibrosis was also scored as 0 = no fibrosis; 1 = periportal or perisinusoidal fibrosis; 2 = perisinusoidal and portal/periportal fibrosis; 3 = bridging fibrosis; and 4 = cirrhosis. While mild fibrosis was defined with fibrotic score ≤ 1 advanced fibrosis was defined with fibrotic score > 1. Mild fibrosis caused by NASH is shown in Figure 1A and advanced fibrosis in Figure 1B.
Figure 1.
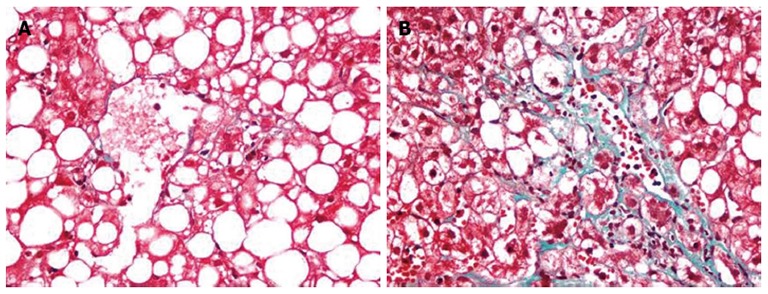
Mild perivenular-perisinusoidal fibrosis (A) and advanced perivenular-perisinusoidal, and periportal fibrosis (B) in non-alcoholic steatohepatitis. A, B: Trichrome stain, x 400.
Ethics
The study was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board.
Statistical analysis
Statistical analyses were performed using the SPSS software version 17 and MedCalc version 12. The variables were investigated by using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk’s test) to determine whether or not they were normally distributed. Non-normally distributed and non-parametric variables were compared between groups using the Mann-Whitney U test where appropriate. The χ2 test, where appropriate, was used to compare the propositions in different groups. As the RDW was not normally distributed, the Kruskal-Wallis test was used for comparisons among NASH, simple steatosis and healthy control groups. The Mann-Whitney U test was used to test the significance of pairwise differences, using Bonferroni correction to adjust for multiple comparisons. For the multivariate analysis, the possible factors identified with univariate analyses were further entered into the logistic regression analysis to determine independent predictors of NASH. To assess model fit, we used Hosmer-Lemeshow goodness of fit statistics. Spearman rank correlation coefficients (r) were calculated to assess the correlation between RDW and liver histopathological features (inflammation, NAS score, the degree of steatosis and degree of fibrosis) and clinical characteristics of NASH patients. Receiver operating characteristics (ROC) analysis was used to evaluate the role of RDW in distinguishing subjects with NASH and fibrosis. ROC analysis was also used to distinguish advanced and mild fibrotic stage groups. Cutoff values that maximized both sensitivity and specificity were chosen for the NASH group. P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics and liver histology
Sixty-two biopsy proven NASH patients, 32 biopsy proven simple steatosis patients and 30 healthy controls were recruited. The median ages of patients with NASH, simple steatosis and healthy controls were 49.5 years (22-77 years), 48 years (24-71 years) and 48 years (24-72 years), respectively. There was no statistically significant difference between the ages of participants. The mean BMI (kg/m2) of NASH, simple steatosis and healthy controls were 27.13 ± 0.37, 25.97 ± 0.67 and 24.22 ± 0.45 kg/m2, respectively (P < 0.01). The platelet counts among NASH, simple steatosis and healthy controls were 245.5 ± 34.73, 244.5 ± 13.8 and 260 ± 4.47 × 103/mL, respectively (P > 0.05). However, there was an inversely significant correlation between platelet counts and fibrotic scores (r = -0.335 and P < 0.01). Among the NASH group 30/62 (48.3%), steatosis group 18/32 (56.2%) and healthy controls 18 (60%) were men (P > 0.05). The median RDW values were 14.28% ± 0.25%, 13.37% ± 0.12% and 12.96% ± 0.14%, respectively (P < 0.01). The clinical and laboratory data of patients with NASH, simple steatosis and healthy control groups are summarized in Table 1. The comparison of RDW values among NASH, simple steatosis and healthy control groups is also shown in Figure 2A.
