Abstract
Damage by the Russian wheat aphid (RWA), Diuraphis noxia, significantly reduces wheat and barley yields worldwide. In compatible interactions, virulent RWA populations flourish and susceptible plants suffer extensive leaf chlorophyll loss. In incompatible interactions, RWA reproduction and population growth are significantly reduced and RWA-related chlorophyll loss in resistant plants is minor. The objectives of this study were to develop an understanding of the molecular and phytochemical bases of RWA resistance in plants containing the Dnx resistance gene. Microarray, real-time polymerase chain reaction, and phytohormone assays were conducted to identify transcriptome components unique to RWA-infested Dnx plants and susceptible (Dn0) plants, and to identify and characterize putative genes involved in Dnx plant defense responses. We found that RWA-infested Dnx plants upregulated > 180 genes related to reactive oxygen species, signaling, pathogen defense, and arthropod allelochemical and physical defense. The expression of several of these genes in RWA-infested Dnx plants increased significantly from 6- to 24-h post infestation (hpi), but their expression in Dn0 plants, when present, was delayed until 48- to 96 hpi. Concentrations of 16- and 18-carbon fatty acids, trans-methyl-12-oxophytodienoic acid, and abscisic acid were significantly greater in Dnx foliage than in Dn0 foliage after RWA infestation, suggesting that Dnx RWA defense and resistance genes may be regulated via the oxylipin pathway. These findings provide a foundation for the elucidation of the molecular basis for compatible- and incompatible plant-aphid interactions.
Keywords: Diuraphis noxia, Insect, Microarray, Northern blot, Oxylipin signaling, Plant defense, Real-time polymerase chain reaction (PCR), Russian wheat aphid, Triticum aestivum, Wheat
Introduction
Aphids (Order Homoptera) are major arthropod pests of agriculture worldwide, damaging crops by removing photoassimilates and vectoring numerous devastating plant viruses. The limited tissue damage caused during aphid feeding and the prolonged interactions of aphid mouthparts with plant cells make plant responses to phloem-feeding aphids distinct from those of chewing insects (Walling 2000). Many species of aphids are resistant to insecticides (Devonshire and Field 1991), and some have developed virulence to plant aphid resistance genes. Environmental concerns linked to insecticide use have led to the development and cultivation of many aphid-resistant crop varieties during the past century (Panda and Khush 1995; Smith 2005).
The Russian wheat aphid (RWA), Diuraphis noxia, is a serious pest of wheat, Triticum aestivum, and barley, Hordeum vulgare, and with the exception of Australia, the aphid has been introduced globally since the early 1900s (Quisenberry and Peairs 1998). In compatible interactions with RWA, susceptible wheat plants react to the injection of RWA saliva by rolling the leaves longitudinally around the main leaf vein to form a tubular refuge that protects aphids from predators. As a result, RWA populations flourish and plants suffer extensive leaf chlorophyll and carotenoid loss (Burd and Eliott 1996; Heng-Moss et al. 2003). These reductions also are manifested as significant reductions in photosynthetic efficiency that results in weakened plants with substantially lower grain yields (Smith et al. 1991). In incompatible interactions that involve plants containing the RWA-resistance genes, RWA reproduction and population growth are significantly reduced, and chlorophyll loss is minor.
Heritable resistance to pest insects has been widely documented in many cereal, forage, fruit, and vegetable crop plants (Smith 1989) and single resistance (R) genes inherited as dominant traits control resistance in both monocots and dicots (reviewed in Smith 2005). Resistance may be categorized as antibiosis (an adverse effect on insect biology - including mortality), antixenosis (an adverse effect on insect behavior), or tolerance (the ability of a plant to withstand insect damage). Many insect-resistant plants, including RWA-resistant barley and wheat, contain multi-category resistance (reviewed in Berzonsky et al. 2003; Smith 2005).
Ten RWA (Dn) resistance genes from cereal crops have been identified (Smith 2004; Liu et al. 2005) and are being deployed in the U.S. (Quick et al. 1996) and South Africa (Prinsloo 2000). However, RWA virulence occurs in Africa (Malinga et al. 2007; Tolmay et al. 2007), Asia (Dolatti et al. 2005), Europe (Basky 2003), North America (Burd et al. 2006), and South America (Smith et al. 2004).
An understanding of the molecular bases of plant-aphid interactions is progressing, albeit slowly, based on the identification of plant genes that control aphid resistance. The NBS-LRR gene Mi-1.2 from Lycopersicon peruvianum controls resistance to the potato aphid, Macrosiphum euphorbiae (Thomas), and to three species of the root knot nematode, Meloidogyne spp. (Kaloshian et al. 1997; Vos et al. 1998). No monocot insect R genes have been cloned, but the transcript levels of NBS-LRR genes are affected in plants infested by aphids (Lacock et al. 2003; Klingler et al. 2005; Botha et al. 2006; Park et al. 2006). Aphid feeding activates plant defense signals similar to those involved in response to pathogen infection (Tjallingii and Hogen-Esch 1993; Walling 2000; Kaloshian 2004), but the origin of aphid elicitors of these signals is poorly understood (Urbanska et al. 1998; Miles 1999; Forslund et al. 2000).
The recognition of aphid probing and sustained feeding results in transmission of defense response signal cascades that involve jasmonic acid (JA), salicylic acid (SA), ethylene (ET), abscisic acid (ABA), and gibberellic acid (GA) (Smith and Boyko 2006). At the onset of aphid-plant interactions, aphid feeding results in the altered activation of peroxidases, intercellular chitinases, and β-1,3-glucanases involved in the release of plant cell wall oligosaccharides (Smith and Boyko 2006). Reactive oxygen species (ROS) elicitors that respond to aphid feeding may also upregulate the activity of these enzymes. Peroxidase (PER), glutathione transferase (GST), catalase (CAT), nitrate reductase, and quinone oxidoreductase genes are upregulated in aphid-infested plants (Martinez-de Ilarduya et al. 2004; Zhu-Salzman et al. 2004; Divol et al. 2005; Boyko et al. 2006; Park et al. 2006; Couldridge et al. 2007).
Interactions between aphids and their host plants elicit the activation of both the JA and SA defense response pathways and the related upregulation of genes controlled by each of these plant hormones. Examples of these interactions include greenbug feeding on sorghum (Zhu-Salzman et al. 2004), the peach potato aphid (PPA), Myzus persicae, feeding on Arabidopsis thaliana and potato, Solanum tuberosum (Fidantsef et al. 1999; Moran and Thompson 2001; Moran et al. 2002), and potato aphid feeding on potato and tomato (Fidantsef et al. 1999; Martinez-de Ilarduya et al. 2004). Results of experiments with RWA feeding on wheat, and the tobacco aphid, Myzus nicotianae, feeding on wild tobacco, Nicotiana attenuate, indicate the involvement of JA signaling but only marginal activity of SA signals in these interactions (Voelckel et al. 2004; Boyko et al. 2006).
Ethylene production increases significantly after aphid feeding on the foliage of aphid-resistant barley or wheat plants compared with susceptible plants (Miller et al. 1994; Argandona et al. 2001; Boyko et al. 2006), and sequences that code for proteins involved in ET production are over-expressed in aphid-infested Arabidopsis and celery, Apium graveolens, plants (Moran et al. 2002; Divol et al. 2005). Jasmosinc acid and ET are synergistic in the defense responses of Arabidopsis resistance to the peach potato aphid (Dong et al. 2004) and in the induction of defense responses in squash, Cucurbita moschata, foliage to feeding by the silver leaf whitefly, Bemisia argentifolii, (van de Ven et al. 2000). WRKY proteins (with the conserved amino acid sequence WRKYGQK) modulate JA-SA interactions in Arabidopsis pathogen response (Li et al. 2004) and WRKY upregulation in tobacco plants infested by the tobacco aphid suggests that JA-SA interactions also play a role in plant defense responses to aphids (Voelckel et al. 2004). Sequences putatively involved in ABA and GA biosynthesis are upregulated in aphid-infested foliage of celery, sorghum, and wheat (Zhu-Salzman et al. 2004; Divol et al. 2005; Boyko et al. 2006; Park et al. 2006).
The objectives of this study were to develop an understanding of the molecular and phytochemical bases of RWA resistance in wheat plants that contain the Dnx RWA resistance gene. Microarray, real-time PCR, and phytohormone assays were used to identify transcriptome components and phytohormones that were differentially regulated in RWA-infested Dnx plants and susceptible (Dn0) plants. Previous research (Dong et al. 2004; Voelckel et al. 2004; Boyko et al. 2006; Park et al. 2006), led us to hypothesize that unique components of the Dnx transcriptome in the ROS, oxylipin, ABA, and terpenoid pathways are activated by RWA feeding, and that these proteins may function to mediate the expression of the Dnx plant phenotype.
Methods and Materials
Plant Genotypes and Aphids
A wheat landrace from Afghanistan (USDA Plant Introduction 220127) (Harvey and Martin 1990) containing the RWA resistance gene Dnx (Liu et al. 2001) was crossed to the RWA-susceptible wheat genotype ‘Sando’s Selection 4040’ (Dn0), originally developed at Oklahoma State University. F2-derived F3 family plants originating from this cross were bulked into groups of 10 resistant plants and 10 susceptible plants according to their reaction to RWA biotype 1 (RWA1) infestation (Liu et al. 2001). Bulk lines were advanced to the F4 generation. Seeds of resistant and susceptible F4 lines were planted in 12 cm diam plastic pots containing Jiffy® potting mix and grown in the greenhouse during July 2005 at 30°C day: 22°C night, and a photoperiod of 14:10 (L:D). Supplemental lighting was provided from sodium halide lamps. Experiments were conducted in the greenhouse under the same conditions. Seed stocks are currently maintained by the Plant Resistance Laboratory, Department of Entomology, Kansas State University (KSU), Manhattan, KS, USA. RWA biotype 1 (RWA1) used in all experiments originated from a culture established with aphids collected from Hays, KS, in 2004, courtesy of Dr. J. P. Michaud, KSU Dept. of Entomology, Hays, KS. The culture was maintained on RWA-susceptible ‘Jagger’ wheat plants at the environmental conditions described above for plant growth.
In total, 140 plants with the Dnx gene and 110 plants lacking the gene (Dn0) were assessed across five different experiments. These included plant phenotyping experiments to classify plant and aphid responses to each other (20 plants of each genotype), a microarray experiment to assess wheat EST expression (90 Dnx plants, 60 Dn0 plants), northern blot verification of microarray results (9 plants of each genotype), real-time PCR verification of microarray results (9 plants of each genotype), and phytohormone experiments (12 plants of each genotype).
Phenotype Evaluations
The previously reported resistance of Dnx and susceptibility of Dn0 plants to RWA1 (Liu et al., 2001; Boyko et al. 2006) was confirmed in experiments to measure phenotypic damage expression, tolerance resistance, and antibiosis resistance with protocols of Boyko et al. (2006). Pre-germinated Dnx and Dn0 F4 seeds were planted in pots and allowed to grow to the two-leaf stage. Plants were paired for height and growth (Dnx with Dnx, Dn0 with Dn0), and one plant of each pair was infested with five RWA1 late stage nymphs. The remaining plant of each pair was not infested and used as a control. All pots were covered with individual nylon-mesh cages, and 10 pairs (replicates) of plants were arranged in a randomized complete block design. When the infested Dn0 plants showed complete leaf rolling and 95% chlorosis of the youngest leaf (~21 d), cages were removed and leaf chlorosis, leaf rolling and leaf folding damage sustained from RWA1 feeding was rated on a 0 - 3 scale for each symptom; where 0 = no damage, 1 = < 50% symptoms, 2 = > 50% symptoms and 3 = 100% symptoms/dead plant) (Smith et al., 1991).
