Abstract
Objective
To examine the effect of long-term lower extremity functional electrical stimulation (FES) cycling on the physical integrity and functional recovery in people with chronic spinal cord injury (SCI).
Design
Retrospective cohort, mean follow-up 29.1 months, and cross-sectional evaluation.
Setting
Washington University Spinal Cord Injury Neurorehabilitation Center, referral center.
Participants
Twenty-five people with chronic SCI who received FES during cycling were matched by age, gender, injury level, and severity, and duration of injury to 20 people with SCI who received range of motion and stretching.
Intervention
Lower extremity FES during cycling as part of an activity-based restorative treatment regimen.
Main outcome measure
Change in neurological function: motor, sensory, and combined motor–sensory scores (CMSS) assessed by the American Spinal Injury Association Impairment scale. Response was defined as ≥1 point improvement.
Results
FES was associated with an 80% CMSS responder rate compared to 40% in controls. An average 9.6 CMSS point loss among controls was offset by an average 20-point gain among FES subjects. Quadriceps muscle mass was on average 36% higher and intra/inter-muscular fat 44% lower, in the FES group. Hamstring and quadriceps muscle strength was 30 and 35% greater, respectively, in the FES group. Quality of life and daily function measures were significantly higher in FES group.
Conclusion
FES during cycling in chronic SCI may provide substantial physical integrity benefits, including enhanced neurological and functional performance, increased muscle size and force-generation potential, reduced spasticity, and improved quality of life.
Keywords: Spinal cord injury, Activity-based restorative therapy, Functional electrical stimulation, Rehabilitation, Cycle ergometry, Assistive technology, Paraplegia, Tetraplegia, Rehabilitation, Physical muscle strength, Spasticity, Quality of life
Introduction
Initial beliefs have suggested that substantial recovery was unlikely to occur in people with spinal cord injury (SCI) after the first 1–2 years post-injury. However, over the past decade, evidence has pointed to the effect of activity in maintaining and restoring central nervous system function. Current treatment paradigms view SCI care as continued rehabilitation to maintain and restore function.
Recently, active rehabilitation strategies, including treadmill training and functional electrical stimulation (FES) leg cycling, have been shown to promote physical integrity and functional recovery in people with chronic SCI.1–5 Research in constraint-induced or forced-use training, and treadmill training in stroke and SCI, has spurred interest in a new area of rehabilitative restoration research.6–9 Recent studies suggest that the functional benefits of such strategies go beyond task-specific training to include optimization of functional recovery via mechanisms of plasticity and regeneration.1, 2, 7, 9–18
The highly publicized late-onset recovery in actor/activist Christopher Reeve that was associated with an activity-based restorative therapy (ABRT) program prompted scrutiny of the physical and functional benefits of such rehabilitative regimens in people with chronic SCI.1, 2 The benefits of such therapies, including FES paradigms, on late-onset functional neurological restoration in SCI remain largely empirical. Therefore, in anticipation of a large prospective randomized trial to evaluate the efficacy of ABRT in SCI, we conducted a combined retrospective and cross-sectional evaluation of the therapeutic value of lower extremity FES during cycling as a component of ABRT.
Methods
The study was conducted with consecutive people with chronic SCI who were referred to the Washington University Spinal Cord Injury Neurorehabilitation program from 1 January 1999 to 1 July 2002. Individuals were eligible for participation if they had been diagnosed with chronic SCI, defined as >16 months following injury at the time of initial evaluation at the center. We compared patients assigned to FES as part of their rehabilitation modality with those not assigned to FES. All levels of upper motor neuron traumatic SCI were eligible for inclusion. Participants in the FES group had to have participated in FES during cycling for at least 3 months. Exclusion criteria included lower extremity pressure sores, pathological fracture, severe spasticity, debilitating disease prior to SCI, or lower motor neuron syndrome in lumbosacral zone. We compared the FES group with controls assigned to “standard of care”, matching the two groups by age, gender, level, severity, and duration of injury.
The Washington University Institutional Review Board (IRB) approved the study, and written informed consent was obtained from all participants. The Johns Hopkins Medical Institution IRB approved the analysis of the data.
Baseline characteristics and neurological function were obtained from medical records at initial evaluation. All participants were invited for a follow-up evaluation, which provided cross-sectional measures of the therapeutic effects of ABRT with FES.
FES cycle ergometry
FES lower extremity cycle ergometry was accomplished using ERGYS2 FES cycle ergometers (Therapeutic Alliances Inc., Fairborn, OH, USA; http://www.musclepower.com). The electrical parameters were set according to what was required for the participants to complete the cycling activity and due to the comfort of the participants according to their sensory deficits. The general parameters of electrical stimulation included: (1) maximal electrical intensity of 140 mA, constant current; (2) pulse width of 500 µs, frequency of 100 hz. Electrodes were placed on quadriceps, gluteal, and hamstring muscles bilaterally, with an algorithm inducing bilateral reciprocal leg cycling, typically at 50 RPM. Session duration was 45–60 minutes at a frequency of three sessions per week. Controls received non-center-based passive stretching with no active physical therapy, regimens typical of the chronic phase of SCI.
