Abstract
Hepatocellular adenoma (HCA) is one of the important complications of glycogen storage disease type Ia (GSD-Ia) because it can be transformed into hepatocellular carcinoma. Although surgical resection is a standard treatment of choice for solitary HCA, multiple HCAs in GSD-Ia patients present as therapeutic challenges for curative treatment. Therefore, treatment strategy according to malignant potential is important in management of HCAs in GSD-Ia. The authors present a case of histologically proven multiple HCAs without β-catenin mutations occurred in a GSD-Ia patient treated successfully with percutaneous radiofrequency ablation as a minimally invasive therapy.
Keywords: Glycogen storage disease, Hepatocellular adenoma, Radiofrequency ablation, β-catenin activation, Glycogen storage disease
Core tip: Risk stratification by pathological examination is an important step in deciding therapeutic options of multiple hepatocellular adenoma in glycogen storage disease type Ia patients.
INTRODUCTION
Glycogen storage disease type Ia (GSD-Ia) is an inherited disorder of carbohydrate metabolism caused by glucose-6-phosphatase deficiency in the liver, kidneys and intestinal mucosa[1]. Liver diseases associated with GSD-Ia include hepatocellular adenoma (HCA) which can lead to considerable morbidity and mortality associated with malignant transformation and intratumoral hemorrhage[2]. While HCA associated with oral contraceptives or exogenous androgen is relatively large, single and encapsulated HCAs in GSD-Ia tend to be small, multiple and non-capsulated[3]. Furthermore, HCAs in GSD-Ia tend to transform into hepatocellular carcinoma (HCC) more frequently than sporadic HCAs[3]. Rates of transformation into HCC have been reported approximately as being up to 10%[4]. Recently, sporadic HCAs have been classified into four subgroups; hepatocytic nuclear factor 1 α mutated HCAs, β-catenin-activated HCAs, inflammatory HCA, and unclassified HCA according to molecular markers and immunohistochemistry[5]. Because β-catenin activation is known as a risk factor for malignant transformation, subgroup classification of HCAs has been suggested as an important step in management of HCAs[6,7]. Although several strategies for management of multiple HCAs in GSD-Ia are suggested in literature, the best treatment is still controversial[8]. Surgical resection has been a standard treatment for solitary HCA with high risk of malignancy. However, the role of surgical resection is limited in multiple HCAs in GSD-Ia. Liver transplantation could be another treatment option for management of multiple HCAs as well as correction of most metabolic derangement in GSD-Ia patients[9]. Other options such as ethanol injection and transarterial embolization have been suggested as new and less invasive treatments[10-12]. The authors present a case of multiple HCAs without β-catenin mutations in a patient with GSD-Ia which were successfully treated by ultrasonography guided percutaneous radiofrequency ablation (RFA).