Tolerance resistance, the ability of a plant to withstand arthropod damage and yield significantly greater dry mass than a susceptible plant under similar conditions of infestation (Smith 2005), was measured by calculating the proportional plant dry weight change (DWT) of Dnx and Dn0 plants as DWT = [(WC - WT)/WC] × 100, where WC was the dry weight of the uninfested control plant, and WT was the dry weight of the infested plant (Reese et al. 1994). The shoots from uninfested and infested plants were cut at the soil surface, placed in aluminum foil pouches, dried in an oven at 75° C for 72 h, and tissue weights were determined.
Antibiosis resistance, in which the plant adversely affects the growth and development of the arthropod, was determined by counting the numbers of RWA1 on the infested plants in each of the pairs of plants in the tolerance experiment. Aphids produced on infested plants of each genotype were removed with a camel’s-hair brush, collected on wax paper, funneled into vials of 95% EtOH, and counted. A tolerance index (TI) was calculated by using the equation: plant DWT/number of aphids produced on the infested plants (Reese et al. 1994). The plant TI was determined to compensate for the confounding effect of differing numbers of RWA1 on infested plants. Genotypes with TI values significantly lower than those of the susceptible control plants were considered tolerant. Data for plant damage, plant DWT, plant TI, and RWA1 population development were subjected to ANOVA by using the SAS GLM procedure (SAS 2001). Where significant, treatment means were separated by using the LSD at α = 0.05.
RNA Extraction
For the microarray hybridization experiments, RNA was extracted from leaves of Dnx and Dn0 plants at the two-leaf stage of growth when the 3rd leaf was beginning to unfurl. Plants were grown in the greenhouse as mentioned previously and were not under drought stress. There were three replications of Dnx infested plants, three replications of Dnx uninfested plants, two replications of Dn0 infested plants, and two replications of Dn0 uninfested control plants. Each replicate of each treatment contained 15 plants. Each of the 15 treatment plants was infested with approximately 50 RWA1 late stage nymphs and adults. In each treatment replicate, leaves of all plants were harvested 24 h after infestation, pooled, quick frozen in a freezer at -80°C, and ground in liquid N2. Total RNA was extracted from pooled leaves with TRI ReagentTM following the manufacturer’s protocol (Molecular Research Center Inc., Cincinnati, OH, USA). RNA samples were purified with a RNease Kit following the manufacturer’s instruction from QIAGEN Inc. (Valencia, CA, USA). RNA concentrations were measured by using a NanoDrop-1000 Spectrophotometer from NanoDrop Technologies (Wilmington, DE, USA). RNA quality was checked with an Agilent 2100 Bioanalyzer following the Reagent Kit guide from Agilent Technologies (Foster City, CA, USA).
Microarray Hybridization and Data Analysis
The Affymetrix GeneChip® Wheat Genome Arrays (Santa Clara, CA, USA) contain 61,127 probe sets representing 55,052 transcripts for all 42 chromosomes in the wheat genome on a single array. The array includes ESTs from T. monococcum, T. turgidum, and Ae. tauschii, and GenBank® full-length mRNAs from all species through May 18, 2004. Labeling and hybridization of arrays were performed according to the standard protocol provided by Affymetrix. (www.affymetrix.com/support/technical/manual/expression_manual.affx). After hybridization and washing, arrays were scanned with an Affymetrix GeneChip Scanner 3000. Hybridization quality was verified by scaling factor, overall hybridization rate, and signal strength of several bacterial spike controls. The spike controls were hybridized with labeled targets in different concentrations resulting in particular ratios between different spikes.
Image acquisition, image settings, and raw data generation were accomplished with Affymetrix GeneChip® Operating Software (GCOS). After alignment of the image settings for each chip, raw data were extracted and marked as “present” (well above the background), “marginal,” or “absent” (similar to, or below the background) under default settings. The overall target signal intensity of each chip was scaled to 500 and then loaded into GeneSpring GX 7.3.1 software (Agilent Technologies, Palo Alto, CA, USA) for further analysis. The data were pre-normalized to the 50th percentile per chip and then normalized based on per-gene normalization with the median method, from which the signal of each gene in a particular sample was divided by the median signal of the same gene in all samples.
Normalization per gene and per chip of the log2 values was performed to allow the comparison of the independent replicates performed in the experiment. Normalization was performed for all measurements by using the flags (“present”, “marginal”, or “absent”) assigned by Affymetrix treatment of the arrays. However, only those transcripts that were declared present or marginal in at least 4 of the 6 microarrays hybridized with RWA-resistant Dnx probes and 3 of the 4 arrays hybridized with RWA-susceptible Dn0 probes were taken into account. This procedure facilitated the elimination of transcripts with very low signals in both treatments (declared “absent”).
The resulting genes that exhibited significant changes in expression in comparisons between treated and control Dnx or Dn0 plants were selected by applying a t-test (one-way ANOVA Welch t-test, P > 0.05 with “Benjamini and Hochberg False Discovery Rate” for multiple comparisons α = 0.05). A cutoff value of a 2-fold change, commonly used for microarray analysis, was used to discriminate the expression of genes that were differentially altered in response to RWA feeding. Fold change values were calculated between treatment and control samples based on the normalized average measurement of the signal intensity. The number of sequences examined was further narrowed after eliminating those with raw expression values of < 600 units.
Comparisons of changes in differential up- or downregulation of gene expression between RWA-infested Dnx and Dn0 plants were discriminated by using the CEDA (Comparative EST Data Analysis) virtual subtraction procedure developed by Wang and Zhang (2004). Genes expressed in uninfested control plants were removed from those in infested plants with the formula: [(Dnx infested-Dnx control)-(Dn0 infested-Dn0 control)] for Dnx plants, and [(Dn0 infested-Dn0 control)-(Dnx infested-Dnx control)] for Dn0 plants. The CEDA output provided a list of candidate up- or downregulated genes, uniquely expressed in Dnx infested plants or Dn0 infested plants, and sorted by the statistical significance of change in gene expression.
The nucleotide sequences identified after probing and hybridization of arrays were clustered by using the CAP3 software tool with default settings (Huang and Madan 1999). From 61,290 array probe sequences, a non-redundant dataset containing 7,511 contigs and 42,863 singletons was obtained that allowed the comparison of the expression of different probes mapped to a single contig. To understand gene function, non-redundant sequences were searched against the UniProt reference database (Bairoch et al. 2005) with the BLASTX program downloaded from the NCBI site (ftp://ftp.ncbi.nih.gov/blast/). Queried sequences were annotated by using the best hit in UniProt with an E value threshold of 1e-5. Functional annotation included text description as well as gene ontology terms of each matched reference sequence (Camon et al. 2003), to understand the biological processes involving genes of interest. Unique known transcripts in Dnx and Dn0 plants were compared for similarity to known genes, and these genes were classified by function.
Northern Blot Analyses
To validate the expression of ESTs in the microarray hybridzation experiment, a separate northern blot experiment was conducted with total RNA isolated from fresh leaf tissue of RWA-infested resistant (containing the Dnx gene) and susceptible (containing the Dn0 gene) wheat plants infested with 20 RWA biotype 1 late stage nymphs per plant. Tissues were collected at 6-, 12-, 24-, 72-, and 120 h post-infestation, and RNA was extracted with TRI reagent™ (Molecular Research Center). Each treatment consisted of RNA pooled from two biological replicates, and each replicate contained leaf tissue from 3 plants, for a total of 6 plants. Three separate uninfested Dnx plants and 3 separate uninfested Dn0 plants served as non-infested controls. Tissues were collected from plants at each time point. Five μg of total RNA from each treatment were subjected to electrophoresis in denaturing 1.5% agarose gels containing formaldehyde and transferred onto a GeneScreen membrane (Perkin-Elmer Life Science Inc., Boston, MA, USA). Gels were stained with ethidium bromide, and rRNA levels were compared to control for equal loading. Membranes were baked at 80 °C for 2 h to fix the RNA and then hybridized separately to individual probes. Primers for PCR amplification were generated at the KSU Core Biotechnology Facility by using selected sequences from the Affymetrix GeneChip® Wheat Genome Array EST files (Table 1). Probes were derived by PCR amplification of plant template cDNAs and labeled with 32P-dCTP by using the random labeling kit from Stratagene (La Jolla, CA, USA). Prehybridization (4 h) and hybridization (overnight) were carried out at 42°C in hybridization buffer (Chen et al. 2004). Membranes then were washed at medium stringency [2x SSC at room temperature for 30 min, 2x SSC, 1% sodium dodecyl sulphate (SDS) at 65°C for 30 min, and 0.1x SSC, 0.1% SDS at room temperature for 30 min]. Blots were placed against X-ray film (Kodak) and hybridization patterns were visualized by autoradiography.
Table 1.
Oligonucleotide primers used for isolation and expression analysis of wheat proteins involved in defense responses to Russian wheat aphid biotype 1
| Purpose/Primer Name | Sequence (5′—3′) |
|---|---|
| Northern Blot Analyses | |
| Q9XEN7 β-1,3-glucanase | |
| 5′ | CTCTTCAACCCGGACAAATC |
| 3′ | TGAAGAATTTGGGCGTTTTC |
| Q8W427 Chitinase III | |
| 5′ | CGACAACCTGGACTGCTACA |
| 3′ | ATGGATCGCACCATTATTCG |
| Q01482 WIR1A Membrane protein | |
| 5′ | CTCCTGCAGATCGCTCTCTT |
| 3′ | CCGGTGGTCTACATCCGTAA |
| Q43212 Peroxidase precursor | |
| 5′ | AACACTGTCCGGAACTTTGC |
| 3′ | TGTCGTGCTGGCTAGTATGC |
| Q8S702 Glutathione S-transferase | |
| 5′ | CCTCAGGGACTGCTCTAACG |
| 3′ | GTCCAACGATCCGAAGTTGT |
| Q8H8H7 Flavanone 3-hydroxylase | |
| 5′ | TACCGCAGCTACACCTACGA |
| 3′ | TGAGTAATGCTGCGTCGTG |
| Q9AVM3 Cytochrome P450 | |
| 5′ | CATCATTGACATGTCCTGAAAA |
| 3′ | GGGCTTGCAGTAAGCAAAAA |
| Q5BQ31 Serine/threonine kinase | |
| 5′ | AAAAGGCACATAGCGTCCAT |
| 3′ | AGTGGTGGAGACCAGGTTTG |
| P27357 Thaumatin-like protein PWIR2 precursor | |
| 5′ | GCAGCACCCAGGACTTCTAC |
| 3′ | TGCGACGTATAGAGGCTTCA |
| P29114 LOX1 Lipoxygenase 1 | |
| 5′ | GATCGAGAGCAAGGTGGTG |
| 3′ | TCAGATGGAGATGCTGTTGG |
| Q5NTH3 Shikimate kinase 2 | |
| 5′ | ATCCATACACAGCGGCTTTC |
| 3′ | GTAGGGCCTCGACAGCAATA |
| P12940 Bowman-Birk trypsin inhibitor | |
| 5′ | GACCCATCCCTCAACGTCT |
| 3′ | ACACCTGCTGGCGTATTCAT |
| Q6Z676 phi-1 ABA dehydration signaling | |
| 5′ | CACCTGTTCGACCTTGGTGT |
| 3′ | GAAAGCCAGTGCAGCAATTT |
| P93671 XET Xyloglucan endotransglycosylase | |
| 5′ | GTGGGTGCAGAGCAACTACA |
| 3′ | GGCGTAAATGCCAAAGAAGA |
| Q43210 PAL Phenylalanine ammonia lyase | |
| 5′ | ACCAGGGTAAGCACATCGAC |
| 3′ | ATCTTTGGCAATGGCCTCTA |
| Expression Analysis (qRT PCR) | |
| Q9P3N1 Stress response protein | |
| 5′ | CTTCACATCTAACGGGCATC |
| 3′ | ATGGAGGTGCTTGAGACG |
| Q5ZD81 Calmodulin-like protein | |
| 5′ | AGGGAAGGGAAAGGATAAAGTG |
| 3′ | CGACCTACAGACAGTACGC |
| Q6Z1A3 NAC1 Stress response protein | |
| 5′ | GGAGGTTACATTACATTTGGAGAG |
| 3′ | TGGAGTAGCATTGGACTATTGG |
| Q6YXE1 Cytochrome P450 | |
| 5′ | TCATGGAGAAGAACAAGCAG |
| 3′ | GAGGCGGGTGTAGAAGAG |
| Q7XN01 Transcription protein | |
| 5′ | GCCATTGCGGAGTCACAAG |
| 3′ | TGGTTCGTCCTTCACTATGC |
| Q6I5G9 Mitochondrial ATP synthase | |
| 5′ | TCATGGAGAAGAACAAGCAG |
| 3′ | GAGGCGGGTGTAGAAGAG |
| TA1868 Wheat actin | |
| 5′ | GAGTCGGTGAAGGTTGTTTAC |
| 3′ | CTTAGGCAGCGTTTGGAATAC |
Real-Time Quantitative PCR Analyses
Real-time PCR was used to confirm the expression of six stress response or cellular metabolism genes in leaf tissues from both Dnx and Dn0 plants. These genes had been shown previously to be highly downregulated in the transcriptome of Dnx plants at 24 hpi. Genes included Q9P3N1 (hypothetical stress response protein), Q5ZD81 (calmodulin-like protein), Q6Z1A3 (putative NAC1 stress response protein), Q6Z1A3 (putative cytochrome P450), Q7XN01 (transcription protein), and Q6I5G9 (mitochondrial ATP synthase). Primers were designed based on Affymetrix™ Gene Chip EST sequences and GeneBank by using the software package Beacon Designer. Primers sequences are shown in Table 1. Wheat actin (AB181991) was used as an internal control. Total RNA was isolated as described for northern blotting. After purification with TURBO™ DNA-free (Ambion, Austin, TX), 2 μg total RNA were reverse transcribed into cDNA by using a SuperScript III First-Strand cDNA Synthesis System (Invitrogen) following the manufacture’s protocols.