Neurological assessment
Neurological assessment was performed by the International Standards for Neurological Classification of Spinal Cord Injury and American Spinal Injury Association Impairment scale (AIS) to evaluate motor, sensory, and composite motor–sensory function. The composite motor–sensory score (CMSS) numerically documents changes in overall neurological function. The CMSS comprises the sum of pinprick (112), light touch (112), and motor (100) scores for a maximum attainable score of 324. We defined response in a neurologic function domain as ≥1 point increase on the AIS scale.
Spasticity and strength measurement
Spasticity and strength were measured quantitatively while participants sat in an isokinetic dynamometer (Biodex Medical Systems Inc., Shirley, NY, USA). To quantify spasticity, we measured resistance torque in newton-meters by passively moving the right and left knee and ankle through range of motion at multiple speeds from a minimum of 30 to a maximum of 180 degrees per second for the knee, and a maximum of 120 degrees per second for the ankle.19
Surface electromyography was recorded in the quadriceps, hamstrings, tibialis anterior, and triceps surae muscles bilaterally to verify the presence of a stretch response, indicative of a spastic catch (electromyography data not shown).
Muscle, fat, and bone measurements
For all participants, total thigh, muscle, intra- and inter-muscular fat volumes were measured from magnetic resonance (MR) images acquired with a 1.5-Tesla MRI scanner (Siemens Medical, Malvern, PA, USA). T1-weighted MR images of thigh with and without fat saturation sequences were obtained. MR images of the entire length of the sternocleidomastoid muscle were analysed to serve as control, non-FES stimulated muscle groups. Bone density (lumbar and hip) and total body fat were quantified, using DXA (Discovery W model, Hologic, Bedford, MA, USA).
Laboratory testing, medication use, and complication evaluation
A complete blood count, metabolic profile, and fasting lipid profile were obtained. We assessed medication use and complications by interviewing participants, reviewing their clinical charts, and pharmacy records.
Quality of life and functional measures
We evaluated health-related quality of life and functional status by administering the Medical Outcomes Study 36-Item Short Form Survey,20 and the Functional Independence Measure (FIM).21 Bowel dysfunction was additionally assessed by a multi-field questionnaire that captures neurogenic bowel habits in people with SCI through responses to 21 questions.22
Statistical analysis
We compared baseline and demographic characteristics of FES and control participants, using t-test and χ2 analysis. Change in neurological function from baseline to follow-up was assessed by AIS scores (AIS motor, sensory, pinprick, light touch, and combined motor–sensory scores), using paired t-test and χ2 analysis. Cross-sectional analysis examined the differences between FES and control groups in muscle, bone, and fat measurements at follow-up. To examine differences in spasticity and strength, we calculated the ratio of maximal resistance torque to the maximal voluntary-plus-stimulated torque in the same muscle, expressed as a percentage for the hamstrings, quadriceps, and gastrocnemius muscles, comparing the results across groups. We then compared the maximal resistance torque to the maximal strength in the antagonist muscle for the knee musculature, because the resistance generated by a stretched muscle influences the net torque that can be produced in the antagonist. Spasticity data were analysed using unpaired t-tests and mixed-model analysis of variance methods, with speed as the within-subject (repeated) factor and group as the between-subject factor, and with post hoc tests as indicated. The differences in quality of life and functional measures were examined using t-test. Statistical analyses were performed using Stata Statistical Software: Release 10, 2007 (StataCorp LP, College Station, TX, USA).
Results
A total of 45 participants were included in this analysis. Of these, 20 (44%) received the standard of care for rehabilitation (control group) and 25 (56%) underwent ABRT including lower extremity FES during cycling. The known predominance of male gender associated with traumatic SCI was evident, as 84% of the participants were male. A total of 51% of participants were between 16 months and 5 years from injury and 49% were >5 years from injury with mean duration post-injury of 7.2 years (range 1.3–43.3 years) (Table 1). The mean duration of follow-up was 29.1 months. On average, FES was used for 29.5 months (range 3–168 months), with an average distance of 20 931 half-cycles (half one-legged cycle) traveled per week. Power expended over the duration of FES ranged from 0.1 to 20.0 (mean 5.2) kilogram-force, the gravitational unit of force. There was no significant difference between the FES group and control group with regard to age, gender, severity, level and time post-injury, and duration of follow-up.
Table 1.
