Abstract
Bacillus subtilis spores were killed by CuCl2-ascorbic acid, chloride ions were essential for killing of spores, and spores with defective coats were killed more rapidly. CuCl2-ascorbic acid did not damage spore DNA, and spores killed by this reagent initiated germination. However, spores killed by CuCl2-ascorbic acid may have damage to their inner membrane.
Spores of various Bacillus and Clostridium species are dormant and resistant to environmental stresses, including heat, radiation, and toxic chemicals (13, 31). This resistance, as well as the potential for a number of spore formers, including Bacillus anthracis, to cause disease has heightened interest in new sporicides. Further stimulating the development of new microbial biocides are the facts that some bacteria have become resistant to common sterilization agents and some currently used sterilization agents pose risks to health-care workers (11).
One group of agents that have been tested as microbial biocides are Fenton reagents (34) that use various metal ions plus hydrogen peroxide or ascorbic acid to generate highly reactive hydroxyl radicals (2, 21, 22, 23). The reactivity of hydroxyl radicals means that they are most effective when they are generated in the immediate vicinity of their target, which is often DNA (8, 9, 19). Fenton reagent formulations with Cu2+ and hydrogen peroxide or ascorbic acid kill spores of Bacillus species (2, 21, 22, 23). Bacillus globigii spore killing by CuCl2-ascorbic acid requires oxygen, presumably for the generation of hydroxyl radicals, and killing is increased by the addition of NaCl or a surfactant (2). Since spores killed by Fenton reagent with Cu2+ and ascorbic acid were not visibly damaged, it was suggested that the Cu2+ and ascorbic acid had penetrated into the spore core and with dissolved oxygen had generated hydroxyl radicals that caused spore death by damage to DNA or enzymes (2). While this is a logical possibility, penetration of ascorbic acid into the spore core seems unlikely, since hydrophilic molecules such as monosaccharides do not normally penetrate the core of Bacillus cereus spores (6). Indeed, even a molecule as small as methylamine penetrates the spore core slowly (32). Since Cu2+-ascorbic acid may be used to kill spores in applied settings, it seemed appropriate to determine how this reagent acts on spores. Of particular interest are the following issues: (i) whether this reagent kills spores by DNA damage; (ii) whether the reagent acts in the spore core or on some more external spore layer; and (iii) what spore features are important in protection against this reagent.
Spore DNA is well protected against damage from toxic chemicals by the proteinaceous spore coats, the low permeability of the spore's inner membrane, the spore core's low water content, and the saturation of spore DNA with α/β-type small, acid-soluble spore proteins (SASP) (13, 31). The DNA protection afforded by these mechanisms is so great that many potentially genotoxic agents that kill spores, including many oxidizing agents, do not do so by causing DNA damage (5, 10, 13, 31, 37).
To test whether Cu2+-ascorbic acid kills spores by causing DNA damage, the killing of spores of B. subtilis strains lacking various DNA protective mechanisms was examined. The B. subtilis strains were isogenic derivatives of strain 168. PS533 is the wild-type strain and carries plasmid pUB110, which contains a kanamycin resistance marker (30). Two genes (sspA and sspB) that encode the two major α/β-type SASP were deleted from strain 168 to create strain PS578, which carries pUB110 (30). The recA gene was deleted from strain 168 and replaced with a gene providing resistance to macrolide antibiotics (MLSr) to create strain PS2318 (30). The sspA and sspB genes were deleted from strain 168 and the recA gene was mutated to create strain PS2319 (30). Strain PS3394 carries pUB110 and contains a tetracycline resistance cassette inactivating the cotE gene essential for proper spore coat assembly (3, 37). Strain PS3379 contains the luxAB genes from Vibrio harveyi under the control of the forespore-specific promoter of the sspB gene (10).