The change in expression of these genes was determined at 6-, 12-, 24-, 72-, 96-, and 120 hpi in tissues from Dnx and Dn0 plants infested with 20 RWA biotype 1 late stage nymphs per plant. For each post-infestation - genotype treatment, RNA was collected from two biological replicates, each consisting of leaves pooled from 3 plants. Three separate uninfested Dnx plants and 3 separate uninfested Dn0 plants served as non-infested controls. Real-time PCR was performed with iQ™ SYBR Green Supermix (BIO-RAD) by using the flowing amplification protocol: 5 min denaturation at 95°C, 40 cycles of 30 s at 95°C, 20 s at 53°C, and 45 s at 72°C. This was followed by product melt to confirm a single PCR product. Gene downregulation fold change was calculated as: 2ΔΔCt [ΔΔCt = (CtGOI In - CtGOI Un) - (CtHKG In - CtHKG Un)], where GOI = gene of interest, In = infested sample, Un = uninfested sample, HKG = wheat-actin gene. Data for mean downregulation of each gene were subjected to ANOVA by using the SAS GLM procedure (SAS, 2001). Where significant, treatment means were separated by using the LSD at α = 0.05.
Phytohormone Analyses
Individual two leaf stage plants containing Dnx or Dn0 were grown in 10 cm diam. plastic pots filled with Pro-MixBx® potting mix, in the greenhouse [24°C day: 20°C night, photoperiod of 14:10 (L:D)]. Six Dnx or 6 Dn0 plants were each infested for 12-, 24-, 48-, or 96 h with 20 to 30 RWA1 late stage nymphs and adults per plant. Six uninfested Dnx or Dn0 plants collected at 24 h post-infestation served as uninfested controls for all infested Dnx or Dn0 treatments. At the end of each infestation period, all above ground foliage from plants in each treatment were harvested, placed a in freezer at-80°C, ground to powder in liquid N2, weighed, and analyzed.
Plant powders were extracted in 300 μl cold 1-propanol : H2O : HCl (2:1:0.005), centrifuged for 1 min, the organic layer transferred to a glass tube, and diluted with 20 μl of 2M trimethylsilyldiazomethane:methylene chloride (1:4). Each extract then was combined with 20 μl of (12:88 acetic acid:hexane) : methylene chloride (1:4) and allowed to stand overnight to quench methylation. Samples were placed in SuperQ columns, where phytohormones were removed by vapor phase extraction and eluted from columns with 100-200 μl of methylene chloride into GC vials. The residual solvent from each SuperQ column was removed and added to each sample vial with a N2 stream. Due to sampling errors from damage to tissues or sample loss during vapor phase extraction, the number of replications analyzed for each phytohormone treatment varied from N = 3 to N = 6.
Samples were subjected to quantitative analyses by using an HP 6890 gas chromotograph and HP 5973 mass spectrometer in the KSU Lipidomics Research Center. Total ion counts were acquired and processed by using Agilent Chemstation software, AMDIS (www.amdis.net), and MET-IDEA (Broeckling et al. 2006; http://www.noble.org/plantbio/ms/MET-IDEA/index.html). Plant tissue data were normalized by fresh weight, and sample ion counts were expressed as per cent ng/g standard. Spectral data were subjected to log e transformation and analyzed by using SAS PROC GLM with unequal variance (SAS, 2001). The procedures used for tissue preparation, extraction, sample acquisition, and GC-MS analyses are detailed in Schmelz et al. (2004).
Samples were evaluated for content of the following phytohormones and fatty acids: methyl abscisic acid (ABA); methyl benzoate (BA); methyl trans-cinnamate (CA); methyl salicylate (SA); cis - and trans methyl jasmonate (JA); cis- and trans-methyl-12-oxophytodienoic acid (12-OPDA); the saturated 16:0 and 18:0 fatty acids, and the 16:1, 16:3, 18:1, 18:2, and 18:3 unsaturated fatty acids. Internal standards were as follows: ABA -- (S)-5-(1-hydroxy-2, 6, 6-trimethyl-4-oxo-2-cyclohexen-1-yl)-3-methyl-(2Z,4E)-pentadienoic acid; BA—(ring-13C6) benzoic acid methyl ester; CA -- (E)-3-phenyl-2-propenoic acid methyl ester; SA -- 2-hydroxybenzoic acid methyl ester; and JA -- 3-oxo-2-(2-pentenyl) cyclopentaneacetic acid. The CA internal standard was used to calculate the amount of each methyl fatty acid, OPDA-Me trans, and OPDA-Me cis. In several previous experiments, the relative instrument response of CA/methyl 19:0 fatty acid was 0.137 ng/ng.
Results
Phenotype Evaluations
F4 plants containing the RWA1 resistance gene Dnx displayed limited leaf rolling symptoms (1.8 ± 0.62, mean ± SE) and leaf chlorosis symptoms (1.8 ± 0.13, mean ± SE), compared to F4 plants lacking Dnx (Dn0) (mean ± SE leaf rolling = 2.9 ± 0.12, mean ± SE chlorosis = 3.0 ± 0.34). (Both symptoms rated on a 0 - 3 scale; 0 = no damage, 3 = 100% symptoms).
Both antibiosis and tolerance function in RWA1 resistance imparted by other Dn genes (Hein, 1992; Smith et al., 2004; Voothuluru et al. 2006), and in tolerance experiments infested Dnx plants sustained significantly (P < 0.009) smaller changes in proportional plant dry weight (DWT) compared with Dn0 plants (Fig. 1B). However, when RWA1 population sizes on Dnx and Dn0 plants were used to compute a tolerance index (TI) to compensate for different population sizes, the observed differences in plant DWT were not significant (data not shown). Nevertheless, leaves of Dnx plants exhibited significantly less leaf rolling and leaf chlorosis compared to Dn0 plants, thus demonstrating their ability to tolerate RWA1 feeding and chloroplast destruction.
Fig. 1.
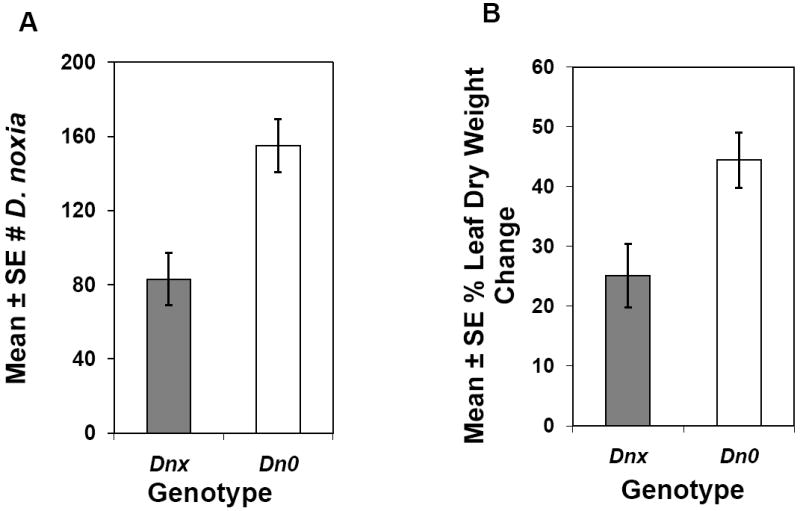
Phenotypic responses of Russian wheat aphid biotype 1 (RWA1) and RWA1-resistant (Dnx) and susceptible (Dn0) plants at 21 d post infestation: (a) Mean ± S.E. RWA population development (differences significant at P = 0.001); (b) Mean ± S.E. percent proportional leaf dry weight changes as determined by DWT = [(dry wt. of uninfested control plant - dry wt. of infested plant) / dry wt. of uninfested control] × 100 (differences significant at P = 0.009); N = 10.
Dnx and Dn0 Plant Transcriptomes
There were distinct and dramatic quantitative differences in the unannotated transcriptomes of RWA1-infested plants and uninfested plants containing Dnx and Dn0. Infested Dnx plants upregulated 1,137 genes and downregulated 171 genes that were expressed at significantly (P≤0.05) greater levels compared to uninfested control plants (Table 2). In contrast, infested Dn0 plants significantly upregulated only 201 genes and downregulated 16 genes.
Table 2.
Numbers of genes exhibiting significant changes in expressiona in comparisons between infestedb and uninfested plants of wheat genotypes resistant (Dnx) and susceptible (Dn0) to Russian wheat aphid biotype 1
| Genotype/Treatment Comparison | Number of Upregulated Genes | Number of Downregulated Genes |
|---|---|---|
| Dnx infested versus Dnx uninfested control | 1,137 | 171 |
| Dn0 infested versus Dn0 uninfested control | 201 | 16 |
P ≤ 0.05, minimum 2-fold change.
24 h post infestation.