Table 1.
Demographic and laboratory features of non-alcoholic steatohepatitis, simple steatosis and healthy control groups
| Factor | NASH group (n = 62) | Steatosis group (n = 32) | Healthy control group (n = 30) | P-value |
| Age mean ± SD | 48.81 ± 12.21 | 47.25 ± 12.58 | 48.03 ± 9.54 | NS |
| Gender (male) | 30 (48.3) | 18 (56.2) | 18 (60) | NS |
| BMI (kg/m2) | 27.13 ± 0.37 | 25.97 ± 0.67 | 24.22 ± 0.45 | < 0.01 |
| Hemoglobin (g/dL) | 14.10 ± 0.16 | 14.51 ± 0.28 | 13.91 ± 0.11 | NS |
| Platelet (× 103/mL) | 245.5 ± 34.73 | 244.5 ± 13.8 | 260 ± 4.47 | NS |
| WBC (k/UL) | 6950 ± 319 | 7250 ± 330 | 7350 ± 146 | NS |
| Neutrophil ratio | 59.39% ± 1.20% | 59.87% ± 1.20% | 59% ± 1.1% | NS |
| Lymphocyte ratio | 31.29% ± 1.09% | 29.88% ± 1.10% | 32% ± 0.78% | NS |
| RDW | 14.28% ± 0.25% | 13.37% ± 0.12% | 12.96% ± 0.14% | < 0.01 |
| MPV (fL) | 8.63 ± 1.30 | 8.49 ± 1.06 | 8.20 ± 0.59 | NS |
| MCV (fL) | 86.34 ± 0.88 | 87.98 ± 1.37 | 85.5 ± 0.82 | NS |
| ALT (IU/L) | 54 ± 8.1 | 45 ± 3.97 | 32.5 ± 1.49 | < 0.01 |
| AST (IU/L) | 40.05 ± 6.04 | 40.05 ± 3.01 | 24 ± 1.39 | < 0.01 |
| ALP (IU/L) | 87.5 ± 9.09 | 81.5 ± 3.91 | 61 ± 1.87 | < 0.01 |
| GGT (IU/L) | 61 ± 13.4 | 32 ± 3.4 | 27 ± 2.5 | < 0.01 |
| Albumin (g/dL) | 4.36 ± 0.73 | 4.5 ± 0.42 | 4.9 ± 0.75 | < 0.01 |
| Creatinine | 0.78 ± 0.033 | 0.79 ± 0.023 | 0.72 ± 0.014 | NS |
| BUN (mg /dL) | 12.25 ± 0.85 | 14.2 ± 0.67 | 13.46 ± 0.71 | NS |
| Diabetes mellitus | 19 (30.6) | 9 (28.1) | 0 (0) | NS |
| Hypertension | 22 (35.4) | 7 (21.8) | 0 (0) | NS |
Values are presented as n (%) frequencies, median ± SE for skewed distributed variables. BMI: Body-mass index; RDW: Red cell distribution width; MPV: Mean platelet volume; NS: Not significant; ALT: Alanine aminotransaminase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GGT: γ glutamyl transferase; WBC: White blood cell; MCV: Mean corpuscle volume; BUN: Blood urine nitrogen.
Figure 2.
