Abstract
AIM: To explore the effects of curcumin (CMN) on hepatic injury induced by acetaminophen (APAP) in vivo.
METHODS: Male mice were randomly divided into three groups: group I (control) mice received the equivalent volumes of phosphate-buffered saline (PBS) intraperitoneally (ip); Group II [APAP + carboxymethylcellulose (CMC)] mice received 1% CMC (vehicle) 2 h before APAP injection; Group III (APAP + CMN) mice received curcumin (10 or 20 mg/kg, ip) 2 h before before or after APAP challenge. In Groups II and III, APAP was dissolved in pyrogen-free PBS and injected at a single dose of 300 mg/kg. CMN was dissolved in 1% CMC. Mice were sacrificed 16 h after the APAP injection to determine alanine aminotransferase (ALT) levels in serum and malondialdehyde (MDA) accumulation, superoxide dismutase (SOD) activity and hepatocyte apoptosis in liver tissues.
RESULTS: Both pre- and post-treatment with curcumin resulted in a significant decrease in serum ALT compared with APAP treatment group (10 mg/kg: 801.46 ± 661.34 U/L; 20 mg/kg: 99.68 ± 86.48 U/L vs 5406.80 ± 1785.75 U/L, P < 0.001, respectively). The incidence of liver necrosis was significantly lowered in CMN treated animals. MDA contents were significantly reduced in 20 mg/kg CMN pretreatment group, but increased in APAP treated group (10.96 ± 0.87 nmol/mg protein vs 16.03 ± 2.58 nmol/mg protein, P < 0.05). The decrease of SOD activity in APAP treatment group and the increase of SOD in 20 mg/kg CMN pretreatment group were also detected (24.54 ± 4.95 U/mg protein vs 50.21 ± 1.93 U/mg protein, P < 0.05). Furthermore, CMN treatment efficiently protected against APAP-induced apoptosis via increasing Bcl-2/Bax ratio.
CONCLUSION: CMN has significant therapeutic potential in both APAP-induced hepatotoxicity and other types of liver diseases.
Keywords: Acetaminophen, Acute hepatic injury, Apoptosis, Free radicals, Curcumin
Core tip: Acetaminophen (APAP) and curcumin (CMN) were administrated intraperitoneally. The aim of the study was to explore whether CMN has effect on APAP-induced hepatic toxicity in vivo. The findings revealed that CMN protects against APAP-induced lipid peroxidation, oxidative stress and hepatocyte apoptosis.
INTRODUCTION
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug that is safe and effective when taken at therapeutic dose[1]. However, it can cause severe liver damage or even acute liver failure that can be fatal in experimental animals and humans when administered in an acute or cumulative overdose[2,3]. APAP overdose is the leading cause for calls to Poison Control Centers (> 100000/year) and accounts for more than 56000 emergency visits, 2600 hospitalizations, and an estimated 458 deaths each year in the United States[3].
APAP is metabolized by cytochrome P450 to N-acetyl-p-benzoquinone imine (NAPQI). NAPQI can react rapidly with glutathione (GSH), so large doses of APAP may result in a profound depletion of hepatocellular GSH[1,4]. Once GSH is exhausted, any remaining NAPQI will covalently bind to cellular proteins and induce mitochondrial dysfunction, lipid peroxidation, oxidative stress, and DNA fragmentation, eventually leads to massive hepatocyte necrosis, liver damage or death[5]. N-acetyl cysteine has been currently used in the treatment of APAP-induced liver toxicity[6]. In addition to its adverse reaction, a major concern when using N-acetyl cysteine is its relatively narrow therapeutic windows and drug toxicity[7,8]. Therefore, new and safe preventive measures against APAP toxicity are eagerly needed.
In recent years, natural products from plants have received considerable attention as a rich resource for drug development. Curcumin (CMN) is a yellow pigment purified from the root tubers of Curcuma longa Linn (commonly known as turmeric), which has long been used as a food colorant and preservative[9]. CMN also has a variety of biological and pharmacological activities, such as anti-inflammatory, anti-oxidant, antifungal, antibacterial and anticancer activities[10]. It was reported that CMN attenuates liver injury induced by ethanol[11], iron overdose[12] and carbon tetrachloride intoxication[13].