Spores were prepared at 37°C on 2×SG medium plates without antibiotics and washed and cleaned as described previously (14, 15). Spore preparations used were free (>98%) from growing cells, cell debris, or spores that had germinated, as determined by examination with a phase-contrast microscope. Spores were treated at an optical density at 600 nm (OD600) of ∼1.0 (∼108 CFU/ml) at room temperature (24°C) with 54 mM CuCl2 and 90 mM ascorbic acid unless noted otherwise; the pH of this solution was 1.5. At various times, aliquots were diluted 100-fold in a solution containing 2.5 g of Na2S2O3 per liter to neutralize the Cu2+-ascorbic acid, and further diluted in water, and aliquots were spotted in duplicate on Luria-Bertani (LB) medium (15, 33, 37) plates. The plates were incubated for ∼24 h at 30 to 37°C, and colonies were counted to assess spore viability. Spores of all strains tested were not killed by being incubated at pH 1.5 for 2 h, as found previously (27).
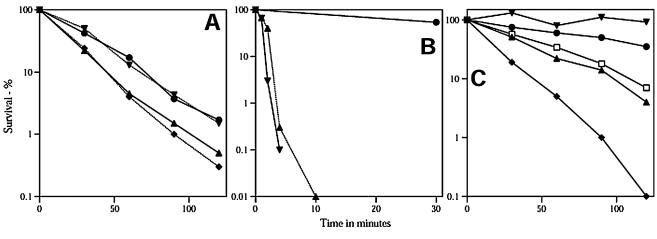
Spores of the wild-type B. subtilis strain (PS533) were killed by CuCl2-ascorbic acid (Fig. 1A), although more slowly than spores of B. globigii (2). Spores of strain PS578 that lack most of the DNA-protective α/β-type SASP (termed α−β− spores) exhibited slightly greater sensitivity to CuCl2-ascorbic acid, but in either a wild-type or α−β− background, loss of the recA gene responsible for much DNA repair in B. subtilis (36) did not reduce spore resistance (Fig. 1A). Previous work has shown that α−β− or recA spores are 3- to 10-fold more sensitive to agents that cause DNA damage than are wild-type spores (13, 33). Thus, the slight (∼1.5-fold) decrease in spore sensitivity to CuCl2-ascorbic acid upon loss of SASP-α and SASP-β may not be due to killing of the α−β− spores through DNA damage. Instead, this small difference is likely due to global changes in gene expression during sporulation of the α−β− strain, because of the absence of accumulation of most α/β-type SASP in the developing α−β− forespore (28). This alteration in gene expression causes slight changes in the properties of the α−β− spores, as seen previously in the decreased resistance of α−β− spores to other agents that do not cause DNA damage in spores (5, 28, 33, 37). In particular, the lack of synthesis of SASP-α and -β during sporulation has major effects on expression of genes coding for spore coat proteins (28), and as shown below, the spore coats are a major factor in spore resistance to CuCl2-ascorbic acid.
FIG. 1.
Killing of spores of B. subtilis strains with Cu2+-ascorbic acid. (A) Spores of B. subtilis strains PS533 (wild type) (•), PS578 (α−β−) (▴), PS2318 (recA) (▾), and PS2319 (α−β− recA) (⧫) were incubated with CuCl2-ascorbic acid. In each experiment shown, the viability of spores of all strains was determined in parallel and at least in duplicate as described in the text. (B) Spores of B. subtilis strains PS533 (wild type) (• and ▴), either intact (•) or decoated (▴), and PS3394 (cotE) (▾) were incubated with CuCl2-ascorbic acid, and spore viability was determined as described in the text. (C) Spores of B. subtilis strain PS533 (wild type) either intact (▴, ▾, □, and ⧫) or decoated (•) were incubated with CuCl2-ascorbic acid (▴), 54 mM CuSO4-90 mM ascorbic acid (▾ and •), 54 mM CuSO4-90 mM ascorbic acid-108 mM NaCl (□), or CuCl2-ascorbic acid-500 mM NaCl (⧫), and spore viability was determined as described in the text. The experiments shown in this figure were carried out two (B and C) or three (A) times with essentially identical results to those shown, and in these experiments, the confidence limits for the relative slopes of the killing curves were at least ±11%. Consequently, the differences between the wild-type and α−β− spores shown in panel A are statistically significant, as are the differences seen in panels B and C, with the exception that the killing curves in panel C for intact spores killed with CuCl2-ascorbic acid or 54 mM CuSO4-90 mM ascorbic acid-108 mM NaCl are not statistically different.