Baseline characteristics of study participants with chronic spinal cord injury by rehabilitation treatment group
| All participants, n = 45, n (%) | Control (no FES), n = 20 | Intervention (FES), n = 25 | P value* | |
|---|---|---|---|---|
| Age (years), mean(SD) | 36.0 (12.2) | 34.6 (12.2) | 37.2 (12.3) | 0.483 |
| Gender | ||||
| Male | 38 (84.4) | 16 (80.0) | 22 (88.0) | 0.462 |
| Female | 7 (15.6) | 4 (20.0) | 3 (12.0) | |
| Months post-injury, mean(range) | 85.8 (16–519) | 66.9 (16–323) | 96.3 (18–519) | 0.280 |
| Time post-injury, ≤60 months | 23 (51.1) | 9 (45.0) | 14 (56.0) | 0.463 |
| >60 months | 22 (48.9) | 11 (55.0) | 11 (44.0) | |
| Duration of follow-up (months), mean (SD) | 29.1 (28.8) | 23.3 (21.2) | 33.7 (33.4) | 0.213 |
| Level of injury | ||||
| Quadriplegia (C1–T1) | 28 (62.2) | 15 (75.0) | 13 (52.0) | |
| Paraplegia (T2–L5) | 17 (37.8) | 5 (25.0) | 12 (48.0) | 0.114 |
| AIS at baseline | ||||
| AIS A | 31 (68.9) | 14 (70.0) | 17 (68.0) | |
| AIS B | 9 (20.0) | 4 (20.0) | 5 (20.0) | |
| AIS C | 5 (11.1) | 2 (10.0) | 3 (12.0) | 0.977 |
| FES therapy | ||||
| Number of 1/2 full cycle (equals one walking step) per week, n (IQR) | — | 20 931 (12 000–19 500) | ||
| Number of 1/2 full cycle (equals one walking step) per week by baseline AIS, AIS A, n (IQR) | 16 547 (12 000–18 000) | |||
| AIS B, n (IQR) | — | 35 520 (10 500–27 000) | ||
| AIS C, n (IQR) | — | 20 000 (18 000–21 000) | ||
| Duration of FES use (months), mean (range) | — | 29.5 (3–168) | ||
| Duration of FES use by baseline AIS (months), mean (range) AIS A | — | 34.6 (3–168) | ||
| AIS B | — | 22 (7–62) | ||
| AIS C | — | 11 (9–13) | ||
| Power expended (kg-force) by baseline AIS, mean (range) | 5.2 (0.1–20.0) | |||
| AIS A (n = 16) | — | 5.3 (0.1–20.0) | ||
| AIS B (n = 5) | — | 6.1 (1.0–13.0) | ||
| AIS C (n = 2) | — | 2.3 (1.1–3.6) |
*P value: χ2 test for categorical variables, t-test for continuous variables.
IQR, Inter-quartile range; kg-force: kilogram-force, the gravitational unit of force.
In retrospective analysis, motor function improvement was observed in 20 of 25 (80%) FES subjects but in only 9 of 20 (45%) controls (P = 0.02) (Table 2). The average improvement in motor score was +8.1 (SD = 10.0) for FES compared with +0.6 (SD = 6.5) for controls (P = 0.004) (Table 3). Improvement in pinprick sensation was observed in 56% of the FES group compared with 25% of controls (P = 0.04) (Table 2). The average change in pinprick sensory score was +5.8 (SD = 15.1) in the FES group vs. −5.6 (SD = 11.7) in the controls (P = 0.008) (Table 3). Fourteen of 25 (56%) FES subjects showed improvement in light touch scores compared with six of the 20 (30%) controls (P = 0.08). The mean change in light touch score was +6 (SD = 10.6) for FES vs. −4.6 (SD = 14.9) in controls (P = 0.008).
Table 2.
