Abstract
Objectives
To (1) assess food intake; (2) establish the prevalence of dietary supplement usage and its associated cost (oral nutritional supplements (ONS); vitamin and mineral supplements (VMS)) and; (3) identify the characteristics of nutritional supplement users among patients admitted to a spinal cord injury (SCI) center.
Study design
A single center survey.
Methods
Standardized questionnaires were used to collect demographic information, food consumption over a 24-hour period, and the use of nutritional supplements. Multivariate logistic regression was used to determine the characteristics of dietary supplement usage and those using them.
Results
Seventy-three patients with SCI completed and returned the questionnaires (69.5% response rate). From 67 questionnaires with food intake data, 21 patients (31.3%) consumed three full meals a day. Nine of the full 73 patients (12.3%) received artificial nutritional support, 14 of 73 (19.1%) received ONS, 34 of 73 (46.5%) received VMS, and 31 of 73 (42.4%) required assistance in order to eat. The three supplements most often prescribed were multivitamins (19.1%), vitamins B (17.8%), and vitamin D (13.6%). VMS use was associated with age (years: >60 vs. ≤60: 62.1 vs. 34.1%, P = 0.019), nutrition risk (Spinal Nutrition Screening Tool (≥11 vs. <11: 65.7 vs. 28.9%, P = 0.001), and serum albumin concentration (<35 vs. ≥35 g/l: 59.6 vs. 16%, P < 0.01). Patients at nutrition risk were found to consume more ONS than the lower risk group (28.5 vs. 10.5%, P = 0.05). The expenditures on ONS and VMS were higher in the group at greater nutritional risk (£1878.3 vs. £914.3, P = 0.005).
Conclusion
The use of nutritional supplements is common in patients with SCI, particularly in older adults and patients with poor nutritional state. However, the present study identified only small numbers of patients consuming all of their hospital meals, which may well contribute to undernutrition risk. Given that a high proportion of patients with SCI require assistance to eat, we suggest that further efforts focus on the feasibility of providing feeding assistants, and on reviewing the nature of the hospital menu.
Keywords: Nutritional supplement, Vitamins and Minerals, Malnutrition, Spinal cord injuries
Introduction
Diet and nutrition play an important role in the maintenance of health and prevention of disease.1,2 Malnutrition is an important but often unrecognized co-morbidity that continues to impact on the health of hospitalized spinal cord injured (SCI) patients in the UK.3,4 The prevalence of malnutrition is more pronounced in those who have a high cervical SCI, those who are on mechanical ventilatory support,3 and those with a previous intensive care unit stay.4 Identifying malnutrition in patients in SCI centers appears to present a considerable challenge. Despite the availability of several guidelines and tools for detecting and managing malnutrition,1,5,6 there is still substantial under-reporting of malnutrition in patient records.7 Moreover, patients with SCI, who are at increased nutritional risk tend to receive more prescribed medications3 and have poorer overall clinical outcomes.8
Patients at risk of undernutrition may require dietary supplements such as oral nutritional supplements (ONS) and/or vitamin and mineral supplements (VMS) in order to correct their nutritional deficits. Opperman et al.9 reported that the use of dietary supplements was high in patients with SCI living in the community, but there is no current literature reporting on the usage and cost of supplements in an in-patient setting (SCI center).
The present study therefore aimed to assess food intake and to establish the prevalence of ONS and VMS usage in a representative sample of hospitalized SCI patients; to report the cost of the use of these supplements; and to identify the characteristics of supplement users.
Methods
Participants were enrolled in the annual nutrition audit program commissioned by the nutrition steering group at the National Spinal Injuries Centre (NSIC) at Stoke Mandeville Hospital, looking at patients’ nutrient intakes and their satisfaction with hospital food, for which complete study methods have been published.10
Survey administration
The questionnaires were given to all in-patients (n = 105) in the NSIC during February 2012. Patients were reminded by a personal visit from the investigator 2 weeks after the initial distribution if questionnaires had not by then been returned.
The questionnaires were completed anonymously, and participants were asked to complete them without conferring with friends or fellow patients.