The CEDA virtual subtraction procedure (Wang and Zhang 2004) was applied to the data in Table 2 to identify genes uniquely expressed in Dnx infested plants or Dn0 infested plants. This was accomplished by using the formulae [(Dnx infested-Dnx control)-(Dn0 infested-Dn0 control)] for Dnx plants, and [(Dn0 infested-Dn0 control)-(Dnx infested-Dnx control)] for Dn0 plants. CEDA subtraction yielded 551 upregulated genes and 43 downregulated genes in infested Dnx plants (≥2-fold expression differences, P≤0.05). Of these genes, 401 of the upregulated transcripts and 27 of the downregulated transcripts were of unknown function. After ontology and grouping, 161genes unique to RWA1-infested Dnx plants were significantly upregulated and only 17 genes were significantly downregulated (Table 3). After virtual subtraction of the Dn0 transcriptome, followed by ontology and grouping, 38 genes were found to be significantly upregulated and 14 genes were found to be significantly downregulated (Table 3). The putative functions of affected transcripts in both genotypes were related to plant cell wall disruption; the initiation of defense responses; ROS production; ABA-, ET-, JA-, and SA signaling; phytopathogen defense responses; and arthropod allelochemical and physical defenses (Table 3). We found differences in the Dnx and Dn0 transcriptomes after annotation (Table 3), compared to before annotation (Table 2), as well as before and after application of the CEDA virtual subtraction procedure.
Table 3.
Numbers of annotated genes in different functional classes expresseda in wheat plant leaves containing the Dnx resistance gene or Dn0 susceptibility gene after phloem feeding by Russian wheat aphid biotype 1b as determined by the Comparative EST Data Analysis virtual subtraction procedurec
| Number of Upregulated Genes | Number of Downregulated Genes | |||
|---|---|---|---|---|
|
| ||||
| Gene Functional Class | Dnx | Dn0 | Dnx | Dn0 |
| β-glucanases | 7 | 0 | 0 | 0 |
| Chitinases | 5 | 0 | 0 | 0 |
| Membrane proteins | 7 | 2 | 4 | 1 |
| Protein kinases | 12 | 0 | 1 | 1 |
| GST, Ca++, Fe++ binding | 10 | 7 | 4 | 2 |
| Oxidoreductase/Hydroxylase | 20 | 4 | 3 | 1 |
| SA signaling | 16 | 3 | 0 | 1 |
| JA signaling | 8 | 5 | 0 | 0 |
| ABA signaling | 4 | 0 | 0 | 0 |
| ET signaling | 2 | 0 | 0 | 0 |
| AUX signaling | 0 | 4 | 0 | 0 |
| Arthropod allelochemical defense | 18 | 4 | 0 | 0 |
| Pathogen defense | 15 | 1 | 0 | 0 |
| Arthropod structural defense | 37 | 1 | 0 | 2 |
| Metabolism | 0 | 7 | 5 | 6 |
|
| ||||
| Total number of genes | 161 | 38 | 17 | 14 |
P ≤ 0.05, minimum 2-fold change.
24 h post infestation.
Dnx plants = [(Dnx infested-Dnx control)-(Dn0 infested-Dn0 control)];
Dn0 plants = [(Dn0 infested-Dn0 control)-(Dnx infested-Dnx control)].
Plant Cell Wall Disruption
Numerous transcripts with putative functions in the cell wall were 2- to 6-fold upregulated in infested Dnx plants (Supplemental Data Table 1). These transcripts include cereal β-1,3-glucanase (GLG), chitinase (Chia), protein kinase, and WIR1 (WIR1) membrane proteins based on sequence similarity. Among them, GLG and Chia were completely absent in infested Dn0 plants. On the other hand, infested Dn0 plants expressed two membrane proteins (Supplemental Data Table 2) that were not expressed in infested Dnx plants.
On northern blots, GLG, Chia-3, and WIR1B exhibited a similar expression pattern (Fig. 2A). They were expressed very little in uninfested Dnx plants, upregulated slightly at 6 hpi, and strongly at 12 hpi in infested Dnx plants. The elevated transcript levels in infested Dnx plants gradually decreased after 24 hpi. These three genes were either not upregulated or much less upregulated in infested Dn0 plants until 72 or 120 hpi, when a significant elevation of the level of these transcripts was observed.
Fig. 2.
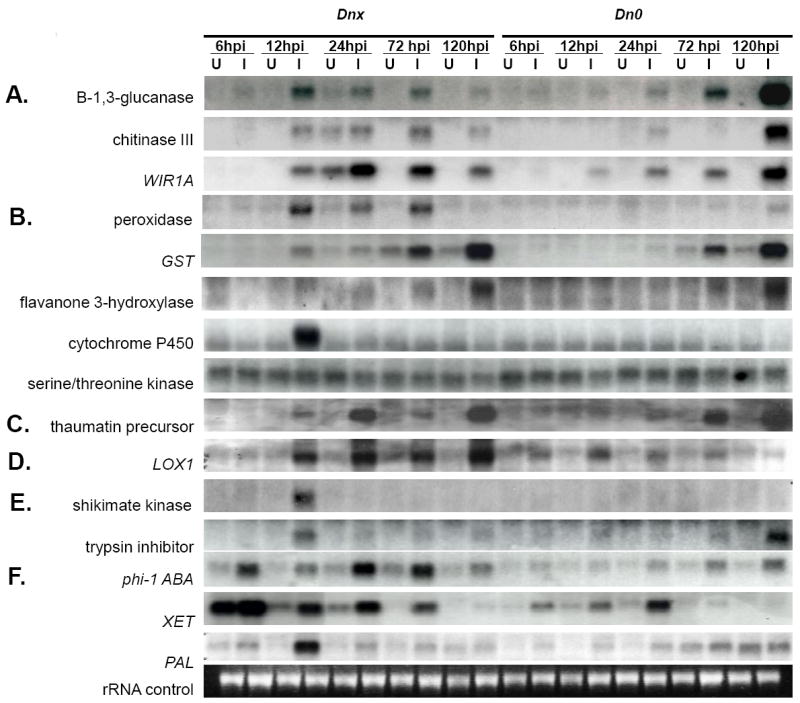
Temporal upregulation of wheat transcripts from Russian wheat aphid biotype 1 (RWA1) -infested (I) and control uninfested (U) plants containing the RWA1 resistant (Dnx) or susceptible (Dn0) genes. (a) Membrane protein genes - Q9XEN7 β-1,3-glucanase, Q8W427 chitinase III, Q01482 WIR1A membrane protein; (b) ROS response genes - Q43212 peroxidase precursor, Q8S702 glutathione S-transferase, Q8H8H7 flavanone 3-hydroxylase, Q9AVM3 cytochrome P450, Q5BQ31 serine/threonine kinase; (c) P27357 thaumatin-like protein PWIR2 precursor (SA metabolism); (d) P29114 LOX1 lipoxygenase 1 (JA precursor); and (e) Aphid anti-digestion/toxin genes - Q5NTH3 shikimate kinase 2, P12940 Bowman-Birk trypsin inhibitor; F. Dehydration response genes - Q6Z676 phi-1 ABA dehydration signaling, P93671 XET xyloglucan endotransglycosylase, Q43210 PAL phenylalanine ammonia lyase. Northern blot analysis of 5 μg of total RNA from the leaves of Dnx or Dn0 plants at 6-, 12-, 24-, 72- and 120 h post infestation (hpi) with RWA biotype 1 adults. Filters were hybridized with probes derived from cDNA clones of Affymetrix EST sequences encoding each gene shown on left. Gels were stained with ethidium bromide and rRNA levels compared to control for equal loading (shown is representative loading on a single gel). Each lane represents RNA pooled from two biological replicates, each consisting of leaves from three plants
ROS Production
Several genes related to ROS metabolism, including GST, Ca++ and Fe++ binding proteins, CYP450, oxidoreductases, and peroxidases, were 2- to 8-fold upregulated in infested Dnx plants responding to RWA1 feeding (Table 3, Supplemental Data Table 1), but their expression was approximately one-half that level in Dn0 plants (Table 3, Supplemental Data Table 2). On northern blots, the levels of these transcripts were strongly upregulated in infested Dnx plants at least at one post infestation time point (Fig. 2B). The levels of these transcripts were either unaffected or less elevated in infested Dn0 plants.
Defense Signaling
Results of microarray analyses produced transcripts putatively associated with the SA signaling pathway. These included Pathogenesis-Related-1 (PR1), PR4, PR5, and WRKY (Yalpani et al. 1991; Dong et al. 2003), which were 2- to 7-fold upregulated in infested Dnx plants (Supplemental Data Table 1). In contrast, transcripts associated with the JA signaling pathway including FAD3C, DAD1 LOX1, ACS1, 12-OPR, OPDA hydrolases, 12-OPDA ABC transporters (Dhondt et al., 2000; Ishiguro et al., 2001; Liechti and Farmer 2002; Theodoulou et al. 2005) were 2- to 4-fold upregulated in infested Dnx plants (Supplemental Data Table 1). Transcripts associated with ABA and ET signaling pathways increased from 2- to 4-fold in infested Dnx plants (Supplemental Data Table 1). These included EIN-3-like proteins, AP2 domain-containing transcription factors, C2 GRAM domain-containing proteins, and putative phi-1 proteins (Zhu 2002; Zhang et al. 2004) (Supplemental Data Table 1). None of these genes were upregulated in infested Dn0 plants (Supplemental Data Table 2).
Pest/Pathogen Defenses
Dnx-based resistance to RWA is manifested as reduced aphid population growth (Boyko et al., 2006; Khan et al. 2009; Lazzari et al. 2009), which may be a result of suppressed aphid feeding, an inhibition of aphid digestion or a combination of both. Plant secondary metabolites suppress insect feeding, and several transcripts associated with secondary metabolite production including cycloartenol synthase, monoterpene synthase, and shikimate kinase were upregulated 2-4 fold in RWA1-infested Dnx plants (Supplemental Data Table 1). On northern blots, the transcript coding for shikimate kinase was expressed in infested Dnx leaves at 12 hpi but was absent in Dn0 plants at all post-infestation time points (Fig. 2E). Further, the transcript encoding a UDP-glucose glucosyltransferase, an enzyme that transfers glucose UDP-glucose to terpenes (Xiong et al. 2001, Scharrenberg et al. 2003), was upregulated 2- to 4-fold (Supplemental Data Table 1). Various enzyme inhibitors limit digestion in the insect gut (Gatehouse and Boulter 1983). A transcript encoding a Bowman-Birk trypsin inhibitor was upregulated at 12 hpi in Dnx infested plants, but this transcript was not significantly affected by RWA1 feeding in Dn0 plants until a very late time point (120 hpi) (Fig. 2E). Interestingly, several phytoalexin and pathogen resistance genes underwent 2- to 4-fold upregulation in infested Dnx plant foliage, including ACRE, HHT, SMM:HSM, and SNAP (Supplemental Data Table 1), but these transcripts were not expressed in Dn0 infested plants (Supplemental Data Table 2).