CASE REPORT
A 24-year-old male patient was referred for multiple liver masses. He was diagnosed as GSD-Ia 10 years ago. He had a family history of two brothers who died of HCC at the age of 22 and 26 years, respectively. His mother died from an undetermined cause in her twenties. After diagnosis, he had never received dietary therapy or other medical treatments. He was undersized with a height of 166 cm and weight of 51 kg. The laboratory findings revealed hyperlipidemia and hyperuricemia after a 12 h fast (Table 1). Serologic tests for viral hepatitis and autoimmune liver disease were all negative. Serum levels of α-fetoprotein and prothrombin induced by vitamin K deficiency or antagonist-II were 2.4 ng/mL and 35 mAU/mL. Mutation analysis of the glucose-6-phosphatase gene revealed compound heterozygosity for p.Leu216X (c.648G > T) and p.Gly222Arg (c.664G > A) in exon 5. On subsequent gadolinium ethoxybenzyl diethylene triamine pentaacetic acid (Gd-EOB-DTPA; Primovist, Bayer Schering Pharma, Berlin, Germany) enhanced magnetic resonance imaging (MRI), four arterial-phase enhancing nodules were noted in both lobes of liver and these nodules showed clear defects on 20 min hepatobiliary phase (Figure 1). Three nodules revealed the same features with intense homogeneous arterial enhancement, nearly iso-signal intensity with capsular enhancement on 3 min delayed phase, and intermediate high signal intensity on axial T2-weighted image (T2WI). There was no evidence of fat component or internal hemorrhage, or the Atoll sign (Figure 1A-C). One nodule showed heterogeneous signal intensity on 3 min delayed phase, axial T2WI, and 20 min hepatobiliary phase (Figure 1D). These features were consistent with multiple hepatic adenomas associated with GSD-Ia. We performed percutaneous liver biopsies on each nodule to identify HCA subtype. Each biopsy specimen underwent standard histopathological examination and immunohistochemistry for β-catenin, glutamine synthetase and amyloid A (Figure 2). Immunohistochemistry confirmed all the adenomas as inflammatory type HCA without malignant transformation. As all the nodules were small without malignant cells and β-catenin activation, we performed ultrasonography guided RFA as a less invasive therapeutic option. RFA was successfully performed under local anesthesia using a cool-tip electrode with a 3 cm exposed tip (ACT2030, Covidien, Mansfield, MA, United States). Multiple overlapping ablation methods were applied for each adenoma. There was no procedure-related complication after RFA. Three months follow-up computed tomogram scans revealed gradual reduction of the RFA zone without any residual tumor (Figure 3).
Table 1.
Laboratory findings on admission
| Blood chemistry | Value |
| Total bilirubin | 0.42 mg/dL |
| ALP | 218 U/L |
| AST | 87 U/L |
| ALT | 37 U/L |
| LDH | 275 U/L |
| γ-GTP | 152 U/L |
| Total protein | 8.9 g/dL |
| Albumin | 4.8 g/dL |
| Total cholesterol | 380 mg/dL |
| TG | 1688 mg/dL |
| Uric acid | 9.4 mg/dl |
| BUN | 8.5 mg/dL |
| Creatinine | 1.15 mg/dL |
| Glucose | 93 mg/dL |
| Lactic acid | 3.5 mmol/L |
| Hemoglobin A1c | 5.0% |
| Hematology | |
| Hemoglobin | 11.3 g/dL |
| White blood cell | 4.14 × 103/μL |
| Platelet | 269 × 103/μL |
| Serology | |
| AFP | 2.2 ng/mL |
| PIVKA-II | 35 mAU/mL |
| Hepatitis B surface antigen | Negative |
| Anti HBs Ab | Negative |
| Hepatitis B core antibody | Negative |
| Anti HCV Ab | Negative |
| Hemostasis | |
| Prothrombin time | 11.2 s |
ALP: Alkaline phosphatase; AST: Aspartate transaminase; ALT: Alanine aminotransferase; LDH: Lactate dehydrogenase; γ-GTP: Gammaglutamyl transpeptidase; TG: Triglyceride; BUN: Blood urea nitrogen; AFP: α-fetoprotein; PIVKA-II: Prothrombin induced by vitamin K deficiency or antagonist-II.
Figure 1.