Baseline demographics, level and cause of SCI, presence of co-morbidities, routine blood biochemistry, nutritional-risk score (Spinal Nutrition Screening Tool (SNST) score6), and degree of appetite10 were collected prospectively from the patients’ medical records. The use of ONS, vitamins, and minerals was established from patients’ prescription charts.
Cost analysis
The costs of dietary supplements were calculated by multiplying the resource volumes by unit costs. Unit costs in UK pounds (£) (£1 = €1.19/US$1.59; 21 January 2013) were based on the information supplied by the British National Formulary (http://www.bnf.org) and validated by the hospital pharmacist.
Ethics
This study was conducted according to the guidelines outlined in the Declaration of Helsinki. Formal ethical permission to conduct the study was not required by the hospital review board as this was considered a clinical audit not involving additional active patient participation.11 The questionnaires had already been approved by the local clinical audit department for the phrasing and grammar of the questions and were in use prior to the study period reported here.
Statistical analysis
Descriptive statistics were used to calculate the response frequency and proportions. Summary data are presented as medians and ranges, or means and standard deviations as indicated. Differences in frequencies between groups were tested with the χ2 test. The Mann–Whitney test was used to test for differences between groups when there were ordinal data.
Univariate logistic regression was conducted to determine the odds ratios and confidence intervals between the risk factor variables and the outcome variables (use of ONS and use of VMS). Variables considered significant risk factors for use of dietary supplements (by the univariate analysis) were entered into a multivariate logistic regression model, to determine the best predictors of dietary supplements usage.
The data were analyzed using SPSS Version 18 (PASW Statistics for Windows, Version 18.0. (SPSS Inc., Chicago, IL, USA)) and significance was accepted if P < 0.05.
Results
A total of 105 questionnaires were distributed. Responses were received from 73 patients (69.5%) (age range: 20–91 years (median: 54), 19.2% were female, 65.7% were tetraplegic; 47.9% had complete SCI). Twenty (27.3%) patients required assistance from hospital staff to complete their questionnaires. Of the 73 patients with a nutrition-risk score, 35 (47.9%) were at risk of undernutrition (SNST ≥ 11).6
Food intake
Sixty-seven of the 73 returned questionnaires included full food intake data. Twenty-one of the 67 (31%) patients consumed three full meals a day. Approximately 1 in 10 patients (7 of 67) ate less than half of their breakfast (Table 1). Nineteen (28.2%) patients were found to miss one or more meals. A significantly higher proportion of patients missed their evening meal than either breakfast or lunch (28.2, 10.4, and 8.9%, respectively, χ2: 11.67, P = 0.003)
Table 1.
Distribution of food intake
| Breakfast |
Lunch |
Supper |
||||
|---|---|---|---|---|---|---|
| Amount eaten | n | % | n | % | n | % |
| All eaten | 50 | 75 | 41 | 61 | 30 | 45 |
| More than half eaten | 3 | 4 | 8 | 12 | 9 | 13 |
| Half eaten | 6 | 9 | 8 | 12 | 7 | 10 |
| Less than half eaten | 1 | 1 | 4 | 6 | 2 | 3 |
| None eaten | 7 | 10 | 6 | 9 | 19 | 28 |
Snacks were not provided to the great majority of patients (90%), and 31 (42.5%) of patients reported that they required assistance to eat.
Prevalence of supplementary enteral nutrition
Nine of 73 (12.3%) patients received enteral tube feeding, and 14 (19.1%) received ONS. Five different types of ONS were recorded. The most commonly prescribed ONS was a 1.5 kcal/ml milky oral sip feed.
No differences were observed in gender, level of SCI, body mass index (BMI), presence of pressure ulcers, use of mechanical ventilation, or age, between users and non-users of enteral nutritional supplements. Undernutrition risk (SNST ≥ 11) was associated with ONS use (Table 2).
Table 2.