The aim of this study was to explore the effect of CMN on the prevention of APAP-induced hepatic toxicity in vivo and investigate whether CMN affects the production of lipid peroxidation, oxidative stress or hepatocyte apoptosis to attenuate liver damage.
MATERIALS AND METHODS
Materials
APAP and CMN were purchased from Sigma Aldrich (Saint Louis, MO, United States). Detection kits for superoxide dismutase (SOD) and malondial dehyde (MDA) were purchased from Nanjing Jiancheng Bioengineer Institute (Nanjing, China). Transferase-mediated dUTP-biotin nick end labeling (TUNEL) detection kit was purchased from Boster Biological Technology Co., Ltd (Wuhan, China).
Animals and treatment
Male BALB/c mice (6-8 wk of age) were purchased from the Center for Animal Experiment of Wuhan University (Wuhan, China). The mice were raised at an animal facility under special pathogen-free conditions with a 12-h light/dark cycle and free access to food and water at least 1 wk prior to treatment. All animal experiments were approved by the institutional animal care and use committee at the Yangtze University, and all efforts were made to minimize the number of animals used and their sufferings. Mice were randomly divided into three groups: group I (control) mice received the equivalent volumes of PBS intraperitoneally (ip); Group II (APAP + CMC) mice received 1% CMC (vehicle) 2 h before APAP injection; and Group III (APAP + CMN) mice received CMN (10 or 20 mg/kg, ip) 2 h before or after APAP challenge. In Group II and Group III, APAP was dissolved in pyrogen-free PBS and injected at a single dose of 300 mg/kg; the dose was selected on the basis of a previous related study[14]. CMN was dissolved in 1% CMC. Serum and livers were collected at 16 h after APAP treatment.
Biochemical analysis
Alanine amino-transaminase (ALT) activity was determined using a diagnostic assay kit (Sichuan Maker Science and Technology Co., Ltd., Chengdu, China) by an automated chemistry analyzer (Olympus AU1000, Japan) by Central Laboratory of the Affiliated Jingzhou Hospital of Yangtze University.
Livers were quickly removed, washed with ice-cold PBS, blotted and weighed, and then a tissue homogenate (1% or 10%, w/v) was prepared in normal saline. The homogenates were then centrifuged at 4000 rpm (4 °C) for 20 min to collect supernatants for determination of SOD and MDA contents at 550 nm and 532 nm, respectively.
Lipid peroxidation was assessed by estimation of MDA in the liver tissues according to the method of Wills (1966). MDA was determined by the thiobarbituric acid assay using a MDA assay kit, according to the manufacturers’ instructions. Liver tissue protein was measured using Coomassie Brilliant Blue protein reagent, and MDA content was expressed as nmol/mg protein. SOD activity was determined by measuring the inhibition of formation of NADPH-phenazine methosulphate nitroblue tetrazolium.
Histochemistry
Liver tissues fixed in 10% formalin were embedded in paraffin, sectioned at 4 μm and stained with hematoxylin-eosin.
TUNEL staining
Paraffin-embedded liver tissues were assayed for DNA fragmentation using a terminal deoxynucleotidyl TUNEL reaction, according to the manufacturer’s instructions. Slides were developed with diaminobenzidine substrate, counterstained with HE, and then examined for evidence of apoptosis. The number of brown apoptotic cells was normalized to total cells as detected by HE. Four fields of each image were counted.