To further examine whether spore killing by CuCl2-ascorbic acid is through DNA damage, wild-type and α−β− spores surviving CuCl2-ascorbic acid were tested for the presence of auxotrophic or asporogenous mutations by examining growth of survivors on plates containing rich, minimal, and sporulation media as described previously (4, 5, 10, 33, 37). In contrast to spores that survive treatments that kill by damaging DNA, 15% of which have obvious mutations (4, 33), wild-type spores surviving CuCl2-ascorbic acid (8% survival) had no more mutations (0 in 416 survivors) than the untreated spores (0 in 416 spores tested). The same finding was made for α−β− spores (83% killed) treated with CuCl2-ascorbic acid (6 mutants in 416 survivors; 6 mutants in 416 untreated α−β− spores examined). The significant level of mutations in untreated α−β− spores has been seen previously and ascribed to mutagenesis during spore preparation and storage (4, 5, 10, 33, 37). In contrast to the absence of DNA damage detected in spores killed by CuCl2-ascorbic acid, growing bacteria treated with a Fenton reagent do exhibit significant mutagenesis, and cell killing is accelerated by a recA mutation (35).
The spore coat helps protect spores against many chemical agents (3, 5, 10, 31, 37). Indeed, B. subtilis spores with defective coats are 10- to 100-fold times more sensitive to many oxidizing agents than intact spores are (5, 10, 37). To assess the role of the coat in spore resistance to CuCl2-ascorbic acid, we examined wild-type spores that were chemically decoated (1) as well as cotE spores (3). Not surprisingly, both the decoated and cotE spores were much more sensitive to this reagent than were untreated wild-type spores (Fig. 1B). The protection against CuCl2-ascorbic acid by the spore coat may explain the observation that a surfactant sensitized B. globigii spores to this reagent (2). Perhaps the surfactant used in that study (2) removes some coat protein, allowing easier access to targets located inside the spore.
It was reported that CuCl2-ascorbic acid killing of spores is increased significantly by the addition of NaCl (2), and we also found this to be the case (Fig. 1C). Why NaCl would increase spore killing by CuCl2-ascorbic acid if these chemicals were to react in the spore core to generate hydroxyl radicals is not clear. However, perhaps the agent killing the spores is not the hydroxyl radical but an oxidizing agent formed by reaction of hydroxyl radicals with chloride ions in free solution. A number of chlorine-containing species can be generated by the Fenton reaction in the presence of chloride ions, including chlorine atoms and hypochlorite (24, 25), and hypochlorite kills spores efficiently, especially as the pH is decreased (37). If the reaction of hydroxyl radicals with chloride ions does generate the killing species, then spores incubated with CuSO4-ascorbic acid should not be killed. This was indeed the case for both intact and decoated wild-type spores, and spore killing by CuSO4-ascorbic acid was restored by the addition of NaCl (Fig. 1C). The lack of spore killing by CuSO4-ascorbic acid further indicates that enhancement by NaCl of spore killing by Cu2+-ascorbic acid was not an ionic strength effect but is more likely a specific effect of the Cl− ion. Indeed, 0.5 M Na2SO4 did not enhance spore killing by CuCl2-ascorbic acid (data not shown).
The data noted above suggest that spore killing by CuCl2-ascorbic acid is through generation of oxidizing chlorine-containing species. In addition, since there is no evident DNA damage in spores killed by CuCl2-ascorbic acid, even in spores lacking the DNA protective α/β-type SASP, it seems likely that the killing agent does not act on the spore core but on some more exterior spore layer. Recent studies examining spore killing by hypochlorite and chlorine dioxide, as well as the new disinfectant Sterilox (which also contains a variety of oxidizing chlorine-containing compounds), have shown that these agents do not damage the DNA in the spore core but appear to kill spores by damaging the spore's inner membrane in some fashion (5, 10, 37). This damage, in particular by hypochlorite, can also greatly diminish the spore's ability to germinate (37).