Downregulated Transcripts
Transcripts downregulated in plants of each genotype possessed various functions (Table 3, Supplemental Data Tables 3 and 4) and several representative downregulated transcripts were chosen for real-time PCR to confirm microarray results (Fig. 3, Supplemental Data Table 5, Supplemental Data Figs. 1 - 14.). There were no significant differences in downregulation of the stress response protein in either Dnx or Dn0 tissues at 6-, 12-, 24-, or 72 hpi (Fig. 3A). A calmodulin-like gene displayed similar patterns, and in both Dnx and Dn0 infested tissues, downregulation was significantly greater at 24- and 120 hpi than at 6- or 12 hpi (Fig. 3B). A transcript encoding a NAC domain pathogen response protein (Collinge and Boller 2001) was strongly downregulated in infested Dnx plants (Fig. 3C), and was significantly greater in Dnx tissues at 72- and 120 hpi than at 6-, 12-, or 24 hpi. There were no differences in expression between any post-infestation times in Dn0 tissues. Expression of CYP450 CA619079 was relatively low in Dnx and Dn0 tissues (Fig. 3D), but was significantly greater in Dnx infested tissues at 24- and 120 hpi compared to 6- and 12 hpi. There were no differences in expression between Dnx infested tissues collected at 72 hpi and any other post-infestation time (Fig. 3D). In Dn0 infested tissues, there were no differences in the expression of CYP450 at any post-infestation time. Downregulation of a transcription protein and a mitochondrial ATP synthase in Dnx plants was significantly greater in Dnx tissues at 120 hpi than at 6 hpi (Fig. 3E,F). There were no significant temporal differences in downregulation of either protein in Dn0 tissues.
Fig. 3.
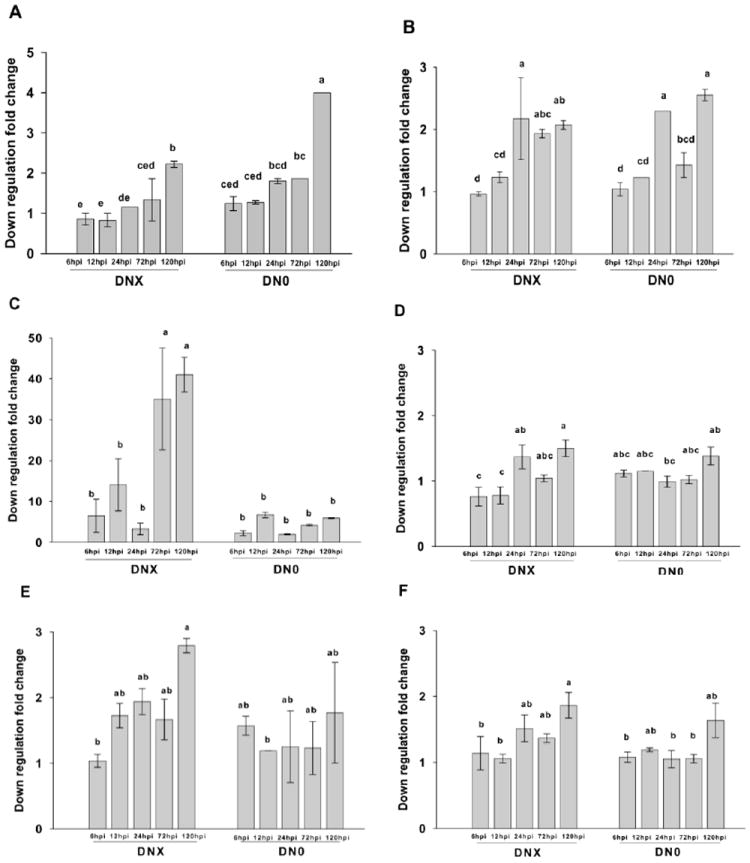
Temporal downregulation (mean ± S.E fold change) of wheat transcripts from plants containing the RWA resistant (Dnx) or susceptible (Dn0) gene at 6-, 12-, 24-, 72- and 120 h post infestation with RWA biotype 1 adults. (a) Q9P3N1 hypothetical stress response protein; (b) Q5ZD81 calmodulin-like protein; (c) Q6Z1A3 NAC1 stress response protein; (d) Q6YXE1 cytochrome P450 (e) Q7XN01 transcription protein; and (f) Q6I5G9 mitochondrial ATP synthase. Each treatment mean represents two biological replicates, each consisting of leaves pooled from three Dnx or Dn0 plants.
Phytohormone Analyses
The concentration of the 16:1 fatty acid in infested Dnx and Dn0 plants increased significantly (P<0.001) at 12-, 24-, and 48 hpi, but returned to the control level at 96 hpi (Table 4). The concentration of 16:3 fatty acid increased dramatically in infested Dnx and Dn0 plants at 48 hpi, where it was significantly greater in Dnx plants than Dn0 plants. There were comparatively fewer significant differences between treatments for the 18:1 and 18:3 fatty acids. The 18:1 fatty acid content of both Dnx and Dn0 infested tissues was significantly (P<0.001) greater than uninfested controls at 96 hpi. The 18:3 fatty acid content of Dnx infested plants was significantly (P<0.001) greater than the uninfested control at 12 hpi, and in Dn0 plants, significantly (P<0.001) greater than the control at 12- and 24 hpi. This trend was similar for the 18:2 fatty acid, but the only significant increase in Dnx tissues over Dn0 tissues occurred at 12 hpi (data not shown). There were no significant differences between two genotypes at any time point for 16:0 or 18:0 fatty acids (data not shown).
Table 4.
Mass (% ng/g standard) of unsaturated 16:1 (7-hexadecenoic), 16:3 (7,10, 13-hexadecatrienoic), 18:1 (9-octadecenoic), and 18:3 (9,12,15-octadecatrienoic) fatty acids in leaves of Russian wheat aphid biotype 1 - resistant (Dnx) and susceptible (Dn0) wheat genotypes at 12-, 24-, 48-, and 96 h post-aphid infestation (hpi), and in 0 h uninfested control plants
| post infestation (h) | Mass (mean ± s.e. in % ng/g)
|
|||
|---|---|---|---|---|
| 16:1 fatty acid | 16:3 fatty acid | |||
| Dnx | Dn0 | Dnx | Dn0 | |
| 0 | 0.52 ± 0.03 ab a | 0.66 ± 0.07 bc | 0.04 ± 0.04 a | 0.10 ± 0.12 b |
| 12 | 1.72 ± 0.09 e | 1.65 ± 0.05 e | 0.08 ± 0.03 b | 0.08 ± 0.04 b |
| 24 | 1.02 ± 0.04 d | 1.16 ± 0.06 de | 0.14 ± 0.05 b | 0.13 ± 0.07 b |
| 48 | 0.79 ± 0.05 cd | 0.36 ± 0.02 a | 2.08 ± 0.14 d | 0.47 ± 0.23 c |
| 96 | 0.51 ± 0.08 ab | 0.89 ± 0.05 cd | 0.03 ± 0.03 a | 0.12 ± 0.04 b |
|
| ||||
| post infestation (h) |
Mean ± SE (% ng/g)
|
|||
|
18:1 fatty acid
|
18:3 fatty acid
|
|||
| Dnx | Dn0 | Dnx | Dn0 | |
|
| ||||
| 0 | 1.81 ± 0.11 ab a | 2.00 ± 0.34 ab | 2.28 ± 0.20 a | 4.45 ± 2.6 a |
| 12 | 2.43 ± 0.22abcd | 1.88 ± 0.36 ab | 14.43 ± 3.11c | 11.25 ± 3.01bc |
| 24 | 2.10 ± 0.13 abc | 2.30 ± 0.20abcd | 5.08 ± 1.08 a | 12.44 ± 3.14bc |
| 48 | 2.63 ± 0.30 bcd | 1.63 ± 0.12 a | 7.26 ±1.47 ab | 2.12 ± 0.65 a |
| 96 | 2.86 ± 0.45 cd | 3.06 ± 0.42 d | 2.59 ± 0.61 a | 2.40 ± 0.42 a |
For each fatty acid, means within a column followed by the same letter are not significantly different at P < 0.001.
The cis- and trans -Me-OPDA content differed significantly (P<0.001) between post infestation times in leaves of Dnx and Dn0 plants, and between the genotypes at each post infestation time (Table 5). In Dnx plants, cis-Me-OPDA content increased dramatically to 2.3 % at 24 hpi, and was significantly (P<0.001) greater than the uninfested control or any other Dnx post infestation time. In contrast, cis-Me-OPDA content in Dn0 plants was reduced at each hpi time compared to that produced in uninfested plants, and reductions were significant (P<0.001) at 12- and 24 hpi (Table 5). With the exception of the 24 hpi treatment, cis-Me-OPDA content was significantly greater in Dn0 plants (including uninfested controls) than the corresponding Dnx plant treatments. Expression of trans-Me-OPDA was more pronounced than cis-Me-OPDA. In Dnx plants, trans-Me-OPDA content was significantly greater at 12-, 24-, and 48 hpi than in uninfested controls, and decreased to the level of the 0 h uninfested control at 96 hpi. As with cis-Me-OPDA, production of trans-Me-OPDA in uninfested Dn0 plants was significantly greater than in uninfested Dnx plants (Table 5). However, the trans-Me-OPDA content of Dn0 infested plants at all hpi intervals was significantly less than untreated Dn0 control plants and significantly lower than that of each corresponding Dnx plant hpi time. In contrast to Me-OPDA, there were no significant trends in differences in cis- or trans-methyl jasmonate (meJA) content at different time points or between Dnx and Dn0 plants (data not shown).
Table 5.
Mass (% ng/g standard) of cis- and trans-methyl-12-oxo-phytodienoic acid (12-OPDA) in leaves of Russian wheat aphid biotype 1 - resistant (Dnx) and susceptible (Dn0) wheat genotypes at 12-, 24-, 48-, and 96 h post-aphid infestation, and in 0 h uninfested control plants
| post infestation (h) | Mass (mean ± s.e. in % ng/g)
|
|||
|---|---|---|---|---|
| cis Me-OPDA | trans Me-OPDA | |||
| Dnx | Dn0 | Dnx | Dn0 | |
| 0 | 0.21 ± 0.13 aba | 1.76 ± 0.04 c | 0.08 ± 0.08 a | 1.15 ± 0.33 b |
| 12 | 0.14 ± 0.14 a | 0.25 ± 0.07 b | 0.90 ± 0.01 b | 0.05 ± 0.07 a |
| 24 | 2.29 ± 0.03 c | 0.22 ± 0.16 ab | 2.01 ± 0.14 b | 0.04 ± 0.09 a |
| 48 | 0.24 ± 0.06 b | 1.28 ± 0.05 c | 2.24 ± 0.28 b | 0.06 ± 0.10 a |
| 96 | 0.28 ± 0.11 b | 1.52 ± 0.04 c | 0.04 ± 0.06 a | 0.04 ± 0.08 a |
For each OPDA isomer, means within a column followed by the same letter are not significantly different at P < 0.001.
Abscisic acid (ABA) content in Dnx plant foliage was significantly (P<0.001) greater at 12- and 48 hpi than in uninfested plants (Table 6) and was significantly greater in Dnx plants than in Dn0 plants at 48 hpi. ABA content in the foliage of Dn0 plants was elevated at 12- and 24 hpi, but these amounts were no different from those of the uninfested control. The leaf content of benzoate, a salicylic acid intermediate, increased significantly (P<0.001) in Dnx foliage at 12-, 24-, and 48 hpi compared to the 0 h control, and a similar trend was absent in Dn0 plants (Table 6). In addition, the benzoate content of infested Dnx plants was significantly greater than in Dn0 plants at one time point (48 hpi). However, there were no differences in methyl salicylate leaf content between any of the Dnx or Dn0 post infestation treatments (data not shown). The amounts of the related SA intermediate trans-cinnamate also increased over time in both Dnx and Dn0 plants, but the quantities were no different from those of uninfested control plants (data not shown).
Table 6.