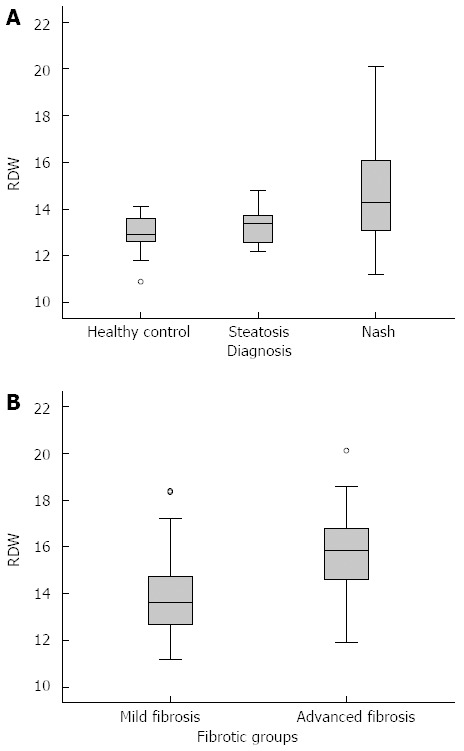
Red cell distribution width values of healthy controls, simple steatosis and non-alcoholic steatohepatitis groups (A), and fibrotic subgroups of non-alcoholic steatohepatitis (B). RDW: Red cell distribution width.
Relationship between RDW and the presence of NASH and fibrosis
The ROC analysis suggested that a cutoff value of 13.56 has the highest sensitivity (61.3%) and specificity (72.6%) for detecting patients with NASH with an area under the curve (AUC) of 0.70 (95%CI: 0.600-0.805), (P < 0.01), as shown in Figure 3A.
Figure 3.
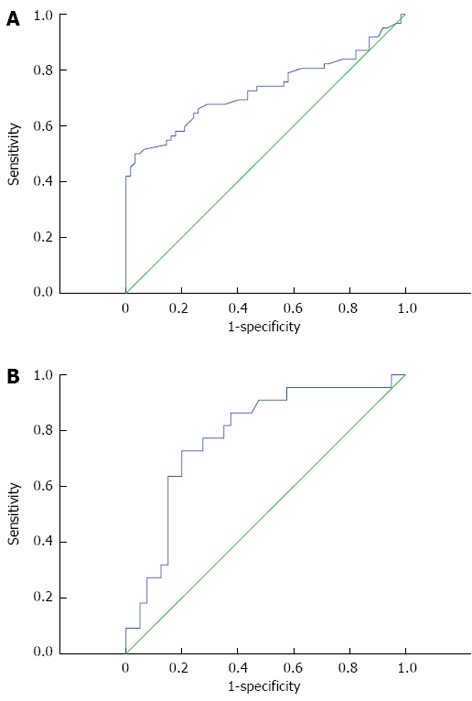
Receiver operating characteristics curve of red cell distribution width values for the identification of patients with non-alcoholic steatohepatitis (A), and the identification of fibrosis in non-alcoholic steatohepatitis (B).
According to the liver histopathological features, while the mild fibrosis group consisted of 40 patients, there were 22 patients in advanced fibrosis group. We compared RDW values between the mild and advanced fibrosis subgroups of NASH and found a statistically significant difference (13.95% ± 1.74% and 15.85% ± 1.89%, respectively, P < 0.01), as seen in Figure 2B. The ROC curve of RDW for the identification of advanced fibrosis in NASH was statistically significant (AUC = 0.78, 95%CI: 0.660 -0.903, P < 0.01), as shown in Figure 3B.
Correlation between RDW and clinical/histopathological features of patients with NASH
In Spearman correlation analysis, there were statistically significant correlations between RDW values and fibrotic scores, age and gender, as seen in Table 2. The correlation between fibrotic scores and RDW values reached statistically significance, such that Spearman’s correlation coefficients were r = 0.42, and P < 0.001, as shown in Figure 4.
Table 2.
Red cell distribution width correlated with clinical characteristics and histological features of non-alcoholic steatohepatitis
| Factor | r-value | P-value |
| Age (yr) | 0.245 | < 0.01 |
| BMI (kg/m2) | 0.131 | NS |
| Gender | -0.251 | < 0.01 |
| Fibrosis | 0.579 | < 0.01 |
| Inflammation | 0.207 | NS |
| Steatosis | 0.121 | NS |
| Ballooning | 0.175 | NS |
| NASH | 0.217 | NS |
BMI: Body mass index; RDW: Red cell distribution width; NASH: non-alcoholic steatohepatitis; NS: Not significant.