Response in motor, sensory, and AIS impairment scores in participants with chronic spinal cord injury by rehabilitation treatment group
| Control (no FES), n = 20 | Intervention (FES), n = 25 | P value* | |
|---|---|---|---|
| AIS motor scores | n (%) | n (%) | |
| AIS motor score response | |||
| Yes | 9 (45.0) | 20 (80.0) | |
| No | 11 (55.0) | 5 (20.0) | 0.02 |
| AIS motor score response by baseline AIS | |||
| AIS A: Yes | 6 (42.9) | 13 (76.5) | |
| No | 8 (57.1) | 4 (23.5) | 0.06 |
| AIS B: Yes | 2 (50.0) | 5 (100.0) | |
| No | 2 (50.0) | 0 (0) | 0.07 |
| AIS C: Yes | 1 (50.0) | 2 (67.7) | |
| No | 1 (50.0) | 1 (33.3) | 0.71 |
| Pinprick AIS scores | |||
| Pinprick AIS score response | |||
| Yes | 5 (25.0) | 14 (56.0) | |
| No | 15 (75.0) | 11 (44.0) | 0.04 |
| Pinprick AIS response by baseline AIS | |||
| AIS A: Yes | 3 (21.4) | 10 (58.8) | |
| No | 11 (78.6) | 7 (41.2) | 0.04 |
| AIS B: Yes | 1 (25.0) | 3 (60.0) | |
| No | 3 (75.0) | 2 (40.0) | 0.29 |
| AIS C: Yes | 1 (50.0) | 1 (33.3) | |
| No | 1 (50.0) | 2 (66.7) | 0.71 |
| Light touch AIS scores | |||
| Light touch AIS score response | |||
| Yes | 6 (30.0) | 14 (56.0) | |
| No | 14 (70.0) | 11 (44.0) | 0.08 |
| Light touch AIS response by baseline AIS | |||
| AIS A: Yes | 4 (28.6) | 10 (58.8) | |
| No | 10 (71.4) | 7 (41.2) | 0.09 |
| AIS B: Yes | 1 (25.0) | 3 (60.0) | |
| No | 3 (75.0) | 2 (40.0) | 0.29 |
| AIS C: Yes | 1 (50.0) | 2 (66.7) | |
| No | 1 (50.0) | 1 (33.3) | 0.71 |
| Combined motor sensory scores (CMSS) | |||
| CMSS response | |||
| Yes | 8 (40.0) | 20 (80.0) | |
| No | 12 (60.0) | 5 (20.0) | 0.006 |
| CMSS response by baseline AIS | |||
| AIS A: Yes | 6 (42.9) | 13 (76.5) | |
| No | 8 (57.1) | 4 (23.5) | 0.06 |
| AIS B: Yes | 1 (25.0) | 5 (100) | |
| No | 3 (75.0) | 0 | 0.02 |
| AIS C: Yes | 1 (50.0) | 2 (66.7) | |
| No | 1 (50.0) | 1 (33.3) | 0.709 |
| AIS conversions | |||
| AIS grade improvement | |||
| Yes | 4 (20.0) | 7 (28.0) | |
| No | 16 (80.0) | 18 (72.0) | 0.535 |
| AIS grade improvement by baseline AIS | |||
| AIS A: Yes | 2 (14.3) | 3 (17.6) | |
| No | 12 (85.7) | 14 (82.3 | 0.800 |
| AIS B: Yes | 2 (50.0) | 4 (80.0) | |
| No | 2 (50.0) | 1 (20.0) | 0.343 |
| AIS C: Yes | 0 | 0 | |
| No | 2 (100) | 3 (100) | — |
Response defined as ≥1 point increase in a specific functional domain on the AIS scale.
*P value for χ2 and t-test.
Table 3.
Change in AIS motor, pinprick, light touch, and combined motor-sensory scores (CMSS) during study period in participants with chronic spinal cord injury by rehabilitation treatment group
| Control (no FES), n = 20 |
Intervention (FES), n = 25 |
||||||
|---|---|---|---|---|---|---|---|
| AIS score | Baseline | Follow-up | Mean change | Baseline | Follow-up | Mean change | P value* |
| Motor mean | 26.1 (19.4) | 26.7 (18.4) | 0.6 (6.5) | 34.6 (20.6) | 42.8 (19.2) | 8.1 (10.0) | 0.004 |
| Pinprick mean(SD) | 40.0 (30.0) | 34.0 (28.2) | −5.6 (11.7) | 41.1 (23.9) | 47.0 (26.8) | 5.8 (15.1) | 0.008 |
| Light touch mean (SD) | 46.7 (30.1) | 42.1 (31.8) | −4.6 (14.9) | 45.4 (24.4) | 51.4 (26.9) | 6.0 (10.6) | 0.008 |
| CMSS Mean (SD) | 112.7 (71.2) | 103.2 (72.8) | −9.6 (22.0) | 121.1 (62.2) | 141.1 (65.0) | 20.0 (29.0) | <0.001 |
*P value for t-test comparing mean AIS score change between control and intervention groups.
CMSS improved in 20 of 25 (80%) FES subjects compared with eight of 20 (40%) controls (P = 0.006) (Table 2). CMSS declined by a mean of 9.6 points (SD = 22.0) in controls compared with an improvement of 20 points (SD = 29.0) in the FES group (P < 0.001). There was no significant difference in AIS grades following FES during cycling (Table 2). AIS grade improved in 28% in the FES group compared with 20% in controls.
In cross-sectional analysis, we compared spasticity and strength measurements, muscle, bone, and fat measurements, commonly tested laboratory parameters, medication use, quality of life, and functional measures in the FES and control group.