Reverse transcriptase polymerase chain reaction
RNA was extracted from the livers using TRIzol® Reagent (Invitrogen) according to the manufacturer’s instruction. cDNA was synthesized from 2 μg of total RNA using PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara Biotechnology, Co., Ltd., Da Lian, LiaoNing, China). PCR amplifications were performed by standard methods using following specific primers: for Bcl-2: Sense: 5’-GGC ATC TTC TCC TTC CAG-3’, Antisense: 5’-CTA CCC AGC CTC CGT TAT-3’; for Bax: Sense: 5’-TTT CAT CCA GGA TCG AGC AGG-3’, Antisense: 5’-GCA AAG TAG AAG AGG GCA ACC AC-3’[15].
Statistical analysis
A computer program (SPSS 13.0) was used for statistical analysis. Data were presented as mean ± SE. Student’s t test (two groups) or one way ANOVA (multiple groups) were used. P < 0.05 indicated statistical significance.
RESULTS
CMN treatment attenuates APAP-induced liver injury
To explore the protective effect of CMN on APAP-induced hepatic toxicity, the animals were injected intraperitoneally with CMN (10 or 20 mg/kg body weight) 2 h before APAP (300 mg/kg body weight), and serum ALT was analyzed after 16 h of administration. Compared with PBS control (41.70 ± 2.82 U/L), APAP treatment significantly increased serum ALT levels (5406.80 ± 1785.75 U/L, 126.9-fold of the control, Figure 1A, P < 0.001 vs control). As expected, CMN pretreatment significantly suppressed the plasma ALT activity in a dose-dependent manner (10 mg/kg: 801.46 ± 661.34 U/L; 20 mg/kg: 99.68 ± 86.48 U/L, Figure 1A, P < 0.001 vs the model group). This protective effect was further confirmed by analysis of histological findings, as shown in Figure 1B, severe sinusoidal congestion and hemorrhage, inflammatory cell infiltration and gross necrosis were observed in the liver of mice treated with APAP. However, these pathological changes were dramatically suppressed by CMN treatment. To evaluate its potential therapeutic role, CMN was administrated after 2 h of APAP injection, and a marked reduction of serum ALT was also observed (395.40 ± 133.52 U/L, Figure 1A). Of note, CMN alone did not influence serum transaminase (Figure 1A) and urea, creatinine (data not shown) in normal control mice.
Figure 1.
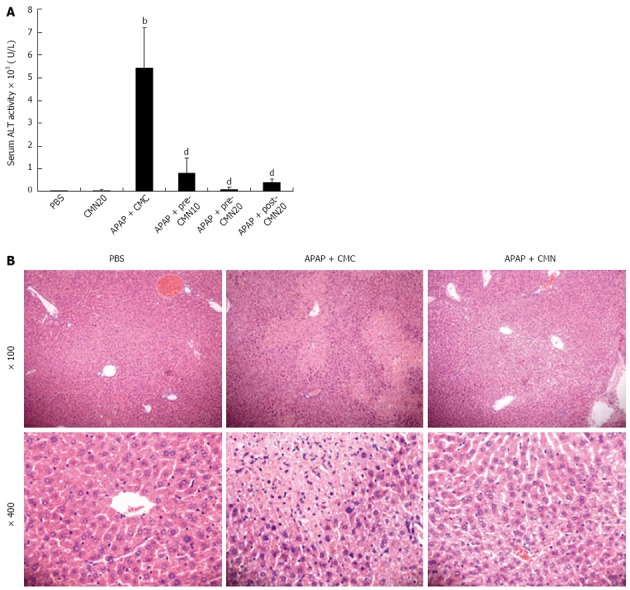
Curcumin treatment protects against acetaminophen-induced hepatic injury in mice. A: Serum alanine aminotransferase (ALT) levels were determined 16 h after acetaminophen injection. Data are expressed as mean ± SE; n = 10 mice per group. bP < 0.01 vs control; dP < 0.001 vs acetaminophen (APAP) + carboxymethylcellulose (CMC); B: Hematoxylin-eosin stained liver sections from animals treated with PBS, APAP + CMC and APAP + CMN (original magnification: ×100 and ×400). Severe inflammatory cell infiltration and gross necrosis of the entire centrilobular areas were obvious in APAP group, and the results were significantly ameliorated in CMN-treated animals. CMN: Curcumin.