Characteristics of supplement user vs. non-user
| Characteristics | n | ONS user | Non-ONS user | Vitamin and minerals supplement user | Non-vitamin and minerals supplement user |
|---|---|---|---|---|---|
| Total participants | 73 | 14 (19.1%) | 59 (80.9%) | 34 (46.4%) | 39 (53.4%) |
| Gender | |||||
| Male | 59 | 10 (16.9%) | 49 (83.1%) | 27 (45.7%) | 32 (54.3%) |
| Female | 14 | 4 (28.6%) | 10 (71.4%) | 7 (50%) | 7 (50%) |
| Level of SCI | |||||
| Tetraplegia | 48 | 11 (22.9%) | 37 (77.1%) | 24 (50%) | 24 (50%) |
| Paraplegia | 25 | 3 (12%) | 22 (88%) | 10 (40%) | 15 (60%) |
| BMI (kg/m2) | |||||
| >25 | 27 | 5 (18.5%) | 22 (81.5%) | 11 (40.7%) | 16 (59.3%) |
| ≤25 | 42 | 9 (21.4%) | 33 (78.6%) | 21 (50%) | 21 (50%) |
| Pressure ulcer‡ | |||||
| Yes | 26 | 7 (26.9%) | 19 (73.1%) | 15 (57.5%) | 11 (42.3%) |
| No | 47 | 7 (14.9%) | 40 (85.1%) | 18 (38.3%) | 29 (61.7%) |
| Ventilation | |||||
| Yes | 9 | 3 (33.3%) | 6 (66.7%) | 5 (55.6%) | 4 (44.4%) |
| No | 64 | 11 (17.2%) | 53 (82.8%) | 28 (43.1%) | 36 (56.3%) |
| Age (years)‡ | |||||
| ≤60 | 44 | 7 (15.9%) | 37 (84.1%) | 15 (34.1%) | 29 (65.9%) |
| >60 | 29 | 7 (24.1%) | 22 (75.9%) | 18 (62.1%) | 11 (37.9%) |
| Nutritional risk*,§ | |||||
| At-risk | 35 | 10 (28.5%) | 25 (71.5%) | 23 (65.7%) | 11 (34.3%) |
| Not at-risk | 38 | 4 (10.5%) | 34 (89.5%) | 11 (28.9%) | 27 (71.1%) |
| Serum albumin*,§ | |||||
| ≥35 g/l | 25 | 2 (8%) | 23 (92%) | 4 (16%) | 21 (84%) |
| <35 g/l | 47 | 12 (25.5%) | 35 (74.5%) | 28 (59.6%) | 19 (40.4%) |
| Hemoglobin*,‡ | |||||
| <13.1 g/l | 56 | 13 (23.2%) | 43 (76.7%) | 28 (50%) | 28 (50%) |
| ≥13.1 g/l | 16 | 1 (6.3%) | 15 (93.7%) | 4 (25%) | 12 (75%) |
ONS, oral nutritional supplement; SCI, spinal cord injury; BMI, body mass index.
*P < 0.05: oral nutritional supplement comparison.
‡P < 0.05: vitamin and mineral supplement comparison.
§P < 0.01: vitamin and mineral supplement comparison.
Prevalence of vitamin and mineral usage
Thirty-four of 73 (46.5%) patients reported taking VMS. Eleven different types of VMS were recorded. The three most commonly prescribed supplements were multivitamins (19.1%), vitamin B supplements (17.8%), and vitamin D supplements (13.6%). Fig. 1 summarizes the distribution of vitamin and mineral use.
Figure 1.
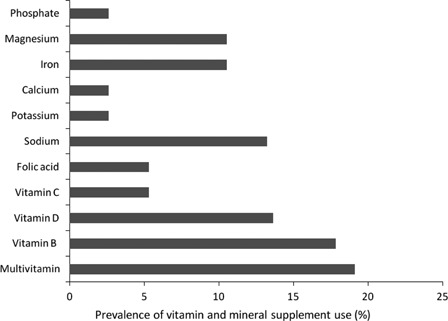
Prevalence of VMS use (%).