FES enhances muscle volume and reduces fat volume
The FES and control groups showed no significant difference (P = 0.24) in total thigh volume. However, the volumes of the anterior and posterior thigh compartment muscles (FES-stimulated muscles) were significantly higher in the FES group than in controls; 36% greater (96.3 cc; P ≤ 0.001) for the mean anterior compartment and 30% greater (63.9 cc; P = 0.005) for the mean posterior compartment (Fig. 1A). Volumes of non-FES stimulated muscle groups below the lesion level (triceps surae and medial thigh compartment) and muscle groups above the injury level (sternocleidomastoid), which were not stimulated and not predicted to change, were similar in both groups.
Figure 1.

(A) Quantitative volume measurements of component thigh muscles and sternocleidomastoid muscle (internal control). Data represent the mean volume with SE bars (n = 20 and 25, controls and FES subjects, respectively) (*P < 0.001, **P < 0.01). (B) Peak resistance torque (spasticity) in hamstrings during right knee extension showing a decreasing amount of spasticity with increasing velocity in the FES group compared to controls.
Total thigh fat measured by MRI was 44% less in the FES group than in the controls (462 vs. 828 cc; P = 0.003; Fig. 1A). This is an important observation, as intra-muscular fat is associated with glucose intolerance, which afflicts nearly two-thirds of people with chronic SCI.23
A correlation of 0.46 (P = 0.003) was observed between intra- and inter-muscular fat measured by MRI and total body fat measured by DXA scanning.
FES enhances predicted and actual muscle strength measurements while not increasing spasticity
Strength values were significantly greater in the trained muscles of the FES group (quadriceps, P = 0.006; hamstrings, P = 0.011) than in the controls, but not in the untrained ankle (triceps surae muscles, P = 0.234). The control and FES groups were comparable in their ability to generate voluntary muscle activation in the quadriceps or hamstrings (4 of 18 (22%) controls vs. 7 of the 25 (28%) FES group). We observed increasing resistance torque with increasing speed of movement in the hamstrings and quadriceps. Resistance torque in the hamstrings was higher in the control group than in the FES group at speeds of 120 degrees per second (Fig. 1B). Higher resistance torque values and/or a higher rate of increase typically indicate greater spasticity. This same effect was not evident in the triceps surae muscles, which were not stimulated.
The ratio of maximal resistance torque to the maximal voluntary-plus-stimulated torque in the same muscle was approximately 40–50% greater in the control group than the FES group for all comparisons at the knee joint (P < 0.05). The ratio was not significantly different at the ankle (P = 0.60).
The quadriceps and hamstring strength values were positively related to total muscle volume (r = 0.49; P = 0.001 for the quadriceps and r = 0.39; P = 0.02 for the hamstrings (data not shown)). Hamstring strength was inversely related to total fat and intramuscular fat measures (r = −0.39; P = 0.02 and r = −0.36; P = 0.02, respectively).
The lower level of spasticity observed in the FES group is unlikely due to higher doses of anti-spasticity medication as the mean dose of the anti-spasticity medication baclofen was significantly lower (P = 0.02) in the FES group (20.2 mg, SD = 29.6) than in the controls (56.0 mg, SD = 59.2). The percentage of baclofen users and the average daily baclofen dose were also lower in the FES group (48% (12 of 25) FES vs. 70% (14 of 20) control; average daily dose, 42 ± 30 mg FES vs. 80 ± 55 mg control). More subjects in the control group (40% (8 of 20)) than in the FES group (20% (5 of 25)) used multiple agents to manage spasticity (P = 0.15).
FES is associated with improvements in quality of life and functional ability measures
The FES and control groups obtained significantly different scores on both the FIM and 36-Item Short Form Survey in domains of physical abilities and functioning (Figs. 2A and B). The FIM also showed improvements in transfer and bladder self-care. FES users had better physical functioning and fewer role limitations due to physical problems. Mean bowel function scores were significantly higher in the FES group than in the controls (36.7 FES vs. 33.6 control; P = 0.04).
Figure 2.
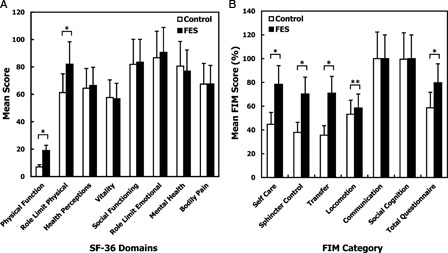
(A) Health-related quality of life assessed by the Medical Outcomes Study 36-Item Short Form Survey (SF-36) among FES and control group after mean 29.1 month follow-up. Data represents mean scores with SE bars (*P < 0.05). (B) Comparison of Functional Independent Measures (FIM) scores after mean 29.1-month follow-up showing improvements in quality of life and functional measures in selective FIM domains with FES use. Data represents mean scores with SE bars. *P ≤ 0.001; **P < 0.05.