To assess the effect of CuCl2-ascorbic acid on spore germination, wild-type spores (94 to 99% of wild-type spores killed by CuCl2-ascorbic acid) were tested for spore germination with nutrients, with a 1:1 mixture of Ca2+ and dipicolinic acid (DPA), and with dodecylamine. Ca2+-DPA and dodecylamine initiate germination of spores of many Bacillus species, including Bacillus subtilis, but are not nutrients (16, 26). The CuCl2-ascorbic acid-killed spores initiated germination normally with nutrients, judging from the conversion of the spores from the bright to dark phase using a phase-contrast microscope (Table 1). Spores killed ∼99% by CuCl2-ascorbic acid also germinated in LB medium plus l-alanine as rapidly as untreated spores, as assessed by the decrease in OD600 of spore suspensions (data not shown), and after 90 min, these germinated spores appeared viable in an assay based on the inability of bacteria with a severely damaged plasma membrane to exclude the dye propidium iodide (Table 1). As judged by their ability to give rise to colonies, ≥95% of these spores were actually dead, indicating that the spores have indeed been damaged in some fashion. A similar discrepancy between these two viability assays was seen when chlorine dioxide or a superoxidized water, Sterilox, was used to kill spores, as a large fraction of spores killed by these reagents as assessed by colony formation appeared alive as measured by the exclusion of propidium iodide (10, 37). Presumably, the CuCl2-ascorbic acid-treated spores had accumulated sufficient damage such that they could not give rise to colonies but not so much damage that spores germinated 90 min in LB medium do not exclude propidium iodide.
TABLE 1.
Germination of B. subtilis spores with or without prior treatment with CuCl2-ascorbic acida
| Treatment | % Killing | % Spore germination with
|
|||
|---|---|---|---|---|---|
| l-Alanine | LB medium plus l-alanine | Ca2+- DPA | Dodecyl- amine | ||
| None | 0 | 82 | 84 (97)b | 89 | 98c; 37d (97)e |
| CuCl2-ascorbic acid | 94 | 80 | 82 (97)b | 5 (5)f | 98c; 35d (3)e |
| CuCl2-ascorbic acid | 99 | 86 | 85 (95)b | NDg | (0.5)e |
Spores of strain PS533 (wild type) were either not treated or treated with CuCl2-ascorbic acid, and percent killing was assessed on plates as described in the text. Spores at an OD600 of 1 (l-alanine, LB medium plus l-alanine, and Ca2+-DPA) or 2 (dodecylamine) were germinated by the following treatments: (i) 3 h at 37°C with l-alanine (8 mM l-alanine, 25 mM Tris-HCl [pH 8.6]); (ii) 90 min at 37°C with LB medium with 4 mM l-alanine added to ensure as rapid germination as possible; (iii) 2 h at 24°C with Ca2+-DPA (60 mM CaCl2, 60 mM DPA, 20 mM Tris-HCl [pH 8.4]), followed by centrifugation and incubation in 20 mM Tris-HCl (pH 8.4) at 37°C for 1 h; and (iv) dodecylamine (1 mM dodecylamine, 20 mM Tris-HCl [pH 7.5]) at 37°C for 3 h or 45°C for 4 h. Germination was assessed by examining the conversion of ∼200 spores from the bright to dark phase with a phase-contrast microscope (l-alanine, LB medium plus l-alanine, and Ca2+-DPA) or measuring DPA release (dodecylamine) as described previously (25).
The values in parentheses are the viability of ∼200 spores that had germinated, as assessed by their staining either red (dead) or green (alive) with the BacLight viability stain (Molecular Probes, Eugene, Oreg.) as described previously (5, 12). However, note that this assay is different from the determination of percent killing as assessed on plates.
Value for spores incubated for 4 h at 45°C.
Value for spores incubated for 3 h at 37°C.
Values in parentheses are the maximum viability of spores that germinated after dodecylamine treatment compared to the viability of untreated spores determined without dodecylamine treatment.
The value in parentheses is the viability of the CuCl2-ascorbic acid-killed spores after incubation with Ca2+-DPA compared to the viability of the untreated spores that had also been incubated with Ca2+-DPA.
ND, not determined.
