Abstract
Objective/Context
Dermatomal somatosensory-evoked potentials (dSEPs) may be valuable for diagnostic purposes in selected cases with spinal disorders.
Design
Reports on cases with successful use of dSEPs.
Findings
Cases 1 and 2 had lesions causing multiple root involvement (upper to middle lumbar region in Case 1 and lower sacral region in Case 2). Cystic lesions in both cases seemed to compress more than one nerve root, and stimulation at the center of the involved dermatomes in dSEPs helped to reveal the functional abnormality. Cases 3 and 4 had lesions involving the spinal cord with or without nerve root impairment. In Case 3, an magnetic resonance imaging (MRI)-verified lesion seemed to occupy a considerable volume of the lower spinal cord, causing only very restricted clinical sensory and motor signs. In Case 4, a cervical MRI showed a small well-circumscribed intramedullary lesion at right C2 level. All neurophysiological investigations were normal in the latter two patients (motor, tibial, and median somatosensory-evoked potentials in Case 3, and electromyography in both) except for the dSEPs.
Conclusions
Objectifying the presence and degree of sensory involvement in spinal disorders may be helpful for establishing diagnoses and in therapeutic decision-making. Valuable information could be provided by dSEPs in selected patients with multiple root or spinal cord involvement.
Keywords: Dermatomal somatosensory-evoked potentials, Syringomyelia, Arachnoid cysts, Electromyography, Radiculopathy, Multiple root involvement, Spinal cord lesions
Introduction
Dermatomal somatosensory-evoked potentials (dSEPs) are techniques of recording cerebral-evoked responses to the stimulation of certain areas innervated by solitary nerve roots, such stimulation being intended to provide pure sensory input to the central nervous system through individual spinal segments. However, somatosensory-evoked potentials (SEPs) performed by stimulating the mixed nerves carry multisegmental inputs to the central nervous system and, therefore, their segmental specificity is expected to be much less than those of dSEPs. In the assumption of these theoretical characteristics, dSEPs have been used for more than 30 years to investigate the abnormalities of sensory nerve roots with or without spinal cord involvement. Although there is no clear evidence about their contribution to the clinical diagnoses, observations in individual cases may emphasize their positive role in documenting spinal lesions, sometimes as the sole electrophysiological evidence for the pathological processes.1 We report four cases in which dSEPs provided useful information about the presence and localization of the lesions with the goal of encouraging the use of these techniques.
Methods
Electrophysiological studies comprising motor and sensory nerve conduction studies (NCS), SEP, dSEPs and needle electromyography (EMG) of the relevant muscles were conducted using a Medelec Synergy electromyography device (Oxford Instruments, Surrey, UK). All the NCS were carried out with surface stimulation and recording. Motor conduction studies were performed by using stimulation electrodes with inter-electrode distances of 2.5 cm and by placing the recording electrodes according to the belly-tendon technique. Orthodromic sensory NCS in the upper extremities (median and ulnar nerves) were performed by stimulating the relevant fingers (second and fifth) with ring electrodes and recording at the wrist. In the lower extremities, sural and superficial peroneal nerves were stimulated at the calves and antidromic recordings were made at the ankle level.
All the SEP recordings were performed with the patient positioned supine on a couch as relaxed as possible. In dSEP studies, the signature area for each clinically suspected dermatome was electrically stimulated bilaterally with an intensity of 2.5 to 3 times the sensory threshold, by using a bipolar surface electrode with an inter-electrode distance of 2.5 cm2. The recordings were made through the input channels connected to electrodes placed at Cz-Fz and Cc-Fz positions according to the international 10–20 system (Cc is C3′ for right- and C4′ for left-sided stimulation). The ground electrode was placed between the stimulation and the recording sites, slightly proximal to the stimulation electrodes. The analysis time was 100 ms, amplifier sensitivity was 1–2 µV/division and band-pass was between 10 Hz–2 kHz. At least two recordings with a minimum of 500 averaged responses were stored. The N1, P1, N2, P2 peaks of the recorded cortical responses were marked. Unilaterally absent responses or inter-side latency differences of more than 5 ms were deemed pathological.