No difference was observed in gender, level of SCI, BMI, or need for mechanical ventilation between users and non-users. Older adults (>60 years old), and those with pressure ulcers, lower hemoglobin (<13.1 g/l), lower albumin (<35 g/l), and undernutrition risk (SNST ≥ 11) were more likely to be on VMS (Table 2).
Cost of supplements
Overall, £2792.6 was spent on ONS and VMS over the 1-month audit period (£38.25 per patient). Patients at risk of undernutrition were found to have cost more than those at low risk ((£53.6 vs. £24.1 per patient); Mann–Whitney Z = −2.831, P = 0.005).
Characteristics of supplement users
Oral nutritional supplements
Abnormalities of the SNST score (P = 0.005), serum albumin (P = 0.012), and hemoglobin level (P = 0.043) were identified by the univariate logistic regression analysis as predictors of ONS usage (Tables 3 and 4).
Table 3.
Univariate analyses of risk factors for ONS use
| Variables | β | P value | Odds ratio | Confidence interval |
|---|---|---|---|---|
| Tetraplegic | 0.779 | 0.269 | 2.18 | 0.55–8.68 |
| Male | −0.673 | 0.327 | 0.51 | 0.13–1.96 |
| Pressure ulcer | −0.744 | 0.217 | 0.475 | 0.15–1.55 |
| Ventilated | −0.879 | 0.260 | 0.415 | 0.09–1.92 |
| Caucasian | −1.20 | 0.27 | 0.301 | 0.04–2.54 |
| Age | 0.006 | 0.722 | 1.006 | 0.97–1.04 |
| Weight | −0.04 | 0.096 | 0.961 | 0.92–1.01 |
| BMI | −0.04 | 0.465 | 0.961 | 0.86–1.07 |
| Hemoglobin* | −0.44 | 0.043 | 0.643 | 0.42–0.98 |
| Albumin* | −0.166 | 0.012 | 0.847 | 0.75–0.96 |
| Total protein | −0.076 | 0.121 | 0.927 | 0.84–1.02 |
| Potassium | −0.508 | 0.399 | 0.602 | 0.19–1.96 |
| Sodium | −0.096 | 0.161 | 0.909 | 0.80–1.04 |
| SNST score† | 0.211 | 0.005 | 1.24 | 1.07–1.43 |
BMI, body mass index; SNST, Spinal Nutrition Screening Tool.
*P < 0.05.
†P < 0.01.
Table 4.
Multivariate logistic regression model considering risk factors for ONS use
| Variables | β | P value | Odds ratio | Confidence interval |
|---|---|---|---|---|
| SNST score* | 0.210 | 0.005 | 1.24 | 1.07–1.43 |
| Constant | −3.974 | 0.019 | – | – |
SNST, Spinal Nutrition Screening Tool.
*P < 0.01.
Vitamin and mineral usage
The SNST score (P = 0.021), the presence of pressure ulcers (P = 0.04), increasing age (P = 0.019), lower hemoglobin (P = 0.024), lower serum albumin (P = 0.002), and lower serum sodium (P = 0.019) were identified by the univariate logistic regression analysis as predictors of vitamin and mineral use (Tables 5 and 6).
Table 5.
Univariate analyses of risk factors for VMS use
| Variables | β | P value | Odds ratio | Confidence interval |
|---|---|---|---|---|
| Tetraplegic | 0.336 | 0.505 | 1.40 | 0.52–3.76 |
| Male | −0.324 | 0.598 | 0.723 | 0.22–2.41 |
| Pressure ulcer* | −1.052 | 0.04 | 0.349 | 0.13–0.96 |
| Ventilated | −0.382 | 0.594 | 0.682 | 0.17–2.78 |
| Caucasian | −0.052 | 0.932 | 0.949 | 0.29–3.16 |
| Age* | 0.035 | 0.019 | 1.036 | 1.01–1.07 |
| Weight | −0.001 | 0.94 | 0.999 | 0.98–1.02 |
| BMI | 0.012 | 0.741 | 1.012 | 0.94–1.08 |
| Hemoglobin* | −0.415 | 0.024 | 0.660 | 0.46–0.95 |
| Albumin† | −0.167 | 0.002 | 0.846 | 0.76–0.94 |
| Total protein | −0.070 | 0.086 | 0.933 | 0.86–1.01 |
| Potassium | −0.090 | 0.85 | 0.914 | 0.36–2.31 |
| Sodium* | −0.178 | 0.019 | 0.837 | 0.72–0.97 |
| SNST score* | 0.142 | 0.021 | 1.15 | 1.02–1.30 |
BMI: body mass index; SNST: Spinal Nutrition Screening Tool.