Previous work has shown that spores killed by a number of chlorine-containing oxidizing agents do not lose the core's large depot of DPA, but the killed spores do lose DPA upon a normally sublethal heat treatment (10, 37). The latter result has been interpreted as a lower resistance of the inner spore membrane to rupture at elevated temperatures due to membrane damage caused by the oxidizing agents (10, 37). Killing of wild-type spores to 99% with CuCl2-ascorbic acid also caused <5% release of spore DPA, determined as described previously (10, 37). However, incubation for 30 min at 80 or 85°C of wild-type untreated spores or spores killed 99% by CuCl2-ascorbic acid caused more release of DPA from the killed spores than from the untreated spores (8% from the killed spores and 3% from the untreated spores at 80°C; 15% from the killed spores and 8% from the untreated spores at 85°C; all values ±15%), when DPA release was measured as described previously (5, 37). While the greater DPA release from the CuCl2-ascorbic acid-killed spores was significant, the amount was significantly lower than from spores killed by other oxidizing agents (10, 37), suggesting there is only a small amount of damage to the inner membrane in CuCl2-ascorbic acid-killed spores.
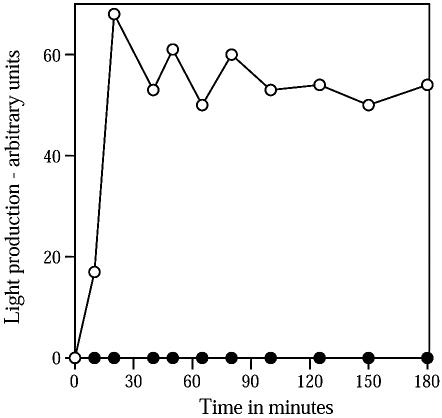
Further evidence for damage to CuCl2-ascorbic acid-killed spores came from analysis of metabolism upon germination using spores that contain high levels of the V. harveyi LuxA and LuxB proteins. Untreated B. subtilis spores initiate metabolism soon after the initiation of germination and generate FMNH2, and FMNH2, LuxA, LuxB, and dodecanal result in light production by germinating spores (5, 7, 10). This was also observed in the current work (Fig. 2), when light production was measured as described previously (5, 10, 37). However, spores killed 99% by CuCl2-ascorbic acid and then germinated exhibited ≤1% of the light production of germinated untreated spores (Fig. 2). Perhaps slight damage by CuCl2-ascorbic acid to the inner spore membrane that becomes the germinated spore's plasma membrane has compromised energy metabolism by these germinated spores.
FIG. 2.
Light production during germination of untreated and CuCl2-ascorbic acid-killed spores carrying V. harveyi LuxA and LuxB proteins. Spores of strain PS3379 (PsspB::luxAB) either not treated (○) or treated with CuCl2-ascorbic acid giving 99% killing (•) were germinated at 37°C and an OD600 of 1 in 2× YT medium (5) containing 4 mM l-alanine. At various times, 0.5-ml samples were mixed with 0.5 ml of fresh medium, 10 μl of 1% dodecanal in ethanol was added, and light production was measured as described in the text. More than 70% of both the treated and untreated spores had germinated by 60 min, as determined by observation with a phase-contrast microscope.
The CuCl2-ascorbic acid-killed spores also initiated germination normally with dodecylamine, an agent that triggers spore germination by causing release of spore DPA (26), although dodecylamine treatment did not revive the CuCl2-ascorbic acid-killed spores (Table 1). However, spores killed with CuCl2-ascorbic acid did not germinate with Ca2+-DPA (Table 1). Germination of B. subtilis spores by Ca2+-DPA requires the enzyme CwlJ, one of the spore's two redundant cortex lytic enzymes (15, 16). In spores, CwlJ is sensitive to exogenous chemicals, including at least one oxidizing agent (15, 37). Consequently, it seems likely that CwlJ is readily inactivated by CuCl2-ascorbic acid treatment. However, inactivation of all spore cortex lytic enzymes is not the reason for spore killing by CuCl2-ascorbic acid, since (i) spores killed by this reagent appear to initiate germination normally with nutrients and (ii) if spores were killed to ∼95% with CuCl2-ascorbic acid and decoated and treated with lysozyme in a hypertonic medium to degrade the spore's peptidoglycan and induce germination, the killed spores became phase dark (data not shown), an indicator of cortex lysis. However, the CuCl2-ascorbic acid-treated spores were not revived by this treatment (data not shown). In contrast, spores not treated with CuCl2-ascorbic acid remained viable after the same treatment (data not shown), as expected (20).