Bilateral median and tibial SEPs were also performed with submaximal stimulation of the relevant nerves at the wrist or ankle levels. Recordings were made from Erb's point, cervical 7, cervical 2, and Cc (all referenced to Fz) for median SEPs and from popliteal, lumbar, Cz-Cc, and Cc-Ci connections for tibial SEPs (Ci is C3′ for left- and C4′ for right-sided stimulation). All electrophysiological recordings were controlled for their quality and assessed by the same author (A.E.Ö.).
Cases
Case 1 was a 27-year-old man presented with a 25-day history of pain in his right buttock and weakness in his right knee. His pain lasted a few days and ameliorated thereafter. A magnetic resonance imaging (MRI) scan showed an ovoid-shaped spinal arachnoid cyst extending from right L2 to L4 vertebral levels with the largest diameter at L3. The patient was referred by his neurosurgeon for evaluation of possible supplementary electrophysiological findings caused by this lesion (Fig. 1).
Figure 1.
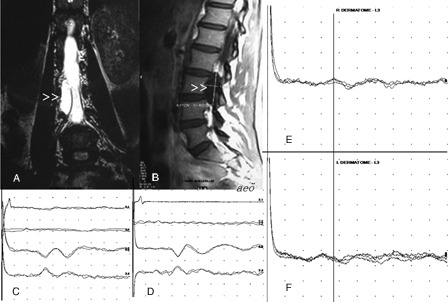
Coronal T2 W (A) and sagittal T1W (B) MR images show a cystic lesion on the right side of the spinal canal (arrowheads). Tibial SEPs are normal bilaterally (C and D, Trace 1: peripheral, Trace 2: spinal, Traces 3 and 4: cortical responses). In dSEP studies, cortical responses to right L3 stimulation (E) has prolonged latency in comparison to that recorded with contralateral stimulation (F).
His neurological examination was normal, except for the slight hypoesthesia found at the right L2–L3–L4 dermatomes. Bilateral sural and superficial peroneal sensory, tibial, and common peroneal motor conduction studies, as well as needle electromyography of lower extremity muscles innervated by bilateral L2–S1 segments, were normal. In tibial SEP studies, peripheral, spinal, and cortical responses had symmetrically normal latencies and amplitudes (Fig. 1). In the dermatomal SEP study, right and left L3 dermatomes were stimulated by the surface electrodes placed cathode proximally on the lower anterior thighs. The cortical responses recorded through the electrode connections described in the ‘Methods’ section had prolonged latencies when elicited with right-sided stimulation as compared to those recorded with contralateral stimulation (Fig. 1).
Case 2 was a 54-year-old woman with a 7-month history of sacral pain accompanying certain movements of her back and intermittent numbness on the mid-outer area of the left buttock. She denied any sexual and sphincteric disturbance. Her neurological examination was normal except for a circumscribed area of hypoesthesia over her left buttock corresponding to the left S3 dermatome. Her external anal sphincter appeared to be hypotonic on examination and the anal reflex seemed to be reduced clinically.
An MRI scan showed huge arachnoid cysts (Tarlov cysts) occupying most of the sacral spinal canal and more pronounced in the center and right side of the upper and the left side of the lower sacral canal (Fig. 3). Needle EMG of the external anal sphincter revealed an increased amount of long duration and polyphasic motor unit potentials more pronounced on the left side, whereas bilateral L3–S1 innervated extremity and girdle muscles were bioelectrically normal. Tibial SEPs and pudendal SEPs, evoked by the electrical stimulation of the clitoris at three times sensory threshold, were normal. In the dSEP study, the S3 dermatome was stimulated at mid-buttock level with the cathode facing upwards. Stimulation on the right side elicited a normal cortical response, whereas there was no response to contralateral S3 stimulation (Fig. 2).
Figure 3.
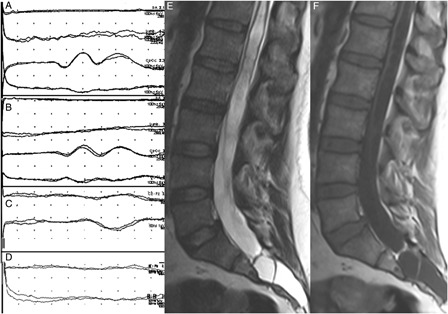
Tibial SEPs were normal bilaterally (A and B; the order of the traces are the same as in Fig. 1). The cortical response in right T11 dSEP study was normal (C) while it was not recordable contralaterally (D). Sagittal MR images show a cerebrospinal fluid containing-cystic lesion completely filling the sacral canal. Conus termination is at the L4 disc level. Distal cord is very thin due to the syringomyelic cavitation within it. Marked scalloping is noted in the posterior margin of the sacrum (E and F).