FES and health determinant measurements
We measured surrogate indices of health status and susceptibility to complications that frequently affect people with chronic SCI.23–30
The ratio of total cholesterol to HDL-cholesterol was significantly lower in the FES group (4.1 (SD = 1.0) FES vs. 5.3 (SD = 1.9) control; P = 0.03), as were mean triglyceride and LDL-cholesterol levels. Mean HDL-cholesterol was higher in the FES group, but the difference was not statistically significant.
A general health index, the variability in red blood cell size, showed that the mean red blood cell distribution width was less in the FES group than in the controls (13.4 (SD = 0.83) FES vs. 14.1 (SD = 1.2) control; P = 0.03). The two groups had similar mean corpuscular volumes.
Mean BUN and creatinine levels were also significantly higher in the FES group than in the controls (BUN 15.6 mg/dl (SD = 5.3) FES vs. 11.6 mg/dl (SD = 3.1) control; P = 0.007; creatinine 0.74 (SD = 0.19) FES vs. 0.58 (SD = 0.24) μmol/l control; P = 0.02), which may be explained by the increased muscle mass.
Bone density measurements by DXA scanning did not reveal any significant differences between the two groups. Importantly, no pathologic fractures occurred in the FES group.
Discussion
Our findings support the hypothesis that advanced active rehabilitative strategies like ABRT can play an important role in promoting physical integrity and functional recovery even when implemented in the chronic phase of SCI. Our primary findings are that lower extremity FES during cycling is associated with (1) clinically important gain in neurological function; (2) attenuation of the slow loss of neurological function associated with immobility in chronic SCI, as reflected by increased motor and sensory function; (3) physical integrity improvements, as reflected by increased muscle volume and reduced muscle fat volume; (4) muscle strengthening; (5) concomitant reduction of spasticity and daily dosage of anti-spasticity medications; and (6) improved general health indices, function, and quality of life.
Improvements in physical integrity and predicted reduction in long-term complications associated with FES, combined with the known benefits of exercise,25,31 are sufficient rationale for people with SCI to participate in FES-assisted activity programs. The reduction in fat and increase in muscle mass may produce better glucose tolerance and potentially reduce the occurrence of type II diabetes.23, 32 The inconsistent results of previous studies of FES during cycling and its ability to enhance physical integrity are likely due to a number of study-specific variables, including time when FES was initiated, duration of therapy, and the load used to promote muscle hypertrophy and cardiovascular conditioning.33–35
Enhanced muscle volume and strength were not associated with greater spasticity, contrasting the general belief that strengthening a spastic muscle exacerbates spasticity. The FES group did not demonstrate higher spasticity, with some values for the stimulated muscles being less than in the controls. These findings may suggest that strengthening does not aggravate spasticity and may, in fact, decrease it. Greater strength relative to spastic resistance may also allow a joint to “move through” or counteract spasticity more effectively. FES-induced neural activity may also induce neuroanatomical plasticity, shifting the balance of excitation and inhibition within the spinal cord.18
Previous studies have showed that electrical stimulation of paralyzed muscles can alter the H reflex parameters, thus potentially reducing spasticity.36 The reduction of spasticity in the FES group is even more substantial given that the use of anti-spasmodic drugs was less in the FES group. This may reflect a lower requirement for anti-spasmodic drugs in the FES group. This finding raises the exciting possibility that FES can be used as an alternative or adjunct to use of anti-spasmodic drugs.
Our study may be limited by its retrospective and cross-sectional design because of potential inherent biases associated with nonrandom treatment assignment. However, the FES and control groups did not differ significantly by important characteristics at baseline.
The strength of our study lies in the improvement in neurological function in people with chronic SCI and the physical integrity benefits, detailed strength and spasticity measurements, and important negative internal controls. The functional improvements also demonstrate internal consistency as the improvements in FIM were confined to those predicted by improved motor function. In addition, they correlated closely with the functional neurological recovery data, as demonstrated by improved bladder and bowel sphincter scores.
The rationale for FES comes from a confluence of principles in developmental neuroscience, exercise physiology, and the emerging field of regenerative medicine. First, there is a growing consensus among neuroscientists that plasticity and regeneration is not limited to the acute phase of injury recovery, but can occur throughout the chronic phase.4, 13–15, 37 Second, there is an understanding that the equation governing recovery and regeneration in humans is more complex than in lower vertebrates. Third, the molecular and cellular events required for regeneration – cell birth, survival, migration, fate choice, neuronal circuit formation and selection, and myelination – are all highly dependent on neural activity during development; therefore, the same processes could be similarly regulated by neural activity following CNS injury.11,16,38,38–40 Fourth, most neurological injuries that produce long-term immobility, particularly SCI, associate with dramatic reductions in global neural activity, especially below the SCI level.7, 11, 16, 17
Based on the activity hypothesis, restoring normal activity levels should optimize the neural regeneration equation. A growing number of regeneration studies in rodent injury models support this hypothesis.11, 14 The concept being that FES-induced leg cycling in turn produces a pattern of normal sensory (muscle spindle) feedback and neural activity to the spinal cord. Thus, animal models and surrogate markers can be used to refine and optimize the FES approach according to known principles of neural regeneration.