The data presented in this communication allow three new conclusions. First, spore killing by Cu2+-ascorbic acid largely if not completely requires Cl−, suggesting that the killing agent is some oxidizing chlorine-containing species. Second, the spore coat provides major protection against spore killing by CuCl2-ascorbic acid, as they do against spore killing by a number of other oxidizing agents (5, 10, 37). Third, spore killing by CuCl2-ascorbic acid is not due to DNA damage, at least DNA damage that is either mutagenic, repaired in a RecA-dependent process, or prevented by α/β-type SASP. In addition, since wild-type and α−β− spores exhibit relatively similar sensitivities to CuCl2-ascorbic acid, it seems unlikely that this reagent generates hydroxyl radicals within the spore core. In contrast, hydrogen peroxide may generate hydroxyl radicals in the spore core, since α−β− spores are much more sensitive to hydrogen peroxide than wild-type spores and α−β− spores (but not wild-type spores) surviving treatment with hydrogen peroxide exhibit high levels of mutations (29).
A remaining area of uncertainty about spore killing by CuCl2-ascorbic acid concerns the mechanism of killing. Previous work on spore killing by oxidizing chlorine-containing agents has shown that these agents do not kill spores by DNA damage but likely cause some damage to the spore's inner membrane that either prevents spore germination or results in loss of membrane integrity in the first minutes of spore germination (10, 37). However, there is no significant defect in the initiation of germination by CuCl2-ascorbic acid-killed spores, and these germinated spores appear to have retained their plasma membrane integrity, at least after 90 min of germination, although their ability to carry out energy metabolism is severely compromised. Consequently, the precise nature of the damage caused by CuCl2-ascorbic acid treatment that kills spores is not clear. Indeed, this damage may be different from that caused by other oxidizing chlorine-containing agents (10, 37), since these latter agents were used at pH values ranging from 3.5 to 11 and the CuCl2-ascorbic acid reagent has a pH of 1.5. An additional possible mechanism for spore killing by the CuCl2-ascorbic acid reagent is the inactivation of one or more enzymes within the spore core, in particular enzymes that are essential for spore metabolism. Inactivation of spore core enzymes accompanying spore killing by hydroperoxides has been observed previously (17, 18), although it is not clear if this is the cause of spore killing by such agents. However, as noted above, the absence of DNA damage in α−β− spores treated with CuCl2-ascorbic acid suggests that this reagent does not exert its killing action in the spore core. Clearly, determining the precise mechanism of spore killing by CuCl2-ascorbic acid remains a subject for further work.
Acknowledgments
We are grateful to DonnaMaria Cortezzo for assistance with some experiments.
This work was supported in part by a grant from the Army Research Office.
REFERENCES
- 1.Bagyan, I., M. Noback, S. Bron, M. Paidhungat, and P. Setlow. 1998. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212:179-188. [DOI] [PubMed] [Google Scholar]
- 2.Cross, J. B., R. P. Currier, D. J. Torraco, L. A. Vanderberg, G. L. Wagner, and P. D. Gladen. 2003. Killing of Bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl. Environ. Microbiol. 69:2245-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fairhead, H., B. Setlow, and P. Setlow. 1993. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J. Bacteriol. 175:1367-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genest, P. C., B. Setlow, E. Melly, and P. Setlow. 2002. Killing of spores of Bacillus subtilis by peroxynitrite appears to be caused by membrane damage. Microbiology 148:307-314. [DOI] [PubMed] [Google Scholar]
- 6.Gerhardt, P., R. Scherrer, and S. H. Black. 1972. Molecular sieving by dormant spore structures, p. 68-74. In H. O. Halvorson, R. Hanson, and L. L. Campbell (ed.), Spores V. American Society for Microbiology, Washington, D.C.