Mass (% ng/g standard) of methyl abscisic acid (Me-ABA) and methyl benzoate (Me-BA) in leaves of Russian wheat aphid biotype 1 - resistant (Dnx) and susceptible (Dn0) wheat genotypes at 12-, 24-, 48-, and 96 h post-aphid infestation, and in 0 h uninfested control plants
| post infestation (h) | Mass (mean ± s.e. in % ng/g)
|
|||
|---|---|---|---|---|
| Me-Abscisic Acid | Me-Benzoate | |||
| Dnx | Dn0 | Dnx | Dn0 | |
| 0 | 1.00 ± 0.05 bca | 1.79 ± 0.10 cde | 0.01 ± 0.04 ab | 0.02 ± 0.09 acb |
| 12 | 2.34 ± 0.05 de | 1.98 ± 0.04 de | 0.43 ± 0.22 e | 0.80 ± 0.31 de |
| 24 | 1.43 ± 0.05 cd | 2.97 ± 0.06 e | 0.03 ± 0.09 cd | 0.03 ± 0.14 cd |
| 48 | 2.10 ± 0.07 de | 0.54 ± 0.09 a | 0.08 ± 0.23 cd | 0.02 ± 0.14 ab |
| 96 | 0.56 ± 0.07 ab | 0.61 ± 0.18 a | 0.01 ± 0.08 a | 0.01 ± 0.11 a |
For Me-abscisic acid or Me-benzoate, means within a column followed by the same letter are not significantly different at P < 0.001.
Discussion
Plant resistance to insects is a complex process, often involving numerous plant biochemical pathways. We hypothesized that the products of unique defense proteins in the Dnx plant transcriptome are activated by RWA1 feeding probes, resulting in expression of the Dnx plant phenotype. Results from microarray hybridizations, northern blot and real-time PCR assays, and vapor phase extraction of foliar phytohormones support this hypothesis. The results that we obtained identify unique differences in the molecular responses of resistant Dnx plants to RWA attack in comparison with those of susceptible Dn0 plants, and suggest that 16 carbon membrane fatty acids; oxylipins, ABA, and ET defense response signals; and downstream defense proteins are likely components for Dnx-mediated resistance to RWA. Most transcripts potentially involved in resistance were upregulated more rapidly and at a greater magnitude than in susceptible plants, suggesting a more rapid and stronger induction of a Dnx-mediated gene network as a likely mechanism for Dnx resistance. This mechanism has been observed with several plant resistance responses to various pathogens (van Loon et al. 2006).
Several results suggest the SA pathway to be less important in mediating Dnx resistance to RWA than the JA pathway. Fewer SA-related transcripts were upregulated in Dn0 plants (3) versus Dnx plants (16), and none of those upregulated in Dnx plants was expressed in Dn0 plants. Over five post-infestation time points, there were no differences between tissues of Dnx and Dn0 infested plants in the content of the trans-cinnamate or SA. Content of the SA intermediate benzoate in Dnx plants was significantly greater than in Dn0 plants at only 48 hpi.
The levels of expression of the majority of transcripts were similar between Dnx and Dn0 plants. However, approximately 30% of the transcripts contained in the microarray were upregulated in Dnx plants, and most of them are plant defense-related genes (Table 3). In comparison, only 19% of the transcripts were upregulated in Dn0 plants, and the proportion of these upregulated transcripts associated with plant defense was much lower. The transcripts upregulated in susceptible plants, therefore, may be related to general plant stress responses. Most, if not all ABA, ET, and JA signaling genes upregulated in Dnx plants were absent in Dn0 plants. On the other hand, approximately 9% of the upregulated Dn0 transcripts were related to auxin (AUX) signaling and these were absent in Dnx plants (Table 3). The expression of AUX-related transcripts may result in Dn0 plants increasing their ROS production, as demonstrated by Boyko et al. (2006) and Kawano (2003). An additional 27% of the upregulated Dn0 transcripts included metabolism genes (Table 3), which were not upregulated in Dnx plants. The downregulation of metabolic transcripts in Dnx plants at 120 hpi (Fig. 3) may decrease some plant metabolic activities that can enable the production of Dnx-related defense compounds. A related downregulation of stress response transcripts in Dnx plants could be due to a reduced need for these transcripts, in favor of downstream plant defenses.
At the onset of insect feeding, components of the cell wall membrane such as GLG and Chita, which were highly expressed in infested Dnx plants, participate in insect defense responses in plants (Kempema et al. 2007; Park et al. 2007; see review of Smith and Boyko, 2006). The increased upregulation of defense response transcripts related to ROS metabolism in Dnx leaves (Fig. 2B) is similar to that induced by other plant feeding insects (Schmidt et al. 2005; Couldridge et al. 2007). The expression of GLG, Chita, and ROS-related transcripts in Dnx infested tissues suggests their involvement in Dnx defense responses to RWA feeding probes.
Further, the putative role of WIR1 membrane proteins in Dnx resistance is strengthened by the results of Gaupels et al. (2008), who identified a WIR1A-like protein in barley phloem sap, obtained from stylets of actively feeding Rhopalosiphum padi aphids. Our results were similar to those of Zhu-Salzman et al. (2004) and Park et al. (2006), who demonstrated expression of CYP450 monooxygenase (MO) in aphid-resistant sorghum plants. The precise role of the CYP450MOs in Dnx-infested plants is difficult to determine, because these compounds function in the synthesis of JA, SA, chemical defenses, and the detoxification of exogenous chemicals such as RWA salivary components.
LOX, a gene whose transcripts are associated with the JA signaling pathway, is strongly induced by foliar feeding of numerous insects (Sardesai et al. 2005; and see Smith and Boyko 2006 review), but plant JA-induced defense responses may be antagonized by those induced by SA (Spoel et al. 2003; Koornneef et al. 2008). For example, silver leaf whitefly herbivory suppresses LOX2 and FAD expression and elevates PR1 and PR5 expression (Kempema et al. 2007). However, the upregulation of JA- and SA-related transcripts in Dnx-infested plants was similar to that noted by Salzman et al. (2005), in sorghum response to mechanical wounding and by Li et al. (2008) in soybean plant response to aphid feeding. We observed induction of homologs of genes composing much of the JA and SA pathways but expression of SA transcripts was ~ 2x greater than expression of JA synthesis transcripts (Supplemental Data Table 1). Nevertheless, although SA transcripts were more highly expressed in microarrays than JA transcripts, results of northern blot and phytohormone assays indicated that SA does not contribute to Dnx resistance (Fig. 2C, Table 6). Liu et al. (2007) reported similar results in Hessian fly resistant wheat plants.
Aphid feeding stimulates WRKY expression (Voelckel et al. 2004; Park et al. 2006; Li et al. 2008) (Supplemental Data Table 1), yet WRKY transcription factors suppress JA (Kalde et al. 2003; Li et al. 2004; Mao et al. 2007), indicating that WRKY expression in Dnx tissues may partially explain the comparatively reduced expression of JA-signaling genes. Wound-induced JA production is regulated by the supply of substrate available to allene oxide synthase (Turner et al. 2002). Thus, the minimal foliar damage and related chloroplast loss sustained by Dnx plants in our experiments (Fig. 1B), may have limited the release of fatty acids from chloroplast lipids for JA metabolism.
Oxylipin analyses suggest that LOX, trans-OPDA, and the 18- and 16 carbon OPDA fatty acid precursors function in Dnx resistance (Fig. 2D, Table 4 and 5). The direct role of OPDA in insect defense responses (Stintzi et al. 2001) and the production of OPDA-specific response gene homologs by mechanical wounding (Taki et al. 2005) support these results. Expression of oxylipin metabolites in Dnx plants appears to stimulate production of downstream feeding inhibitors and toxins (Fig. 2E), several of which have been reported as resistance factors (Moraes et al. 2000; Miller et al. 2005; Lou and Baldwin 2006; Smith and Boyko 2006; Liu et al. 2007). RWA1 phloem ingestion decreases markedly on Dnx plants within 8 hpi (Lazzari et al. 2009), further supporting the possibility that Dnx feeding inhibitors and/or toxins contribute to the significant (P < 0.001) antibiotic effects exhibited in the reduced RWA1 population development shown in Fig. 1A. These antibiotic effects shown by RWA1 substantiate similar results of Khan et al. (2009) with plants containing Dnx in different genetic backgrounds. The ~50% RWA1 population reduction on Dnx plants in Fig. 1A is similar to that observed by Boyko et al. (2006) (57%, 66%) and Khan et al. (2009) (34%).
The lack of leaf wilting and interveinal collapse in RWA1 incompatible interactions indicates that Dnx resistance also may result from reduced tissue and water loss. For example, PAL and CAD5, which mediate lignin synthesis, were highly expressed in infested Dnx tissues (Fig. 2F), and both have been implicated in insect resistance (Ciepiela 1989; Kempema et al. 2007; Liu et al. 2007). Elevated expression of latex-abundant-, fiber (CA609522), and sorbitol transporter (BT009301) proteins (Supplemental Data Table 1) provide additional evidence of physical components of Dnx resistance and an additional explanation of the low tissue dry weight changes occurring in RWA-infested Dnx plants (Fig. 1B).
ABA- and ET- dehydration responses were uniquely upregulated in Dnx plants during RWA feeding (Fig. 2F, Table 6), as reported by Salzman et al. (2005) and Park et al. (2006). ABA- and ET over-expression in response aphid-infested plants (Moran et al. 2002; Divol et al. 2005; Boyko et al. 2006) and the upregulation of the ET signaling genes in Dnx plants (Supplemental Data Table 1) strongly suggests their role in Dnx resistance. Related dehydration response transcripts were highly expressed, and one - XET - was differentially expressed in Dnx plants (6-72 hpi) and Dn0 plants (12-24 hpi) (Fig. 2F), also demonstrating their contribution to Dnx resistance.
Our results indicating the upregulation of homologs of the pathogen resistance genes ACRE, AP, Bet, HHT, and SNAP in RWA1-infested Dnx tissues is the first report of their expression in response to insect feeding. Validation of the role(s) of these genes and other candidate genes in Dnx resistance awaits functional confirmation experiments, likely involving gene silencing. In the interim, more than 400 Dnx expressed transcripts are presently of unknown function. Elucidation of their function will provide additional information about putative genes and their expression patterns involved in wheat plant responses to RWA herbivory.
Supplementary Material
Acknowledgments
We thank Nanyan Lu for helpful comments and support in the Affymetrix array data acquisition. This research was supported by a Kansas Crop Improvement Association grant to CMS and is contribution No. 08-151-J of the Kansas Agricultural Experiment Station. This research was performed in the Gene Expression Facility at Kansas State University, which is supported through the National Science Foundation grant, DBI 0421427. Plant phytohormone analyses were performed at the Kansas Lipidomics Research Center Analytical Laboratory, supported by the Functional Genomics Consortium initiative of Kansas State University’s Targeted Excellence Program. The Kansas Lipidomics Research Center is supported by National Science Foundation (EPS 0236913, MCB 0455318, DBI 0521587), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20RR16475), and Kansas State University.