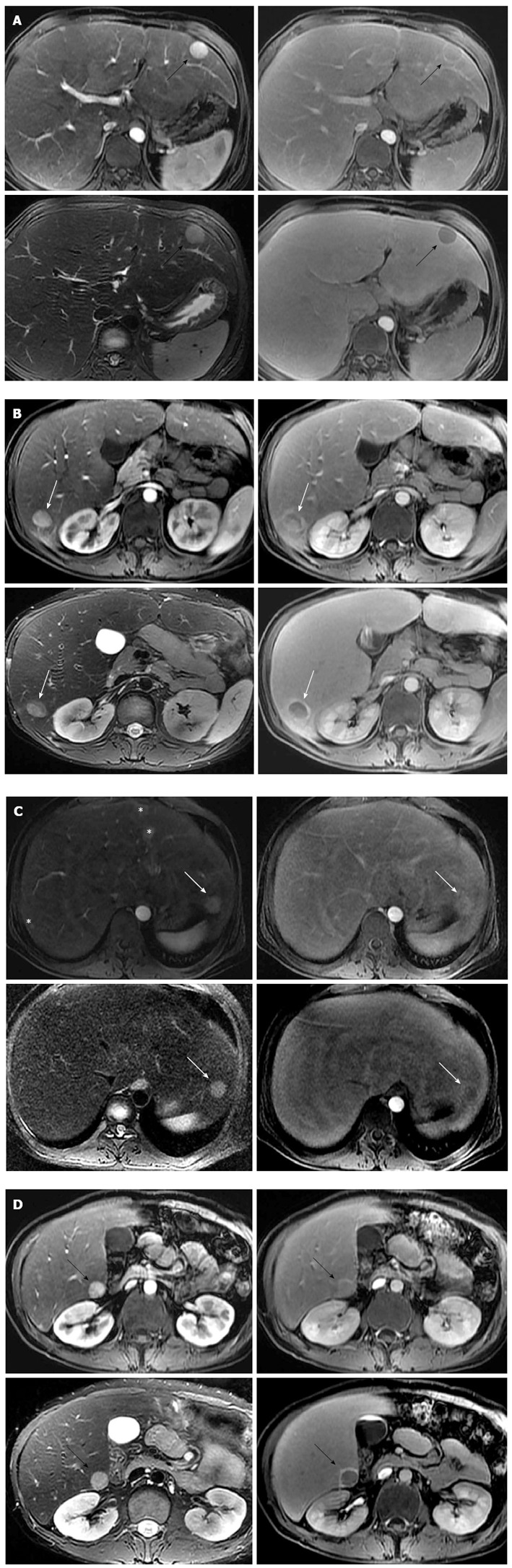
Hepatic adenomas associated with glycogen storage disease type Ia. A: Gd-EOB-DTPA enhanced axial T1-weighted image on arterial phase (left upper) reveals approximately 2 cm intense enhancing mass in the segment 3 of liver (black arrows). The mass shows nearly iso-signal intensity with capsular enhancement on 3 min delayed phase (right upper), intermediate high signal intensity on axial T2-weighted image (left lower), and clear defect on 20 min hepatobiliary phase (right lower); B: Approximately 1.8 cm sized intense enhancing mass on arterial phase (left upper) is noted in the segment 6 of liver. This mass shows suspicious focal eccentric wash-out enhancement (white arrows) on 3 min delayed phase (right upper). This portion reveals relatively low signal intensity on axial T2-weighted image (left lower) and 20 min hepatobiliary phase (right lower), compared to other tumor area. Surrounding rim enhancement in the tumor is seen on 20 min hepatobiliary phase; C: Approximately 1.8 cm arterial enhancing mass (white arrows) is seen in the segment 2 of liver. The mass reveals slightly high signal intensity on 3 min delayed phase (right upper), intermediate high signal intensity on axial T2-weighted image (left lower), and fuzzy defect on 20 min hepatobiliary phase (right lower). Multifocal arterioportal shunts (asterisks) are noted in the segments 3 and 7 of liver; D: Approximately 1.8 cm sized intense enhancing mass (black arrows) on arterial phase (left upper) is noted in the segment 6 of liver. This mass shows slightly high signal intensity with capsular enhancement on 3min delayed phase (right upper), and intermediate high signal intensity on axial T2-weighted image (left lower). On 20 min hepatobiliary phase (right lower), the tumor reveals clear defect with surrounding rim enhancement. Gd-EOB-DTPA: Gadolinium ethoxybenzyl diethylene triamine pentaacetic acid.
Figure 2.