- 7.Hill, P. J., L. Hall, D. A. Vinicombe, C. J. Soper, P. Setlow, W. M. Waites, S. Denyer, and G. S. A. B. Stewart. 1994. Bioluminescence and spores as biological indicators of inimical processes. Soc. Appl. Bacteriol. Symp. Ser. 76:129S-134S. [DOI] [PubMed] [Google Scholar]
- 8.Imlay, J. A., and S. Linn. 1988. DNA damage and oxygen radical toxicity. Science 240:1302-1309. [DOI] [PubMed] [Google Scholar]
- 9.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 93:13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loshon, C. A., E. Melly, B. Setlow, and P. Setlow. 2001. Analysis of killing of spores of Bacillus subtilis by a new disinfectant, Sterilox®. J. Appl. Microbiol. 91:1051-1058. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray, T., D. L. Popham, C. B. Pearson, A. R. Hand, and P. Setlow. 1998. Analysis of the outgrowth of Bacillus subtilis spores lacking penicillin-binding protein 2a. J. Bacteriol. 180:6493-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 15.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In J. A. Hoch, R. Losick, and A. L. Sonenshein (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 17.Palop, A., G. C. Rutherford, and R. E. Marquis. 1996. Hydroperoxide inactivation of enzymes within spores of Bacillus megaterium ATCC 19213. FEMS Microbiol. Lett. 142:283-287. [PubMed] [Google Scholar]
- 18.Palop, A., G. C. Rutherford, and R. E. Marquis. 1998. Inactivation of enzymes within spores of Bacillus megaterium ATCC 19213 by hydroperoxides. Can. J. Microbiol. 44:465-470. [PubMed] [Google Scholar]
- 19.Park, S., and J. A. Imlay. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 185:1942-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic acid lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 93:15405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagripanti, J.-L. 1992. Metal-based formulations with high microbiocidal activity. Appl. Environ. Microbiol. 58:3157-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagripanti, J.-L., and A. Bonifacino. 1996. Comparative sporicidal effect of liquid germicides on three medical devices contaminated with spores of Bacillus subtilis. Am. J. Infect. Control 24:364-371. [DOI] [PubMed] [Google Scholar]
- 23.Sagripanti, J.-L., and A. Bonifacino. 1999. Bacterial spores survive treatment with commercial sterilants and disinfectants. Appl. Environ. Microbiol. 65:4255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saran, M., I. Beck-Speier, B. Fellerhoff, and G. Bauer. 1999. Phagocytic killing of microorganisms by radical processes: consequences of the reaction of hydroxyl radicals with chloride yielding chlorine atoms. Free Radical Biol. Med. 26:482-490. [DOI] [PubMed] [Google Scholar]
- 25.Saran, M., and W. Bors. 1997. Radiation chemistry of physiological saline reinvestigated: evidence that chloride-derived intermediates play a key role in cytotoxicity. Radiat. Res. 147:70-77. [PubMed] [Google Scholar]
- 26.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637-648. [DOI] [PubMed] [Google Scholar]
- 27.Setlow, B., C. A. Loshon, P. C. Genest, A. E. Cowan, C. Setlow, and P. Setlow. 2002. Mechanisms of killing of spores of Bacillus subtilis by acid, alkali and ethanol. J. Appl. Microbiol. 92:362-375. [DOI] [PubMed] [Google Scholar]
- 28.Setlow, B., K. A. McGinnis, K. Ragkousi, and P. Setlow. 2000. Effects of major spore-specific DNA binding proteins on Bacillus subtilis sporulation and spore properties. J. Bacteriol. 182:6906-6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setlow, B., and P. Setlow. 1993. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl. Environ. Microbiol. 59:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setlow, P. 2000. Resistance of bacterial spores, p. 217-230. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 32.Swerdlow, B. M., B. Setlow, and P. Setlow. 1981. Levels of H+ and other monovalent cations in dormant and germinating spores of Bacillus megaterium. J. Bacteriol. 148:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tennen, R., B. Setlow, K. L. Davis, C. A. Loshon, and P. Setlow. 2000. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J. Appl. Microbiol. 89:330-338. [DOI] [PubMed] [Google Scholar]
- 34.Wardman, P., and L. P. Candeias. 1996. Fenton chemistry: an introduction. Radiat. Res. 147:523-531. [PubMed] [Google Scholar]
- 35.Woodmansee, A. N., and J. A. Imlay. 2003. A mechanism by which nitric oxide accelerates the rate of oxidative DNA damage in Escherichia coli. Mol. Microbiol. 49:11-22. [DOI] [PubMed] [Google Scholar]
- 36.Yasbin, R., D. Cheo, and D. Bol. 1993. DNA repair systems, p. 529-538. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 37.Young, S. B., and P. Setlow. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 95:54-67. [DOI] [PubMed] [Google Scholar]