References
- ARGANDONA VH, CHAMAN M, CARDEMIL L, MUNOZ O, ZUNIGA GA, CORCHERA LJ. Ethylene production and peroxidase activities in aphid-infested barley. J Chem Ecol. 2001;27:53–68. doi: 10.1023/a:1005615932694. [DOI] [PubMed] [Google Scholar]
- BAIROCH A, APWEILER R, WU CH, BARKER WC, BOECKMANN B, FERRO S, GASTEIGER E, HUANG H, LOPEZ R, MAGRANE M, MARTIN MJ, NATALE DA, O’DONOVAN C, REDASCHI N, YEH LS. The universal protein resource (UniProt) Nucleic Acids Res. 2005;33:D154–D159. doi: 10.1093/nar/gki070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASKY Z. Biotypic and pest status differences between Hungarian and South African populations of Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae) Pest Manag Sci. 2003;59:1152–1158. doi: 10.1002/ps.750. [DOI] [PubMed] [Google Scholar]
- BERZONSKY W, SHANOWER T, LAMB R, MCKENZIE R, DING H, HARRIS MO, PEAIRS F, HALEY S, PORTER D, RATCLIFFE RH. Breeding wheat for resistance to insects. Plant Breed Rev. 2003;22:221–296. [Google Scholar]
- BOTHA A-M, LACOCK L, NIEKERK CV, MATSIOLOKO MT, DU PREEZ FB, LOOTS S, VENTER E, KUNERT KJ, CULLIS CA. Is photosynthetic transcriptional regulation in Triticum aestivum L. cv. ‘TugelaDN’ a contributing factor for tolerance to Diuraphis noxia (Homoptera: Aphididae)? Plant Cell Reports. 2006;25:41–54. doi: 10.1007/s00299-005-0001-9. [DOI] [PubMed] [Google Scholar]
- BOYKO EV, SMITH CM, VANKATAPPA T, BRUNO J, DENG Y, STARKEY SR, KLAAHSEN D. The molecular basis of plant gene expression during aphid invasion: wheat Pto- and Pti-like sequences modulate aphid-wheat interaction. J Econ Entomol. 2006;99:1430–1445. doi: 10.1603/0022-0493-99.4.1430. [DOI] [PubMed] [Google Scholar]
- BROECKLING CD, REDDY IR, DURAN AL, ZHAO X, SUMNER LW. MET-IDEA: a data extraction tool for mass spectrometry-based metabolomics. Analytical Chem. 2006;78:4334–41. doi: 10.1021/ac0521596. [DOI] [PubMed] [Google Scholar]
- BURD JD, ELLIOTT NC. Changes in chlorophyll α fluorescence induction kinetics in cereals infested with Russian wheat aphid (Homoptera: Aphididae) J Econ Entomol. 1996;89:1332–1337. [Google Scholar]
- BURD JD, PORTER DR, PUTERKA GJ, HALEY SD, PEAIRS FB. Biotypic variation among North American Russian wheat aphid (Homoptera: Aphididae) populations. J Econ Entomol. 2006;99:1862–1866. doi: 10.1603/0022-0493-99.5.1862. [DOI] [PubMed] [Google Scholar]
- CAMON E, MAGRANE M, BARRELL D, BINNS D, FLEISCHMANN W, KERSEY P, MULDER N, OINN T, MASLEN J, COX A, APWEILER R. The gene ontology annotation (GOA) project: implementation of GO in SWISS-PROT, TrEMBL, and InterPro. Genome Res. 2003;13:662–672. doi: 10.1101/gr.461403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN, et al. 2004 [Google Scholar]
- CIEPIELA A. Biochemical basis of winter wheat resistance to the grain aphid, Sitobion avenae. Entomol Exp Appl. 1989;51:269–275. [Google Scholar]
- COLLINGE M, BOLLER T. Differential induction of two potato genes, Stprx2 and StNAC, in response to infection by Phytophthora infestans and to wounding. Plant Mol Biol. 2001;46:521–529. doi: 10.1023/a:1010639225091. [DOI] [PubMed] [Google Scholar]
- COULDRIDGE C, NEWBURY HJ, FORD-LLOYD B, BALE J, PRITCHARD J. Exploring plant responses to aphid feeding using a full Arabidopsis microarray reveals a small number of genes with significantly altered expression. Bull Entomol Res. 2007;97:523–532. doi: 10.1017/S0007485307005160. [DOI] [PubMed] [Google Scholar]
- DEVONSHIRE AL, FIELD LM. Gene amplification and insecticide resistance. Annu Rev Entomol. 1991;36:1–23. doi: 10.1146/annurev.en.36.010191.000245. [DOI] [PubMed] [Google Scholar]
- DHONDT S, GEOFFROY P, STELMACH BA, LEGRAND M, HEITZ T. Soluble phospholipase A(2) activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes. Plant J. 2000;23:431–440. doi: 10.1046/j.1365-313x.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- DIVOL F, VILAINE F, THIBIVILLIERS S, AMSELEM J, PALAUQUI JC, KUSIAK C, DINANT S. Systemic response to aphid infestation by Myzus persicae in the phloem of Apium graveolens. Plant Mol Biol. 2005;57:517–40. doi: 10.1007/s11103-005-0338-z. [DOI] [PubMed] [Google Scholar]
- DOLATTI L, GHAREYAZIE B, MOHARRAMIPOUR S, NOORI-DALOII MR. Evidence for regional diversity and host adaptation in Iranian populations of the Russian wheat aphid. Entomol Exp Appl. 2005;114:171–180. [Google Scholar]
- DONG J, CHEN C, CHEN Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51:21–37. doi: 10.1023/a:1020780022549. [DOI] [PubMed] [Google Scholar]
- DONG H-P, PENG J, BAO Z, MENG X, BONASERA JM, CHEN G, BEER SV, DONG H. Downstream divergence of the ethylene signaling pathway for harpin-stimulated arabidopsis growth and insect defense. Plant Physiol. 2004;136:3628–3638. doi: 10.1104/pp.104.048900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIDANTSEF AL, STOUT MJ, THALER JS, DUFFEY SS, BOSTOCK RM. Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogensis-related protein P4 in the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol. 1999;54:97–114. [Google Scholar]
- FORSLUND K, PERRERSSON J, BRYNGELSSON T, JONSSON L. Aphid infestation induces PR-proteins differentially in barley susceptible or resistant to the bird cherry-oat aphid. Physiol Plant. 2000;110:496–502. [Google Scholar]
- GATEHOUSE AMR, BOULTER D. Assessment of the antimetabolic effects of trypsin-inhibitors from cowpea (Vigna unguiculata) and other legumes on development of the bruchid beetle Callosobruchus maculatus. J Sci Food Agric. 1983;34:345–350. [Google Scholar]
- GAUPELS FA, BUHTZ T, KNAUER S, DESHMUKH F, WALLER AJE, VAN BEL K-H, KOGEL, KEHR J. Adaptation of aphid stylectomy for analyses of proteins and mRNAs in barley phloem sap. J Exp Botany. 2008;59:3297–3306. doi: 10.1093/jxb/ern181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY TL, MARTIN TJ. Resistance to Russian wheat aphid Diuraphis noxia, in wheat (Triticum aestivum) Cereal Res Commun. 1990;18:127–129. [Google Scholar]
- HEIN GL. Influence of plant growth on Russian wheat aphid, Diuraphis noxia (Homoptera: Aphididae). Reproduction and damage symptom expression. J Kansas Entomol Soc. 1992;65:369–376. [Google Scholar]
- HENG-MOSS TM, NI X, MACEDO T, MARKWELL JP, BAXENDALE FP, QUISENBERRY SS, TOLMAY V. Comparison of chlorophyll and cartenoid concentrations among Russian wheat aphid (Homoptera: Aphididae)-infested wheat isolines. J Econ Entomol. 2003;96:475–481. doi: 10.1093/jee/96.2.475. [DOI] [PubMed] [Google Scholar]
- HUANG X, MADAN A. CAP3: a DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIGURO S, KAWAI-ODA A, UEDA J, NISHIDA I, OKADA K. The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG J, WON S, SUH S, KIM H, WING R, JEONG Y, HWANG I, KIM M. The barley ERF-type transcription factor HvRAF confers enhanced pathogen resistance and salt tolerance in Arabidopsis. Planta. 2007;225:575–588. doi: 10.1007/s00425-006-0373-2. not cited in paper. [DOI] [PubMed] [Google Scholar]
- KALDE M, BARTH M, SOMSSICH IE, LIPPOK B. Members of Arabidopsis WRKY group III transcripton factors are a part of different plant defense signaling pathways. Molecular Plant-Microbe Interactions. 2003;16:295–305. doi: 10.1094/MPMI.2003.16.4.295. [DOI] [PubMed] [Google Scholar]
- KALOSHIAN I, KINSER MG, ULLMAN DE, WILLAMSON VM. The impact of Meu1-mediated resistance in tomato on longevity, fecundity, and behavior of the potato aphid, Macrosiphum euphorbiae. Entomol Exp Appl. 1997;83:181–187. [Google Scholar]
- KALOSHIAN I. Gene-for-gene disease resistance: bridging insect pest and pathogen defense. J Chem Ecol. 2004;30:2419–2438. doi: 10.1007/s10886-004-7943-1. [DOI] [PubMed] [Google Scholar]
- KAWANO T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003;21:829–837. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- KEMPEMA LA, XINPING C, HOLZER FM, WALLING LL. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 2007;143:850–865. doi: 10.1104/pp.106.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN SA, MURUGAN M, STARKEY S, MANLEY A, SMITH CM. Inheritance and categories of resistance in wheat to Russian wheat aphid (Hemiptera: Aphididae) biotype 1 and biotype 2. J Econ Entomol. 2009;102:654–1662. doi: 10.1603/029.102.0433. [DOI] [PubMed] [Google Scholar]
- KLINGLER J, CREASY R, GAO L, NAIR RM, CALIX AS, JACOB HS, EDWARDS OR, SINGH KB. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 2005;137:1445–1455. doi: 10.1104/pp.104.051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOORNNEEF A, LEON-RAYES A, RITSEMA T, VERHAGE A, DEN OTTER FC, VAN LOON LC, PIETERSE CMJ. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 2008;147:1358–1368. doi: 10.1104/pp.108.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACOCK LC, VAN NIEKERK S, LOOTS F, DU PREEZ, BOTHA AM. Functional and comparative analysis of expressed sequences from Diuraphis noxia infested wheat obtained utilizing the conserved nucleotide binding site. African J Biotechnol. 2003;2:75–81. [Google Scholar]
- LAZZARI S, STARKEY S, REESE J, RAY-CHANDLER A, SMITH CM. Feeding behavior of Russian wheat aphid (Hemiptera: Aphididae) biotype 2 in response to wheat genotypes exhibiting different categories of resistance. J Econ Entomol. 2009;102:1291–1300. doi: 10.1603/029.102.0356. [DOI] [PubMed] [Google Scholar]
- LIECHTI R, FARMER EE. The jasmonate pathway. Science. 2002;296:1649–1650. doi: 10.1126/science.1071547. [DOI] [PubMed] [Google Scholar]
- LI J, BRADER G, PALVA ET. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y, ZOU J, LI M, BILGIN DD, VODKIN LO, HARTMAN GL, CLOUGH SJ. Soybean defense responses to the soybean aphid. New Phytologist. 2008;179:185–195. doi: 10.1111/j.1469-8137.2008.02443.x. [DOI] [PubMed] [Google Scholar]
- LIU X, BAI J, HUANG L, ZHU L, LIU X, WENG N, REESE JC, HARRIS M, STUART JJ, CHEN M-S. Gene expression of different wheat genotypes during attack by virulent and avirulent Hessian fly (Mayetiola destructor) larvae. J Chem Ecol. 2007;33:2171–2194. doi: 10.1007/s10886-007-9382-2. [DOI] [PubMed] [Google Scholar]
- LIU XM, SMITH CM, GILL BS. Allelic relationships among Russian wheat aphid resistance genes. Crop Sci. 2005;45:2273–2280. [Google Scholar]
- LIU XM, SMITH CM, GILL BS, TOLMAY V. Microsatellite markers linked to six Russian wheat aphid resistance genes in wheat. Theor Appl Genet. 2001;102:504–510. doi: 10.1007/s00122-001-0831-y. [DOI] [PubMed] [Google Scholar]