CMN pretreatment inhibits production of MDA
As lipid peroxidation has been reported to be closely related to APAP-induced toxicity, the content of malondialdehyde, the end product of lipid peroxidation, in the liver tissues was detected at 16 h after APAP treatment. Only low levels of MDA were observed in the control mice (10.81 ± 1.46 nmol/mg protein), but a significant increase was found in APAP-treated mice (16.03 ± 2.58 nmol/mg protein). As expected, MDA contents were significantly inhibited by 20 mg/kg CMN pretreatment (10.96 ± 0.87 nmol/mg protein, Figure 2).
Figure 2.
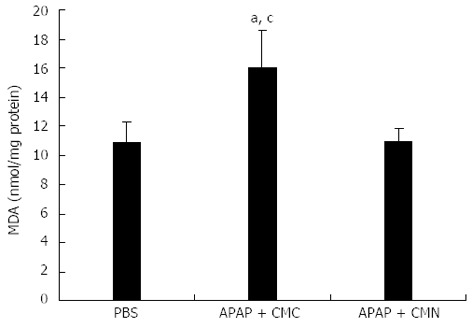
Curcumin pretreatment inhibits malondialdehyde production after acetaminophen induction. Liver homogenate was prepared to analyze the content of malondialdehyde (MDA) 16 h after acetaminophen (APAP) administration. Data are expressed as mean ± SE; n = 10 mice per group. aP < 0.05 vs control; cP < 0.05 vs APAP + curcumin (CMN). CMC: Carboxymethylcellulose.
CMN pretreatment enhances activity of SOD
In addition to lipid peroxidation, oxidative stress is an early events related to radicals generated during the hepatic metabolism of APAP. SOD is an enzyme that neutralizes free radicals[16]. So the activity of SOD was investigated at 16 h after APAP treatment in the liver tissues. A significant decrease in SOD activity was observed in the mice treated with APAP compared with control group (24.54 ± 4.95 U/mg protein vs 45.64 ± 5.96 U/mg protein). However, pretreatment of mice with CMN induced a significant increase in the activity of SOD (50.21 ± 1.93 U/mg protein, Figure 3).
Figure 3.
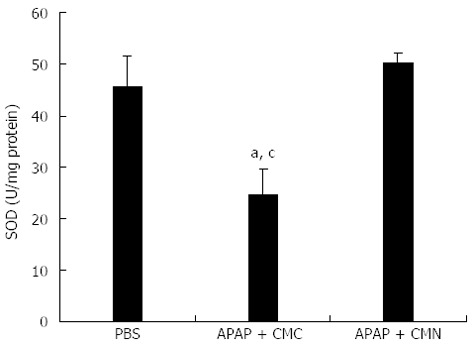
Curcumin pretreatment enhances activity of superoxide dismutase after acetaminophen. Liver homogenate was prepared to analyze the activity of superoxide dismutase (SOD) 16 h after acetaminophen (APAP) administration. Data are expressed as mean ± SE; n = 10 mice per group. aP < 0.05 vs control; cP < 0.05 vs APAP + curcumin (CMN). CMC: Carboxymethylcellulose.
CMN pretreatment prevents hepatocyte apoptosis
Based on the above observations, we further explored the possible mechanisms by which CMN attenuates liver injury induced by APAP. Given the importance of apoptosis in APAP-induced liver injury, the extent of hepatocyte apoptosis was determined by TUNEL assay. As shown in Figure 4A, massive hepatocyte apoptosis was detected in the livers of mice treated with APAP. CMN pretreatment markedly prevented the apoptosis induced by APAP. Furthermore, we examined the mRNA expression of anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bax in the livers. As shown in Figure 4B, CMN pretreatment down-regulated the mRNA expression of Bax and up-regulated the mRNA expression of Bcl-2 compared with APAP-treated group. These data suggest that CMN inhibits hepatocyte apoptosis via regulating the gene expression of Bcl-2 family and then protects the mice from APAP-induced hepatic injury.