The histological features are consistent with the inflammatory type of hepatocellular adenoma. A: A hepatocellular adenoma in GSD-Ia has several unpaired arteries (thick black arrows), sinusoidal dilation (thin black arrows), and steatosis (white arrows), but the atypia of hepatocytes are low without a nuclear pleomorphism; B: A reticulin staining does not show thick hepatic cords; C: The lymphocytic infiltrates are found in a hepatic lobule; D: The lymphocytes infiltrate in the area surrounded a portal and a periportal tracts; E: Staining for glutamine synthetase is diffuse cytoplasmic with focal nuclear expression; F: β-catenin staining does not show abnormal expression. GSD-Ia: Glycogen storage disease type Ia.
Figure 3.

Computed tomogram follow-up after 3 mo showed gradual reduction of radiofrequency ablation zone. A: Post-RFA follow-up CT revealed gradual contraction of ablated lesion in segment 3 to 1 cm; B: Complete ablation of enhancing lesion in segment 6 was observed in Post-RFA follow-up CT; C: Post-RFA follow-up CT showed complete ablation of 1.8 cm sized tumor in segment 2; D: Post-RFA CT revealed no residual lesion without adjacent organ damage. CT: Computed tomogram; RFA: Radiofrequency ablation.
DISCUSSION
Management of HCAs in patients with GSD-Ia is not well established because of the rarity of this disease. As patients with GSD-Ia survive longer with intensive dietary therapy, they have more chances to develop HCAs in their expanded life span[13]. Although there is a case report that HCA in GSD-Ia regressed after strict dietary therapy, maintenance of normoglycemia under intensive dietary therapy is insufficient to regress HCAs or reverse malignant transformation because patients with multiple HCAs sometimes showed sudden progression and metastatic spread[3,14-17]. As HCAs in GSD-Ia tend to be multiple, involving both lobes of liver, the ultimate treatment of HCAs in GSD-Ia is liver transplantation for management of HCAs and to reverse metabolic derangement[9,18]. However, liver transplantation is rarely performed due to uncertainties on timing of transplantation, limited organ availability and combined renal dysfunction with immunosuppression[13]. Surgical resection of HCAs is suggested as an effective intermediate therapy for prevention of HCC until liver transplantation[13]. However, liver resection in GDS-Ia patients showed greater morbidity including intra-abdominal abscess, multi-organ failure, unstable blood glucose control and hemorrhage.
Recently, molecular characterizations of HCAs demonstrated that frequent β-catenin mutations are more frequently observed in HCAs in GSD-Ia and related to increased risk of malignant transformation like sporadic HCAs[19]. To assess the risk of malignant transformation, we biopsied each HCA for analysis of subtype of each tumor by immunohistochemistry. All the HCAs were small and inflammatory type with low risk of malignant transformation. There are reports of treatment of HCAs in GSD-Ia by transarterial embolization and ethanol injection as a minimal invasive procedure[11,12]. As RFA can offer complete necrosis of target lesions compared to transarterial embolization and ethanol injection, we treated HCAs by percutaneous RFA successfully. The present case shows the potential role of RFA in management of multiple HCAs in GSD-Ia patients along with risk stratification by pathological examination.