Figure 2.
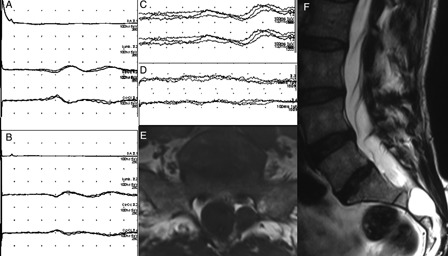
Tibial SEPs were normal bilaterally (A and B; the order of the traces are the same as in Fig. 1). The cortical response to right S3 dermatomal stimulation was normal (C) but was absent contralaterally (D). In the MR examination, very little spinal canal was left at the sacral level because of the huge arachnoid cysts in this patient. Marked scalloping is noted at the posterior margin of S2 vertebra (E and F).
Case 3 was a 41-year-old man referred by a neurosurgeon for further evaluation for his back pain. Two months ago, he almost fell but he managed to prevent a fall with his hands. Shortly after this event, he noted a belt-like pins-and-needles sensation over the left side of his waist. More recently, he experienced numbness on his right foot and calf as well. On examination, his right ankle and toe dorsiflexion were moderately weak (4/5 MRC scale) and there was hypoesthesia to light touch and pinpricks on the right T11 dermatome. Ankle reflexes were bilaterally absent. Sphincteric and sexual functions were reported to be normal.
An MRI scan showed a tethered cord syndrome along with a large syringomyelic cavity occupying the lower spinal cord (Fig. 2). Nerve conduction studies were normal, except for the bilaterally absent soleus H reflexes. A needle EMG of the bilateral lower extremity muscles innervated by L2–S1 segments provided no abnormality. Motor-evoked potentials performed with recordings from bilateral tibialis anterior and gastrocnemius muscles, as well as SEPs to bilateral median and tibial nerve stimulation, were normal. Dermatomal SEP studies were performed by T11 dermatome stimulation with the electrodes placed antero-laterally at the mid-abdominal level, the cathode facing up and laterally. There was no cortical response to left-sided stimulation, but stimulation on the same dermatome contralaterally evoked normal responses (Fig. 3).
Case 4 was a 47-year-old man who presented with a 2-year history of numbness and transient cramps in an area 4–5 cm in diameter over the right-anterior side of his chest. On his neurological examination, superficial sensation was diminished over the same area, which nearly corresponded to the T7 dermatome.
His thoracic computerized tomography and dorsal spinal MRI were normal. The cervical MRI showed a small well-circumscribed T2 hyperintense and T1 hypointense intramedullary lesion at the right C2 level (Fig. 4). In the dSEP study, the stimulator was placed on the T7 dermatome at the lower thoracic level on the mid-clavicular line with the cathode facing medially. Cortical responses to right-sided stimulation were not recordable, although they were recorded as normal on the contralateral side. Needle EMG of the right T5–T7 rectus abdominis muscle was also normal (Fig. 4).
Figure 4.
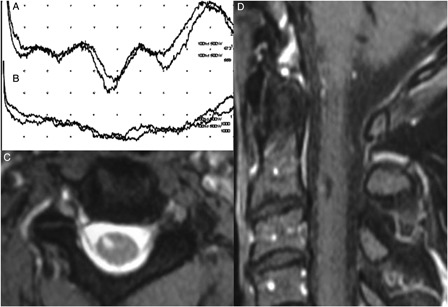
Cortical response to left T7 dermatomal stimulation was normal (A) while it was not recordable on the contralateral side (B). MRI examination shows a cystic intramedullary lesion at the level of C2–C3 intervertebral disc (C and D).
Discussion
Dermatomal SEPs have a theoretical basis for investigating the pathological processes causing isolated radicular or segmental involvement. However, the literature on this method debates its usefulness in clinical practice. The aim of this study is to present examples in which dSEPs provided valuable information about the causative lesions.