Cardiovascular events, glucose intolerance, and diabetes are a leading cause of mortality in both able-bodied persons and people with SCI. Therefore, it is important that people with SCI participate in physical and cardiovascular exercise to achieve the same benefits that able-bodied individuals and the elderly derive from such activity.
Conclusion
In conclusion, our findings suggest that FES as part of a rehabilitation regimen may be associated with substantial functional neurological recovery and physical integrity/health benefits. The current study provides rationale for a prospective randomized clinical trial to evaluate the efficacy of ABRT using FES in people with SCI.
Acknowledgements
This work was supported by funding from the Deans Fund at Washington University School of Medicine (J.W.M.), Barnes-Jewish Hospital Foundation (J.W.M.), the Barnes-Jewish Hospital Auxiliary Foundation (J.W.M.), Christopher Reeve Paralysis Foundation (C.S., J.W.M.), the Nextsteps Foundation (St. Louis, MO; J.W.M.), the Sam Schmidt Foundation (Las Vegas, NV; C.S., J.W.M.), Gateway to a Cure Foundation (St. Louis, MO; J.W.M.), and the Eric Westacott Foundation (St. Louis, MO; J.W.M.) and, in part, by the Intramural Research Program at the NIH Clinical Center. Our appreciation to Anna Schneider for technical assistance. Author J.W.M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.McDonald JW, Becker D, Sadowsky CL, Jane JAS, Conturo TE, Schultz LM. Late recovery following spinal cord injury. Case report and review of the literature. J Neurosurg 2002;97Suppl 2:252–65 [DOI] [PubMed] [Google Scholar]
- 2.Corbetta M, Burton H, Sinclair RJ, Conturo TE, Akbudak E, McDonald JW. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci U S A 2002;99(26):17066–71 doi: i10.1073/pnas.262669099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 2012;939:1508–17 doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 4.McDonald J, Sadowsky C, Stampas A. The changing field of rehabilitaion: optimization of spontaneous regeneration and recovery of function. In: McDonald J, Verhaagen J, (eds.) Handbook of clinical neurology. Spinal Cord Injury Edition, vol. 109 Baltimore, MD and White Plains, NY, USA: Elsevier BV; 2012 [DOI] [PubMed] [Google Scholar]
- 5.Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair 2011;25(5):433–42 doi: 10.1177/1545968310392924 [DOI] [PubMed] [Google Scholar]
- 6.Barbeau H, Nadeau S, Garneau C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J Neurotrauma 2006;23(3/4):571–85 doi: 10.1089/neu.2006.23.571 [DOI] [PubMed] [Google Scholar]
- 7.Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma 2006;23(3/4):560–70 doi: 10.1089/neu.2006.23.560 [DOI] [PubMed] [Google Scholar]
- 8.Hesse S, Werner C, Bardeleben A, Barbeau H. Body weight-supported treadmill training after stroke. Curr Atheroscler Rep 2001;3(4):287–94 [DOI] [PubMed] [Google Scholar]
- 9.Mark VW, Taub E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restor Neurol Neurosci 2004;22(3–5):317–36 [PubMed] [Google Scholar]
- 10.Tator CH. Strategies for recovery and regeneration after brain and spinal cord injury. Inj Prev 2002;8Suppl 4:IV33–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald JW. Repairing the damaged spinal cord: from stem cells to activity-based restoration therapies. Clin Neurosurg 2004;51:207–27 [PubMed] [Google Scholar]
- 12.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001;82(6):818–24 doi: 10.1053/apmr.2001.23752 [DOI] [PubMed] [Google Scholar]
- 13.Belegu V, Oudega M, Gary DS, McDonald JW. Restoring function after spinal cord injury: promoting spontaneous regeneration with stem cells and activity-based therapies. Neurosurg Clin N Am 2007;18(1):143–68, xi. doi: 10.1016/j.nec.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 14.Becker D, Gary DS, Rosenzweig ES, Grill WM, McDonald JW. Functional electrical stimulation helps replenish progenitor cells in the injured spinal cord of adult rats. Exp Neurol 2010;222(2):211–8 doi: 10.1016/j.expneurol.2009.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett 2010;479(2):128–33 doi: 10.1016/j.neulet.2010.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker D, Sadowsky CL, McDonald JW. Restoring function after spinal cord injury. Neurologist 2003;9(1):1–15 doi: 10.1097/01.nrl.0000038587.58012.05 [DOI] [PubMed] [Google Scholar]