Figure 4.
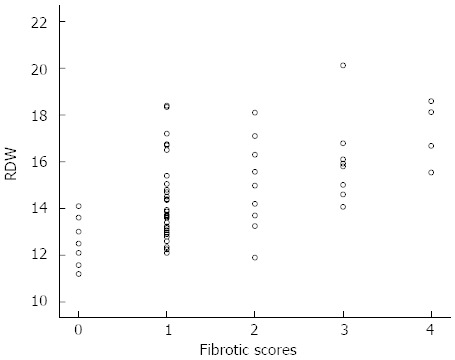
Correlation between red cell distribution width values and fibrotic scores of non-alcoholic steatohepatitis. RDW: Red cell distribution width. Score 0, n = 8; Score 1, n = 32; Score 2, n = 10; Score 3, n = 8; Score 4, n = 4.
Univariate analysis for the presence of NASH indicated that age, gender, platelet counts, ALT, AST, ALP, GGT, albumin, BMI and RDW were statistically significant predictor factors of the presence of the NASH. The results of univariate analysis are shown in Table 3.
Table 3.
Univariate analyses for predicting liver fibrosis
| OR | 95%CI | P-value | |
| Age | 1.05 | 1.004-1.109 | < 0.05 |
| Gender | 1.46 | 0.516-4.171 | NS |
| Platelet | 0.98 | 0.988-0.996 | < 0.01 |
| ALT | 1.08 | 0.976-1.004 | NS |
| AST | 0.98 | 0.963-1.007 | NS |
| ALP | 1.015 | 1.002-1.028 | < 0.05 |
| GGT | 1.002 | 0.998-1.007 | NS |
| RDW | 1.73 | 1.242-2.409 | < 0.01 |
| Albumin | 0.418 | 0.158-1.105 | NS |
| BMI | 1.012 | 0.847-1.209 | NS |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GGT: γ glutamyl aminotransferase; RDW: Red cell distribution width; BMI: Body mass index; NS: Not significant.
Those variables that were statistically significant in univariate logistic regression analyses were furthered subjected to multivariate logistic regression analysis, and RDW continued to be statistically significant and an independent predictor of fibrosis (OR = 1.75, 95%CI: 1.129-2.711, P < 0.001), as seen in Table 4.
Table 4.
Multivariate analyses for advanced liver fibrosis
| OR | 95%CI | P-value | |
| ALP | 1.005 | 0.993-1.018 | NS |
| RDW | 1.75 | 1.129-2.711 | < 0.05 |
| Age | 1.02 | 0.957-1.086 | NS |
| Platelet | 0.991 | 0.981-1.001 | NS |
| Albumin | 1.016 | 0.291-3.545 | NS |
ALP: Alkaline phosphatase; RDW: Red cell distribution width; NS: Not significant.
DISCUSSION
In the present study, we concluded that patients with biopsy proven NASH have higher RDW values compared with simple steatosis patients and healthy controls. The increase in RDW values correlated with disease progression because the patients with advanced fibrosis had higher RDW values than the mild fibrosis group. There was a positive correlation between RDW values and fibrotic scores. As a result of multivariate logistic regression analysis RDW was identified as an independent predictor of NASH and advanced liver fibrosis.
The search for a noninvasive diagnostic marker indicating the histological changes and fibrotic stages observed in NASH is very important. The absence of approved treatment methods for NASH means that care must be taken on the detection of advanced fibrosis. The risk stratification requires an adequate evaluation of fibrosis, which only currently possible by performing a liver biopsy. Biopsy is an invasive method with natural risks, costs, and is disturbing for the patients; therefore, considerable efforts have been made in the development of noninvasive alternatives of determining the degree of liver fibrosis.