Footnotes
P- Reviewer: Bannasch P S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Zhang DN
References
- 1.Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, Boney A, Sullivan J, Frush DP, Chen YT, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang DQ, Fiske LM, Carreras CT, Weinstein DA. Natural history of hepatocellular adenoma formation in glycogen storage disease type I. J Pediatr. 2011;159:442–446. doi: 10.1016/j.jpeds.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakellariou S, Al-Hussaini H, Scalori A, Samyn M, Heaton N, Portmann B, Tobal K, Quaglia A. Hepatocellular adenoma in glycogen storage disorder type I: a clinicopathological and molecular study. Histopathology. 2012;60:E58–E65. doi: 10.1111/j.1365-2559.2011.04153.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee PJ. Glycogen storage disease type I: pathophysiology of liver adenomas. Eur J Pediatr. 2002;161 Suppl 1:S46–S49. doi: 10.1007/s00431-002-1002-0. [DOI] [PubMed] [Google Scholar]
- 5.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 6.Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–489. doi: 10.1002/hep.22995. [DOI] [PubMed] [Google Scholar]
- 7.Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888–902. doi: 10.1053/j.gastro.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 8.van Aalten SM, Witjes CD, de Man RA, Ijzermans JN, Terkivatan T. Can a decision-making model be justified in the management of hepatocellular adenoma? Liver Int. 2012;32:28–37. doi: 10.1111/j.1478-3231.2011.02667.x. [DOI] [PubMed] [Google Scholar]
- 9.Reddy SK, Austin SL, Spencer-Manzon M, Koeberl DD, Clary BM, Desai DM, Smith AD, Kishnani PS. Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009;51:483–490. doi: 10.1016/j.jhep.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Rocourt DV, Shiels WE, Hammond S, Besner GE. Contemporary management of benign hepatic adenoma using percutaneous radiofrequency ablation. J Pediatr Surg. 2006;41:1149–1152. doi: 10.1016/j.jpedsurg.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 11.Karkar AM, Tang LH, Kashikar ND, Gonen M, Solomon SB, Dematteo RP, D’ Angelica MI, Correa-Gallego C, Jarnagin WR, Fong Y, et al. Management of hepatocellular adenoma: comparison of resection, embolization and observation. HPB (Oxford) 2013;15:235–243. doi: 10.1111/j.1477-2574.2012.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa M, Fukui K, Kuriyama S, Tsujimoto T, Nakatani Y, Toyokawa Y, Kurematsu Y, Awata J, Shiroi A, Fukui H, et al. Hepatic adenomas treated with percutaneous ethanol injection in a patient with glycogen storage disease type Ia. J Gastroenterol. 2001;36:52–61. doi: 10.1007/s005350170155. [DOI] [PubMed] [Google Scholar]
- 13.Reddy SK, Kishnani PS, Sullivan JA, Koeberl DD, Desai DM, Skinner MA, Rice HE, Clary BM. Resection of hepatocellular adenoma in patients with glycogen storage disease type Ia. J Hepatol. 2007;47:658–663. doi: 10.1016/j.jhep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I) Eur J Pediatr. 2002;161 Suppl 1:S20–S34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- 15.Yiu WH, Pan CJ, Mead PA, Starost MF, Mansfield BC, Chou JY. Normoglycemia alone is insufficient to prevent long-term complications of hepatocellular adenoma in glycogen storage disease type Ib mice. J Hepatol. 2009;51:909–917. doi: 10.1016/j.jhep.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zangeneh F, Limbeck GA, Brown BI, Emch JR, Arcasoy MM, Goldenberg VE, Kelley VC. Hepatorenal glycogenosis (type I glycogenosis) and carcinoma of the liver. J Pediatr. 1969;74:73–83. doi: 10.1016/s0022-3476(69)80010-7. [DOI] [PubMed] [Google Scholar]
- 17.Mikuriya Y, Oshita A, Tashiro H, Amano H, Kobayashi T, Arihiro K, Ohdan H. Hepatocellular carcinoma and focal nodular hyperplasia of the liver in a glycogen storage disease patient. World J Hepatol. 2012;4:191–195. doi: 10.4254/wjh.v4.i6.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belingheri M, Ghio L, Sala A, Menni F, Trespidi L, Ferraresso M, Berardinelli L, Rossi G, Edefonti A, Parini R. Combined liver-kidney transplantation in glycogen storage disease Ia: a case beyond the guidelines. Liver Transpl. 2007;13:762–764. doi: 10.1002/lt.21147. [DOI] [PubMed] [Google Scholar]
- 19.Calderaro J, Labrune P, Morcrette G, Rebouissou S, Franco D, Prévot S, Quaglia A, Bedossa P, Libbrecht L, Terracciano L, et al. Molecular characterization of hepatocellular adenomas developed in patients with glycogen storage disease type I. J Hepatol. 2013;58:350–357. doi: 10.1016/j.jhep.2012.09.030. [DOI] [PubMed] [Google Scholar]