Figure 4.
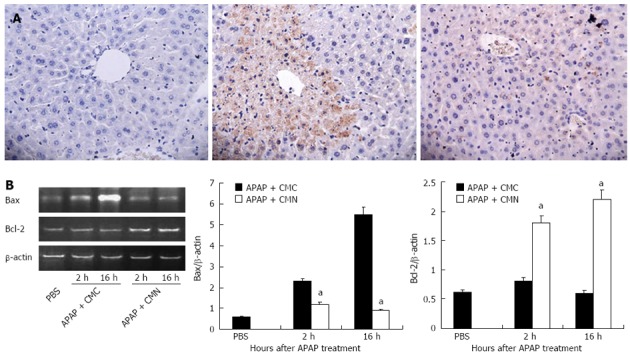
Curcumin pretreatment prevents hepatocyte apoptosis induced by acetaminophen. A: Transferase-mediated dUTP-biotin nick end labeling stained liver sections from animals treated with PBS, acetaminophen (APAP) + carboxymethylcellulose (CMC) and APAP + curcumin (CMN) (original magnification: × 400); B: Liver samples were collected 2 h and 16 h after APAP injection, and the mRNA expression of Bax and Bcl2 was determined by reverse transcriptase polymerase chain reaction. Data are expressed as mean ± SE; n = 6 mice per group. aP < 0.05 vs APAP + CMC.
DISCUSSION
In the present study, for the first time, we examined the effect of CMN on liver injury induced by APAP and the possible mechanisms in mice. Our data demonstrated that CMN pretreatment dose-dependently alleviated APAP-induced acute liver injury. CMN pretreatment markedly decreased ALT levels in plasma and inhibited the necrosis of hepatocytes in Con A-treated mice. Furthermore, CMN could be also considered as a rescue therapy, for it significantly decreased APAP-induced hepatotoxicity when administered 2 h after APAP overdose.
NAPQI is the reactive metabolite product generated from APAP-induced hepatic toxicity, and it was found to be formed by cytochrome P-450 by a direct two electron oxidation of APAP. At low doses, the metabolite was efficiently detoxified by GSH. However, at high doses, NAPQI leads to GSH depletion and subsequently covalently binds to cysteine residues on proteins, which results in lipid peroxidation reaction[14,17]. As a metabolite of free radical, MDA is generally considered as an important indicator of lipid peroxidation. In this study, in line with previous reports, we found that MDA in liver tissues was increased significantly 16 h after APAP administration. As expected, our results demonstrated that CMN treatment could inhibit the increase of MDA induced by APAP, suggesting that CMN has potent beneficial effects on lipid peroxidation.
Our organism has a function to neutralize and scavenge the free radical in order to prevent oxidative damage to cells. Such endogenous mechanisms are mainly provided by a set of antioxidant enzymes such as SOD, glutathione peroxidase, and catalase. SOD represents the first line of defense against free radicals, it converts superoxide anion into hydrogen peroxide, and then hydrogen peroxide is converted into oxygen and water by glutathione peroxidase, making reduced GSH as its substrate[18]. As a antioxidant, CMN has been demonstrated to effectively prevent the decrease in SOD activity in a variety of experimental models, including inflammation, cardiotoxicity and carbon tetrachloride-induced liver injury models[19]. In line with these concepts, we found that the major scavenger enzyme SOD activity was significantly decreased in the liver of APAP-treated mice. As expected, pretreatment with CMN restored SOD activity. Therefore, it is suggested that the protective effect of CMN on APAP-induced injury is associated with its inhibition of oxidative stress.