Lou et al[21] found that RDW values were significantly increased in patients with hepatitis B and that high RDW values were associated with disease severity. Similarly Chen et al[22] concluded that RDW to platelet index is a routinely available and easily calculated index that could predict significant liver fibrosis caused by chronic hepatitis B with a high accuracy. They also estimated that this index may reduce the requirement of liver biopsy in patients infected with chronic hepatitis B. High RDW values are associated with poor outcomes in patients with cardiovascular diseases[23,24]. Although the main mechanism that causes elevation of RDW levels in these different conditions is unknown, it is speculated that inflammation may play an important role[25]. Some pathways may explain the increase in RDW values, such as impaired iron metabolism, suppressed erythropoietin gene expression, inhibition of proliferation of erythroid progenitor cells, downregulation of erythropoietin receptor expression and reduced erythrocyte circulatory half-life[26].
Alkhouri et al[27] found that the neutrophil to lymphocyte ratio was higher in patients with NASH and advanced fibrosis. They hypothesized that this ratio could be used as a novel noninvasive marker to predict advanced liver fibrosis caused by NASH. Tonelli et al[28] reported that higher RDW values might reflect an underlying chronic inflammation, which could result in an increased risk of cardiovascular disease.
These results suggest that inflammation may be a potential underlying biological mechanism for increased RDW values. Chronic inflammation may play a role in disease progression of NASH and fibrosis. Indeed, multiple inflammatory cytokines have been assessed in different studies as noninvasive markers for the presence of NASH and fibrosis[29]. Thus, increased RDW values may be considered as a chronic inflammatory process in the pathogenic basis of NASH. Moreover our findings revealed that RDW could discriminate fibrotic scores of NASH, making it a potentially useful marker for predicting the progression and fibrotic stages of NASH.
We hypothesized that the relationship between RDW and NASH may be a result of an effect of an inflammatory process that suppresses mature erythrocytes and secretes young erythrocytes into the circulation, leading to anisocytosis and high RDW values. To the best of our knowledge, this is the first study to evaluate an association between high RDW values and high fibrotic scores in a well-designed, specifically organized patient group of biopsy proven NASH.
There are some limitations to our study that: RDW was assessed on a single situation instead of multiple consecutive measurements, therefore we could not evaluate any biological variabilities and measurement errors that could be a result of this. Also erythropoietin, reticulocyte count, inflammatory markers such as interleukin (IL)-1, IL-6, tumor necrosis factor-α were not provided; they might provide some important information on the pathophysiology of underlying high RDW physiology. A lack of a marked fall in the platelet count of NASH patients may reflect the small sample size and small number of cirrhotic patients. This must be evaluated in large population based prospective studies.
In summary, our study showed that in patients with biopsy proven NASH, RDW is associated with histological severity and could be used to identify patients with advanced liver fibrosis. Unlike many other noninvasive markers of NAFLD, RDW is inexpensive, widely available and easily repeatable. Although the accuracy of RDW for detecting NASH and significant fibrosis is sufficient, RDW in combination with other markers may help to identify patients at increased risk of having advanced disease. Perhaps independently of anemia and other factors, RDW could be used to estimate the fibrotic process of different diseases. It may become one of the cornerstones of inflammation and fibrosis if confirmed in large population based studies in the future.
COMMENTS
Background
Non-alcoholic steatohepatitis (NASH) is one of the most common causes of chronic liver diseases. Liver biopsy is the only method for distinguishing NASH from other diagnoses and evaluating the damage and fibrosis of liver, but it may have procedure-related clinical complications.
Research frontiers
Therefore, there has been an attempt to find noninvasive methods for detecting the presence of NASH, liver fibrosis and avoiding procedure-related complications. In this study, the authors demonstrated that red cell distribution width (RDW) may indicate the presence of NASH and advanced liver fibrosis.