- 17.Sadowsky CL, McDonald JW. Activity-based restorative therapies: concepts and applications in spinal cord injury-related neurorehabilitation. Dev Disabil Res Rev 2009;15(2):112–6 doi: 10.1002/ddrr.61 [DOI] [PubMed] [Google Scholar]
- 18.Tillakaratne NJ, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res 2000;60(2):219–30 [DOI] [PubMed] [Google Scholar]
- 19.Damiano DL, Quinlivan JM, Owen BF, Payne P, Nelson KC, Abel MF. What does the Ashworth scale really measure and are instrumented measures more valid and precise? Dev Med Child Neurol 2002;44(2):112–8 [DOI] [PubMed] [Google Scholar]
- 20.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31(3):247–63 [DOI] [PubMed] [Google Scholar]
- 21.Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil 1993;74(5):531–6 [DOI] [PubMed] [Google Scholar]
- 22.Lynch AC, Wong C, Anthony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury: a description of bowel function in a spinal cord-injured population and comparison with age and gender matched controls. Spinal Cord 2000;38(12):717–23 [DOI] [PubMed] [Google Scholar]
- 23.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury – a cross-sectional study. Spinal Cord 2004;42(12):711–6 doi: 10.1038/sj.sc.3101652 [DOI] [PubMed] [Google Scholar]
- 24.Bravo G, Guizar-Sahagun G, Ibarra A, Centurion D, Villalon CM. Cardiovascular alterations after spinal cord injury: an overview. Curr Med Chem Cardiovasc Hematol Agents 2004;2(2):133–48 [DOI] [PubMed] [Google Scholar]
- 25.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–56 [DOI] [PubMed] [Google Scholar]
- 26.Tsitouras PD, Zhong YG, Spungen AM, Bauman WA. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm Metab Res 1995;27(6):287–92 doi: 10.1055/s-2007-979961 [DOI] [PubMed] [Google Scholar]
- 27.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet 2002;359(9304):417–25 doi: 10.1016/S0140-6736(02)07603-1 [DOI] [PubMed] [Google Scholar]
- 28.Campagnolo DI, Bartlett JA, Keller SE. Influence of neurological level on immune function following spinal cord injury: a review. J Spinal Cord Med 2000;23(2):121–8 [DOI] [PubMed] [Google Scholar]
- 29.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285(6):785–95 [DOI] [PubMed] [Google Scholar]
- 30.Consortium for Spinal Cord Medicine. Clinical Practice Guidelines Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 2001;24Suppl 1:S40–101 [DOI] [PubMed] [Google Scholar]
- 31.Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med 2004;34(11):727–51 [DOI] [PubMed] [Google Scholar]
- 32.Griffin L, Decker MJ, Hwang JY, Wang K, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614–22 doi: 10.1016/j.jelekin.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 33.Skold C, Lonn L, Harms-Ringdahl K, Hultling C, Levi R, Nash M, et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J Rehabil Med 2002;34(1):25–32 [DOI] [PubMed] [Google Scholar]
- 34.Sloan KE, Bremner LA, Byrne J, Day RE, Scull ER. Musculoskeletal effects of an electrical stimulation induced cycling programme in the spinal injured. Paraplegia 1994;32(6):407–15 doi: 10.1038/sc.1994.67 [DOI] [PubMed] [Google Scholar]
- 35.Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Eser P. Effect of detraining on bone and muscle tissue in subjects with chronic spinal cord injury after a period of electrically-stimulated cycling: a small cohort study. J Rehabil Med 2009;41(4):282–5 doi: 10.2340/16501977-0321 [DOI] [PubMed] [Google Scholar]
- 36.Yuan B, Gomez J, Gonzalez J, Yu W, Ino S. H-reflex measurement and a simulation model for interpreting the effect of an auxiliary electrical stimulation on FES. Conf Proc IEEE Eng Med Biol Soc 2010;2010:5843–6 doi: 10.1109/IEMBS.2010.5627507 [DOI] [PubMed] [Google Scholar]
- 37.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 2004;5(2):146–56 doi: 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- 38.Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature 1987;326(6114):694–7 doi: 10.1038/326694a0 [DOI] [PubMed] [Google Scholar]
- 39.Wang E, Gao J, Yang Q, Parsley MO, Dunn TJ, Zhang L, et al. Molecular mechanisms underlying effects of neural stem cells against traumatic axonal injury. J Neurotrauma 2012;29(2):295–312 doi: 10.1089/neu.2011.2043 [DOI] [PubMed] [Google Scholar]
- 40.Gary DS, Malone M, Capestany P, Houdayer T, McDonald JW. Electrical stimulation promotes the survival of oligodendrocytes in mixed cortical cultures. J Neurosci Res 2012;90(1):72–83 doi: 10.1002/jnr.22717; 10.1002/jnr.22717 [DOI] [PubMed] [Google Scholar]