*P < 0.05.
†P < 0.01.
Table 6.
Multivariate logistic regression model considering risk factors for VMS use
| Variables | β | P value | Odds ratio | Confidence interval |
|---|---|---|---|---|
| Albumin* | 0.167 | 0.002 | 0.846 | 0.76–0.94 |
| Constant | 5.175 | 0.003 | – | – |
*P < 0.01.
Discussion
Data on dietary supplement usage by patients with SCI are scarce in the literature. To our knowledge, this is the first study reporting formally on the pattern of nutritional supplement use in a representative sample of hospitalized patients with SCI. The inevitable disabilities and the need for extended hospitalization for acute care and rehabilitation in this group of patients renders extrapolation from generic hospital populations unwise. We believe, when compared with the published literature, that our sample size and response rate lend sufficient weight for our conclusions to be meaningful.9,10
The study found a high prevalence of patients at risk of undernutrition (47.9%), which is comparable with our previous findings.3 Food is the treatment for most malnourished patients in hospital. However, we found that more than one-third (39.8%) of patients did not eat all the food served; of these patients, almost one in three (28%) were found to miss one or more meals (Table 1). Based on the UK's estimated average requirements and reference nutrient intake,12 the deficit from a single missed meal would be equivalent to 300–600 kcal and 15–20 g protein per day (16–33% of daily requirement). In addition, this study also demonstrated that snacks, which can contribute substantially (e.g. 400 kcal, 6 g protein, or ∼15% of daily requirement), were not provided to the majority of patients (90%), indicating an important missed opportunity in patients with SCI. Although oral nutrition supplements are used to augment food intake in a proportion of patients, artificial nutrition support, such as enteral tube feeding, is only used in a minority of patients with SCI (12.3%).3,4
In tackling hospital malnutrition, the implementation of protected mealtimes is one of the key areas in the Council of Europe resolution: Food and Nutritional Care in Hospitals,13 and this is included in the recent UK Government policy statement entitled “Improving Nutritional Care”.14 Some of our patients and staff reported, however, that meals were not served on time and that there were frequent interruptions during mealtimes, suggesting that this could relate to a lack of implementation of intended best practice. Indeed, recent evidence suggests that only minor improvements in mealtime experience can be expected after the implementation of “protected mealtimes”,15 questioning its effectiveness. Given also that almost half of the patients with SCI would require assistance to eat (especially true for those with high cervical SCI), perhaps further effort should be focused on the possibility of engaging additional feeding assistants.15
It is known that the prevalence of supplement use has increased over recent years. The British National Dietary and Nutrition Survey16 reported that the number of respondents (n = 1724) taking supplements had increased, with 40% of women and 29% of men taking some form of supplement in 2000/2001, compared with 17% of women and 9% of men in 1986/1987. We cannot fully explain why our present study found a higher proportion of patients (50% of females and 45.7% of males) to be receiving VMS, but the very fact of hospitalization is obviously important. Increasing interest in normalizing the micronutrient profile to improve health outcomes may also have contributed. Although our study, unusually,16 showed no gender difference in VMS use, similar consumption by both sexes was also observed in a study of patients with SCI in the community.9
The use of ONS was (unsurprisingly) associated with the risk of undernutrition (Table 2), but nonetheless not all high-risk patients received ONS, an observation explained only partly by enteral tube feeding (n = 9, 25.7%) and/or special diets. Multivariate analysis indicates that only the risk of malnutrition independently predicts use of ORS, but the data are perhaps unduly skewed by use of the ORS, since their prescription obviously reflects professional decisions that may have been dependent on the other parameters identified in the univariate analysis. Low serum albumin was however found to be an independent predictor of VMS use.