Accumulating evidence suggests that hepatocyte apoptosis plays a critical role in APAP-induced hepatic injury, although the mode of cell death inflicted by APAP is still controversial[20,21]. APAP-induced apoptosis is observed not only in primary hepatocytes[22], but also in livers of mice treated with toxic doses of APAP[23]. Also, a recent report showed that hepatic caspase-3 and caspase-9 are activated in both wild type and CXCR2 knock out mice within one hour of APAP treatment[21]. Moreover, inhibiting apoptosis prevents the development of acute liver failure[23]. Based on these concepts, we found that apoptotic hepatocytes were significantly increased in the liver of mice 16 h after APAP treatment and the hepatocyte apoptosis was significantly reduced by CMN pretreatment. Bcl-2 protein is commonly recognized as an anti-apoptotic factor, it inhibits cell apoptosis by preventing mitochondrial membrane depolarization. As a member of the Bcl-2 family, Bax inactivates Bcl-2 by interacting with it to form a heterodimer[24]. In this study, we found that CMN pretreatment down-regulated the mRNA expression of Bax and up-regulated the mRNA expression of Bcl-2 compared with APAP-treated group, suggesting that CMN can increase Bcl-2/Bax ratio, thus reducing APAP-induced apoptosis. The mechanisms of CMN-mediated anti-apoptotic effect remained unclear. A recent study showed that CMN exerts a potent anti-apoptotic effect via inhibition of TGF-β as inducer of caspase-3 mediated apoptosis in kidney and lung tissues[25], however, the precise mechanisms by which CMN modulates cell apoptosis in APAP-induced liver injury need to be further investigated.
In summary, our study revealed that CMN has a protective effect on the acute hepatic injury induced by APAP. Both pre- and post-treatment with CMN resulted in a significant reduction in serum ALT and hepatocyte necrosis. The protection of CMN may be related to its inhibition of lipid peroxidation and oxidative stress. Moreover, we found that CMN restored Bcl-2/Bax ratio, thus reducing the APAP-induced hepatocyte apoptosis.
COMMENT
Background
Acetaminophen (APAP) can cause severe liver damage or even acute liver failure when administered in an acute or cumulative overdose. N-acetyl-p-benzoquinone imine (NAPQI) is the metabolite of APAP by cytochrome P450. Accumulation of NAPQI could induce lipid peroxidation, oxidative stress, mitochondrial dysfunction and DNA fragmentation, even liver failure or death. Thus, new and safe preventive measures against APAP-induced hepatic damage are eagerly needed.
Research frontiers
Previous studies have shown that curcumin (CMN) exerts anti-inflammatory, anti-oxidant and anticancer pharmacological activities. APAP lead to liver injury through increasing oxidative stress, lipid peroxidation and pro-apoptosis. In this study, the authors showed that CMN improved hepatic injury through inhibiting oxidative stress, lipid peroxidation and hepatic apoptosis in APAP induced liver damage model.
Innovations and breakthroughs
This study investigated the effect of CMN on the prevention of APAP-induced hepatic toxicity in vivo and examined whether CMN affects the production of lipid peroxidation, oxidative stress or hepatocyte apoptosis to attenuate liver damage. The results indicated that CMN could protect mice from APAP-induced liver injury.
Applications
CMN was abundant in the root tubers of Curcuma longa Linn and can be purified by modern technology. It has a broad application prospect.
Peer review
It is an interesting study investigating the protection effect of CMN on APAP-caused hepatitis. The experimental evidences presented that APAP induced hepatic injury, with elevated alanine aminotransferase, lipid peroxidation, oxidative stress and apoptosis were improved by CMN.