Innovations and breakthroughs
Recent reports have demonstrated that elevated RDW values were related to negative outcomes in cardiovascular and metabolic disorders, colon cancer and stroke independent of hemoglobin values. The association between RDW values and the severity of liver diseases has also been reported in two recent studies. This is the first study to demonstrate the importance of RDW in the diagnosis of NASH and predicting advanced liver fibrosis.
Applications
By understanding that high RDW values can be seen in the presence of NASH and advanced liver fibrosis, this study may represent a future strategy for non-invasive diagnostic method of patients with NASH.
Terminology
RDW is a widely available, inexpensive and easily repeatable parameter of variation of circulating red cells, reflecting the heterogeneity of red cell volume, a component of the complete blood count. Chronic inflammation plays a significant role in disease progression to NASH. Liver fibrosis is a result of chronic injury and can only be diagnosed invasively by liver biopsy.
Peer review
The results present a new laboratorial and non-invasive marker of NASH and fibrotic stage. The authors concluded that in patients with biopsy proven NASH, RDW is associated with histological severity and could be used to identify patients with advanced liver fibrosis disease. This result is interesting and RDW may be used as a non-invasive marker of indicating the presence of NASH and advanced liver fibrosis.
Footnotes
P- Reviewers: Castiella A, Ji G, Nakano S S- Editor: Zhai HH L- Editor: Stewart GJ E- Editor: Zhang DN
References
- 1.Brunt EM. Nonalcoholic steatohepatitis: pathologic features and differential diagnosis. Semin Diagn Pathol. 2005;22:330–338. doi: 10.1053/j.semdp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 4.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 5.Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, Hu PJ. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafrani ES. Non-alcoholic fatty liver disease: an emerging pathological spectrum. Virchows Arch. 2004;444:3–12. doi: 10.1007/s00428-003-0943-7. [DOI] [PubMed] [Google Scholar]
- 7.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 9.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 10.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 13.Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–108. doi: 10.1016/j.jns.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Spell DW, Jones DV, Harper WF, David Bessman J. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev. 2004;28:37–42. doi: 10.1016/j.cdp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee WS, Kim TY. Relation between red blood cell distribution width and inflammatory biomarkers in rheumatoid arthritis. Arch Pathol Lab Med. 2010;134:505–506. doi: 10.5858/134.4.505.c. [DOI] [PubMed] [Google Scholar]
- 16.Malandrino N, Wu WC, Taveira TH, Whitlatch HB, Smith RJ. Association between red blood cell distribution width and macrovascular and microvascular complications in diabetes. Diabetologia. 2012;55:226–235. doi: 10.1007/s00125-011-2331-1. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z, Sun Y, Wang Q, Han Z, Huang Y, Liu X, Ding C, Hu C, Qin Q, Deng A. Red blood cell distribution width is a potential prognostic index for liver disease. Clin Chem Lab Med. 2013;51:1403–1408. doi: 10.1515/cclm-2012-0704. [DOI] [PubMed] [Google Scholar]
- 18.Milić S, Mikolasević I, Radić M, Hauser G, Stimac D. Clinical utility of red cell distribution width in alcoholic and non-alcoholic liver cirrhosis. Coll Antropol. 2011;35 Suppl 2:335–338. [PubMed] [Google Scholar]
- 19.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Lou Y, Wang M, Mao W. Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B. PLoS One. 2012;7:e37644. doi: 10.1371/journal.pone.0037644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One. 2013;8:e68780. doi: 10.1371/journal.pone.0068780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868–872. doi: 10.1016/j.amjcard.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CK, Chan J, Cembrowski GS, van Assendelft OW. Complete blood count reference interval diagrams derived from NHANES III: stratification by age, sex, and race. Lab Hematol. 2004;10:42–53. doi: 10.1532/lh96.04010. [DOI] [PubMed] [Google Scholar]
- 25.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 26.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 27.Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, Zein NN, Feldstein AE. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 28.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 29.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]