In the present study, the ONS and VMS preparations most often used were comparable with those of other studies,9 and concordant with the assumption that vitamin and mineral deficiency is important in patients with SCI,9 especially those with pressure ulcers17 and those at risk of undernutrition the re-feeding syndrome.1,17,18
The main limitation of this audit was that only 1 day's food intake was assessed, and therefore assumptions have necessarily been made about food provision and net intake across a sometimes very prolonged hospital stay. More data from each patient would provide stronger evidence to permit a more conclusive comparison with the national standard.19 However, it is highly probable, had we asked for longer diary data, that this would have adversely affected response rates and itself diminished the generalizability of the conclusions for different reasons. Residual confounding, such as from varying socio-economic status, could also have been important.20
The higher cost of dietary supplements in those at risk of undernutrition should not be taken to imply that dietary intervention is not cost-effective. The literature is increasingly in agreement that the cost of preventing malnutrition is substantially less than the cost of treating it once it occurs (estimated to be £3.8 billion per annum in the UK alone). We believe that the cost of ONS/VMS is justifiable on this basis through their potential to improve clinical outcomes and quality of life,1,21,22 but controlled trial data are required to add scientific weight to this belief.
Conclusion
In conclusion, this is the first study to describe the links between risk of malnutrition and the frequent use of ONS and VMS in patients in a UK SCI center, an unusual sample that cannot easily be captured in national representative surveys. Nutrient intake from hospital meals alone was often insufficient to meet requirements, and it is likely to be beneficial to use ONS and/or VMS to protect against and to treat both macro- and micronutrient deficiencies and their related complications. Further research is needed on usage patterns of dietary supplements and the potential health economic effects of dietary supplements in patients with SCI. The present study serves as a baseline to which the findings of subsequent efforts can be compared.
Acknowledgements
The authors thank Ms Lynsey Spillman for assisting with data collection, Ms Bhavna Badani for providing cost information for vitamins and minerals. We thank the patients who participated in this audit, and the staff working in the National Spinal Injuries Centre, Stoke Mandeville Hospital for completing the questionnaires. We also thank Fayth Armitage for proofreading the manuscript. Staff receive support from the Comprehensive Biomedical Research Centre funding awarded to UCL and its partner Trust by the National Institute for Health Research.
References
- 1.National Institute for Health and Clinical Excellence (NICE) Nutrition support in adults: oral nutrition support, enteral tube feeding and parenteral nutrition. London: NICE; 2006. Available from: http://www.nice.org.uk/nicemedia/live/10978/29979/29979.pdf [accessed 2013 Jan 17] [PubMed] [Google Scholar]
- 2.Department of Health: Healthy Lives, Healthy People Our strategy for public health in England. London: HMSO; 2010. Available from: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_127424.pdf [accessed 2012 Jun 30] [Google Scholar]
- 3.Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A. The prevalence of malnutrition in spinal cord injured patients – a UK multicentre study. Br J Nutr 2012;108(5):918–23 [DOI] [PubMed] [Google Scholar]
- 4.Wong S, Graham A, Hirani SP, Grimble G, Forbes A. Profile and prevalence of malnutrition in children with spinal cord injuries – assessment of the Screening Tool for Assessment in Paediatrics (STAMP). Spinal Cord 2012;50(1):67–71 [DOI] [PubMed] [Google Scholar]