Footnotes
Supported by National Natural Science Foundation of China, No. 81271872; Health Department of Hubei Province, No. XF2012-5; and Jingzhou Bureau of Science and Technology
P- Reviewers: Hsu CP, Jung YD S- Editor: Zhai HH L- Editor: Ma JY E- Editor: Zhang DN
References
- 1.Rumack BH. Acetaminophen misconceptions. Hepatology. 2004;40:10–15. doi: 10.1002/hep.20300. [DOI] [PubMed] [Google Scholar]
- 2.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–48, vi. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Hennig GE, Manautou JE. Hepatobiliary excretion of acetaminophen glutathione conjugate and its derivatives in transport-deficient (TR-) hyperbilirubinemic rats. Drug Metab Dispos. 2003;31:798–804. doi: 10.1124/dmd.31.6.798. [DOI] [PubMed] [Google Scholar]
- 5.Hinson JA, Reid AB, McCullough SS, James LP. Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev. 2004;36:805–822. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- 6.Zwingmann C, Bilodeau M. Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology. 2006;43:454–463. doi: 10.1002/hep.21075. [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Miki K, He X, Killeen ME, Fink MP. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care. 2009;13:R55. doi: 10.1186/cc7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kheradpezhouh E, Panjehshahin MR, Miri R, Javidnia K, Noorafshan A, Monabati A, Dehpour AR. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur J Pharmacol. 2010;628:274–281. doi: 10.1016/j.ejphar.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 10.Rivera-Espinoza Y, Muriel P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009;29:1457–1466. doi: 10.1111/j.1478-3231.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- 11.Rajakrishnan V, Jayadeep A, Arun OS, Sudhakaran PR, Menon VP. Changes in the prostaglandin levels in alcohol toxicity: effect of curcumin and N-acetylcysteine. J Nutr Biochem. 2000;11:509–514. doi: 10.1016/s0955-2863(00)00112-1. [DOI] [PubMed] [Google Scholar]
- 12.Messner DJ, Sivam G, Kowdley KV. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009;29:63–72. doi: 10.1111/j.1478-3231.2008.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam Clin Pharmacol. 2008;22:417–427. doi: 10.1111/j.1472-8206.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Cheng L, Holt M, Hail N, Maclaren R, Ju C. Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology. 2010;51:1007–1016. doi: 10.1002/hep.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Gong Q, Li JH, Kong XL, Tian L, Duan LH, Tong J, Song FF, Fang M, Zheng F, et al. CpG ODN pretreatment attenuates concanavalin A-induced hepatitis in mice. Int Immunopharmacol. 2010;10:79–85. doi: 10.1016/j.intimp.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 17.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;(196):369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu YL, Jiang YZ, Jin XJ, Lian LH, Piao JY, Wan Y, Jin HR, Joon Lee J, Nan JX. Acanthoic acid, a diterpene in Acanthopanax koreanum, protects acetaminophen-induced hepatic toxicity in mice. Phytomedicine. 2010;17:475–479. doi: 10.1016/j.phymed.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Naik SR, Thakare VN, Patil SR. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol. 2011;63:419–431. doi: 10.1016/j.etp.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Kass GE, Macanas-Pirard P, Lee PC, Hinton RH. The role of apoptosis in acetaminophen-induced injury. Ann N Y Acad Sci. 2003;1010:557–559. doi: 10.1196/annals.1299.103. [DOI] [PubMed] [Google Scholar]
- 21.Hu B, Colletti LM. CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology. 2010;52:691–702. doi: 10.1002/hep.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Singh RL, Kakkar P. Modulation of Bax/Bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem Toxicol. 2011;49:770–779. doi: 10.1016/j.fct.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 23.Hu J, Yan D, Gao J, Xu C, Yuan Y, Zhu R, Xiang D, Weng S, Han W, Zang G, et al. rhIL-1Ra reduces hepatocellular apoptosis in mice with acetaminophen-induced acute liver failure. Lab Invest. 2010;90:1737–1746. doi: 10.1038/labinvest.2010.127. [DOI] [PubMed] [Google Scholar]
- 24.Rajan D, Wu R, Shah KG, Jacob A, Coppa GF, Wang P. Human ghrelin protects animals from renal ischemia-reperfusion injury through the vagus nerve. Surgery. 2012;151:37–47. doi: 10.1016/j.surg.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awad AS, El-Sharif AA. Curcumin immune-mediated and anti-apoptotic mechanisms protect against renal ischemia/reperfusion and distant organ induced injuries. Int Immunopharmacol. 2011;11:992–996. doi: 10.1016/j.intimp.2011.02.015. [DOI] [PubMed] [Google Scholar]