- LOU Y, BALDWIN IT. Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 2006;140:1126–1136. doi: 10.1104/pp.105.073700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINGA JN, KINUYA MG, KAMAU AW, WANJAMA JK, AWALLA JO, PATHAK RS. Biotypic and genetic variation within tropical populations of Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae) in Kenya. J Entomol. 2007;4:350–361. [Google Scholar]
- MAO P, DUAN W, WEI C, LI Y. WRKY62 transcriptional factor acts downstream of cytosolic NPR1 and negatively regulates jasmonate-responsive gene expression. PlantCell Physiol. 2007;48:833–842. doi: 10.1093/pcp/pcm058. [DOI] [PubMed] [Google Scholar]
- MARTINEZ DE ILARDUYA O, NOMBELA G, HWANG CF, WILLIAMSON VM, MUNIZ M, KALOSHIAN I. Rme1 is necessary for Mi-1-mediated resistance and acts early in the resistance pathway. Mol Plant Microbe Interact. 2004;17:55–61. doi: 10.1094/MPMI.2004.17.1.55. [DOI] [PubMed] [Google Scholar]
- MILES PW. Aphid saliva. Biol Rev. 1999;74:41–85. [Google Scholar]
- MILLER B, MADILAO L, RALPH S, BOHLMANN J. Insect-induced conifer defense. White pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol. 2005;137:369–382. doi: 10.1104/pp.104.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER HL, NEESE PA, KETRING DL, DILLWITH JW. Involvement of ethylene in aphid infestation of barley. J Plant Growth Reg. 1994;13:167–171. [Google Scholar]
- MORAES RA, SALES MP, PINTO MSP, SILVA LB, OLIVEIRA AEA, MACHADO OLT, FERNÁNDEZ KVS, XAVIER-FILHO J. Lima bean (Phaseolus lunatus) seed coat phaseolin is detrimental to the cowpea weevil (Callosobruchus maculatus) Brazil J Med Biol Res. 2000;33:191–198. doi: 10.1590/s0100-879x2000000200005. [DOI] [PubMed] [Google Scholar]
- MORAN PJ, CHENG YF, CASSELL JL, THOMPSON GA. Gene expression profiling of Arabidopsis thaliana in compatible plant–aphid interactions. Arch Insect Biochem Physiol. 2002;51:182–203. doi: 10.1002/arch.10064. [DOI] [PubMed] [Google Scholar]
- MORAN PJ, THOMPSON GA. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001;125:1074–1085. doi: 10.1104/pp.125.2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANDA N, KHUSH GS. Host Plant Resistance to Insects. CAB / International Rice Research Institute; Wallingford, Oxon, UK: 1995. [Google Scholar]
- PARK D-S, YI G-H, LEE J-H, KWAK D-Y, YEO U-S, OH B-G, NAM M-H, GOO Y-C, KIM HY. The identification of candidate rice genes that confer resistant to the brown planthopper (Nilaparvata lugens) through representational difference analysis. Theor Appl Genet. 2007;115:537–547. doi: 10.1007/s00122-007-0587-0. [DOI] [PubMed] [Google Scholar]
- PARK SJ, HUANG YH, AYOUBI P. Identification of expression profiles of sorghum genes in response to greenbug phloem-feeding using cDNA subtraction and microarray analysis. Planta. 2006;223:932–947. doi: 10.1007/s00425-005-0148-1. [DOI] [PubMed] [Google Scholar]
- PRINSLOO GJ. Host and host instar preference of Aphelinus sp. nr. varipes (Hymenoptera: Aphelinidae), a parasitoid of cereal aphids (Homoptera: Aphididae) in South Africa. Afr Entomol. 2000;8:57–61. [Google Scholar]
- QUICK JS, ELLIS GE, NORMANN RM, STROMBERGER JA, SHANAHAN JF, PEAIRS FB, RUDOLPH JB, LORENZ K. Registration of ‘Halt’ wheat. Crop Sci. 1996;36:210. [Google Scholar]
- QUISENBERRY SS, PEAIRS FB. Entomol Soc Am. Lanham, MD: 1998. A Response Model for an Introduced Pest- the Russian Wheat Aphid. Thomas Say Publications in Entomology. [Google Scholar]
- REESE JC, SCHWENKE JR, LAMONT PS, ZEHR DD. Importance and quantification of plant tolerance in crop pest management programs for aphids: greenbug resistance in sorghum. J Agric Entomol. 1994;11:255–270. [Google Scholar]
- SALZMAN RA, BRADY JA, FINLAYSON SA, BUCHANAN CD, SUMMER EJ, SUN F, KLEIN PE, KLEIN RR, PRATT LH, CORDONNIER-PRATT M-M, MULLET JE. Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid, and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol. 2005;138:352–368. doi: 10.1104/pp.104.058206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARDESAI N, SUBRAMANYAM S, NEMACHECK J, WILLIAMS CE. Modulation of defense-response gene expression in wheat during Hessian fly larval feeding. J Plant Interact. 2005;1:39–50. [Google Scholar]
- SAS Institute Inc. SAS/STAT Software Version 9.1. SAS Institute; Cary, NC: 2001. [Google Scholar]
- SCHARRENBERG C, FALK J, QUAST S, HAUSSUHL K, HUMBECK K, KRUPINSKA K. Isolation of senescence-related cDNAs from flag leaves of field grown barley plants. Physiol Plant. 2003;118:278–288. [Google Scholar]
- SCHMELZ EA, ENGELBERTH J, TUMLINSON JH, BLOCK A, ALBORN HT. The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J. 2004;39:790–808. doi: 10.1111/j.1365-313X.2004.02168.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT DD, VOELCKEL C, HARTL M, SCHMIDT S, BALDWIN IT. Specificity in ecological Interactions. Attack from the same lepidopteran herbivore results in species-specific transcriptional responses in two solanaceous host plants. Plant Physiol. 2005;138:1763–1773. doi: 10.1104/pp.105.061192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH CM. Plant Resistance to Insects: A Fundamental Approach. John Wiley & Sons; New York: 1989. [Google Scholar]
- SMITH CM. Plant resistance against pests: Issues and strategies. In: Koul O, Dhaliwal GS, Cuperus G, editors. Integrated Pest Management: Potential, Constraints and Challenges. CABI Publ.; Oxon, UK: 2004. pp. 147–167. [Google Scholar]
- SMITH CM. Plant Resistance to Arthropods - Molecular and Conventional Approaches. Springer; The Netherlands: 2005. [Google Scholar]
- SMITH CM, BOYKO EV. Mini Review: The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol Exp Appl. 2006;122:1–16. [Google Scholar]
- SMITH CM, SCHOTZKO DJ, ZEMETRA RS, SOUZA EJ, SCHROEDER-TEETER S. Identification of Russian wheat aphid (Homoptera, Aphididae) resistance in wheat. J Econ Entomol. 1991;84:328–332. [Google Scholar]
- SMITH CM, BELAY T, STAUFFER C, STARY P, KUBECKOVA I, STARKEY S. Identification of Russian wheat aphid (Homoptera: Aphididae) biotypes virulent to the Dn4 resistance gene. J Econ Entomol. 2004;97:112–117. doi: 10.1093/jee/97.3.1112. [DOI] [PubMed] [Google Scholar]
- SPOEL SH, KOORNNEEF A, CLAESSENS SMC, KORZELIUS JP, VAN PELT JA, MUELLER MJ, BUCHALA AJ, METRAUX J-P, BROWN R, KAZAN K, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–770. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STINTZI A, WEBER H, REYMOND P, BROWSE J, FARMER EE. Plant defense in the absence of jasmonic acid: The role of cyclopentenones. Proc Natl Acad Sci U S A. 2001;98:12837–12842. doi: 10.1073/pnas.211311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKI N, SASAKI-SEKIMOTO Y, OBAYASHI T, KIKUTA A, KOBAYASHI K, AINAI T, YAGI K, SAKURAI N, SUZUKI H, MASUDA T, TAKAMIYA K, SHIBATA D, KOBAYASHI Y, OHTA H. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005;139:1268–1283. doi: 10.1104/pp.105.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEODOULOU FL, JOB K, SLOCOMBE SP, FOOTITT S, HOLDSWORTH M, BAKER A, LARSON TR, GRAHAM IA. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005;137:835–840. doi: 10.1104/pp.105.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TJALLINGII WF, HOGEN-ESCH T. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol Entomol. 1993;18:317–328. [Google Scholar]
- TOLMAY VL, LINDEQUE RC, PRINSLOO GL. Preliminary evidence of a resistance-breaking biotype of the Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae), in South Africa. Afr Entomol. 2007;15:228–230. [Google Scholar]
- TURNER JG, ELLIS C, DEVOTO A. The jasmonate signal pathway. Plant Cell. 2002;14(suppl):S153–S164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URBANSKA A, TJALLINGII WF, DIXON AFG, LESZCZYNSKI B. Phenol oxidizing enzymes in the grain aphid’s saliva. Entomol Exp Appl. 1998;86:197–203. [Google Scholar]
- VAN DE VEN WTG, LEVESQUE CS, PERRING TM, WALLING LL. Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell. 2000;12:1409–23. doi: 10.1105/tpc.12.8.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN LOON LC, REP M, PIETERSE CMJ. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- VOELCKEL C, WEISSER WW, BALDWIN IT. An analysis of plant aphid interactions by different microarray hybridization strategies. Mol Ecol. 2004;13:3187–3195. doi: 10.1111/j.1365-294X.2004.02297.x. [DOI] [PubMed] [Google Scholar]
- VOOTHULURU P, MENG J, KHAJURIA C, LOUIS J, ZHU L, STARKEY S, WILDE GE, BAKER CA, SMITH CM. Categories and inheritance of resistance to Russian wheat aphid (Homoptera:Aphididae) Biotype 2 in a selection from wheat cereal introduction 2401. J Econ Entomol. 2006;99:1854–1861. doi: 10.1603/0022-0493-99.5.1854. [DOI] [PubMed] [Google Scholar]
- VOS P, SIMONS G, JESSE T, WIJBRANDI J, HEINEN L, et al. The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nature Biotechnol. 1998;16:1315–1316. doi: 10.1038/4350. [DOI] [PubMed] [Google Scholar]
- WALLING LL. The myriad plant responses to herbivores. J Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- WANG L, ZHANG Y. Data integration and target selection for Medicago genomics. In: Hopkins A, Wang Z-Y, Mian R, Sledge M, Barker RE, editors. Developments in Plant Breeding: Molecular Breeding of Forage and Turf. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2004. pp. 275–288. [Google Scholar]
- XIONG LZ, LEE MW, QI M, YANG YN. Identification of defense-relate rice genes by suppression subtractive hybridization and differential screening. Mol Plant Microbe Interact. 2001;14:685–692. doi: 10.1094/MPMI.2001.14.5.685. [DOI] [PubMed] [Google Scholar]
- YALPANI N, SILVERMAN P, WILSON TMA, KLEIER DA, RASKIN I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991;3:809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H, SREENIVASULU N, WESCHKE W, STEIN N, RUDD S, RADCHUK V, POTOKINA E, SCHOLZ U, SCHWEIZER P, ZIEROLD U, LANGRIDGE P, VARSHNEY RK, WOBUS U, GRANER U. Large-scale analysis of the barley transcriptome based on expressed sequence tags. Plant J. 2004;40:276–290. doi: 10.1111/j.1365-313X.2004.02209.x. [DOI] [PubMed] [Google Scholar]
- ZHU JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU-SALZMAN K, SALZMAN RA, AHN J, KOIWA H. Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol. 2004;134:420–431. doi: 10.1104/pp.103.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