- 5.Gall A, Turner-Stokes L. Guideline Development Group Chronic spinal cord injury: management of patients in acute hospital setting. Clin Med 2008;8(1):70–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A. Validation of the Spinal Nutrition Screening Tool (SNST) in patients with spinal cord injuries (SCI) – result form a multicentre study. Eur J Clin Nutr 2012;66(3):382–7 [DOI] [PubMed] [Google Scholar]
- 7.Wong S, Derry F, Grimble G, Forbes A. How do spinal cord injury centres manage malnutrition? A cross-sectional survey of 12 SCIC in the UK and Ireland. Spinal Cord 2012;50(2):132–5 [DOI] [PubMed] [Google Scholar]
- 8.Wong SS, Derry F, Jamous A, Harini S, Grimble G,, Forbes A. Is nutritional risk associated with adverse clinical outcomes such as length of stay and mortality in spinal cord injured patients admitted to a spinal centre? Gut 2012;61Supp 2:A89 PMO-039. [Google Scholar]
- 9.Opperman EA, Buchholz AC, Darlington GA, Martin Ginis KA, The SHAPE-SCI Research Group Dietary supplement use in the spinal cord injury population. Spinal Cord 2012;48(1):60–4 [DOI] [PubMed] [Google Scholar]
- 10.Wong S, Graham A, Green D, Hirani SP, Grimble G, Forbes A. Meal provision in a UK National Spinal Injury Centre – a qualitative audit of service users and stakeholders. Spinal Cord 2012;50(10):772–7 [DOI] [PubMed] [Google Scholar]
- 11.National Research Ethics Service (NRES) Is your project research? Available from: http://www.nres.nhs.uk/applications/is-your-project-research. NRES; 2011 [accessed 2012 Jun 30]
- 12.Department of Health. Dietary Reference Values of Food Energy and Nutrients for the United Kingdom Report on Health and Social Subjects no. 41. H.M. Stationery Office, London; 1991 [Google Scholar]
- 13.Council of Europe Committee of Ministers Resolution RESAP on food and nutritional care in hospitals. Brussels: Council of Europe; 2003. Available from: https://wcd.coe.int/ViewDoc.jsp?id=85747 [accessed 2012 Jun 30] [Google Scholar]
- 14.Department of Health Improving nutritional care: a joint action plan from the department of health and nutrition summit stakeholders. London: Department of Health, H.M. Stationery Office; 2003. Available from: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_079932.pdf [accessed 2012 Jun 30]. [Google Scholar]
- 15.Hickson M, Connolly A, Whelan K. Impact of protected mealtimes on ward mealtime environment, patient experience and nutrient intake in hospitalised patients. J Hum Nutr Diet 2011;24(4):370–4 [DOI] [PubMed] [Google Scholar]
- 16.Henderson L, Gregory J, Irving K, et al. The national diet and nutrition survey: adults aged 19–64 Years. Volume 3: Vitamin and mineral intake and urinary analyses. London: HMSO; 2003 [Google Scholar]
- 17.National Institute for Health and Clinical Excellence (NICE) The management of pressure ulcers in primary and secondary care: a clinical practice guideline. London: NICE; 2005. Available from: http://www.nice.org.uk/nicemedia/live/10972/29885/29885.pdf [accessed 2012 Jun 30] [Google Scholar]
- 18.Scottish Intercollegiate Guidelines Network (SIGN) Management of osteoporosis: a national clinical guideline. Edinburgh: SIGN; 2003. Available from: http://www.sign.ac.uk/pdf/sign71.pdf [accessed 2012 Jun 30] [Google Scholar]
- 19.Scottish Government: Food in Hospitals National catering and nutrition specification for food and fluid provision in hospitals in Scotland. Edinburgh: Scottish Government; 2008. Available from: http://www.scotland.gov.uk/Publications/2008/06/24145312/0 [accessed 2012 Jun 30] [Google Scholar]
- 20.Vatanparas H, Adolphe JL, Whiting SJ. Socio-economic status and vitamin/mineral supplement use in Canada. Health Rep 2010;21(4):19–25 [PubMed] [Google Scholar]
- 21.Milne AC, Avenell A, Potter J. Meta-analysis: protein and energy supplementation in older people. Ann Intern Med 2006;144(1):37–48 [DOI] [PubMed] [Google Scholar]
- 22.NHS Institute for Innovation and Improvement High impact actions for nursing and midwifery. Coventry: NHS Institute for Innovation and Improvement; 2009. Available from: http://www.institute.nhs.uk/index.php?option=com_joomcart&main_page=document_product_info&products_id=731&cPath=88 [accessed 2012 Jun 30] [Google Scholar]