Our recent PubMed search showed fewer than 100 reports on dSEPs, which constitutes a very small quantity as compared to the number of reports on SEPs as a whole (more than 11 000). Initially, the studies mostly focused on the role of dSEPs in the diagnosis of radiculopathies due to cervical and lumbosacral disc diseases and resulted in contradictory findings.3–21 While some groups reported the high diagnostic sensitivity of dSEPs, exceeding 90%,8–14 others claimed that the technique had poor sensitivity and had no significant advantage over existing electrophysiological methods.15–21 The discrepancy between these opinions could be related to the differences between the case groups (in particular for single vs. multilevel root involvement), between the criteria used over the years to define electrophysiological abnormalities and between the techniques of verification of nerve root lesions (surgical visualization of the compressed root vs. the use of imaging techniques with different sensitivities, from myelography to MRI). Therefore, the final opinion seems to be that dSEPs do not have sufficient sensitivity in detecting single radicular lesions.5,22 The possible explanation for this conclusion is that dSEPs are carried out by stimulating signature areas corresponding to dermatomal/segmental levels, assuming that a single dermatomal segment is excited at each stimulation point and the response is sufficiently selective to reflect the function of a particular sensory root. On the contrary, there is usually some overlap between adjacent dermatomes and the area on the skin chosen for stimulation is rarely innervated by a single nerve root.23,24 Moreover, a segmental conduction defect caused by a focal lesion located in the nerve root may be masked by the normal conduction velocity of the uninjured long afferent pathway through the peripheral nerve.25 These two major factors may be considered as the cause of low sensitivity of dSEPs in detecting focal conduction abnormalities, especially if they are located in single nerve roots.
This unlucky beginning for dSEPs has led to the scepticism toward them that prevails nowadays and limits their use in the daily work of clinical neurophysiology. The aim of this paper is to present some examples of conditions in which dSEPS provide valuable information about the nature and localization of pathological processes.
In our opinion, dSEPs, as the present report illustrates, could be helpful in certain kinds of spinal disorder: lesions involving multiple nerve roots and/or lesions damaging the spinal cord itself. Cases 1 and 2 may be seen as examples of multiple root involvement. Cystic lesions seemed to compress more than one root in both cases, in the upper lumbar region in the first, sacral in the second, and stimulation over almost the center of the involved dermatomes in dSEPs helped to disclose the functional abnormality, although these lesions did not affect the findings of conventional (median and tibial) SEPs or most of the other electrophysiological studies. Cases 3 and 4 are examples of lesions involving the spinal cord itself with or without nerve root impairment. In Case 3, an MRI-verified spinal cord lesion seemed to occupy a considerable volume of the spinal cord, causing only very restricted clinical sensory and motor signs. All neurophysiological investigations were normal in this patient, except for the dSEPs. In Case 4, an extensive evaluation could not find any lesion involving the peripheral or central nervous system, other than the one shown by the cervical spinal MRI. Although it is difficult to claim that the location of the visible lesion was sufficient to cause the complaints of the patient, there was no other abnormality to explain the dSEP-verified restricted hypoesthesia on the same side. These discrepancies between the results of imaging methods, clinical findings and dSEPs could have resulted from the tissue changes beyond the limits that imaging can show or else the continuity of nerve conduction in the tissues imaged as more severely injured, e.g. edema.
Many different methodological studies have reported with both optimistic and negative results on the role of dSEPs for lesions leading to multi-radicular and/or spinal cord involvement.26–29 Kraft26 reports that the improvement of dSEPs provides the best confirmation of the surgical decompression in lumbosacral spinal stenosis (LSSS). Later, Storm and Kraft27 also evaluated the clinical use of dSEPs in the conservative management of LSSS and suggest that they might provide helpful information for the neurophysiological monitoring of the patients. Kramer et al.29,30 also show good correlation between pathological dSEPs and electrical perceptual threshold during recovery from spinal cord injury (SCI) and suggest that dSEPs could be helpful in monitoring the safety of a therapeutic drug or cell transplant in early-phase clinical studies, as well as in documenting the potential efficiency of intervention. However, Katz et al.31 examined the ability of SEPs and dSEPs to predict motor recovery after SCI and report that the evoked potentials could not add any prognostic information to the findings of physical examination in patients with SCI. Shields et al.28 also reported negative results implying that dSEPs were of limited value in determining the level of cervical SCI, as compared to transcranial magnetic motor-evoked potentials.
Although the reports on multiradicular and/or spinal cord lesions are not uniformly optimistic about the sensitivity of dSEPs, it may be worthwhile to include them in the diagnostic arsenal. While it is easier to evaluate motor function by neurological examination, sensory complaints and signs are often difficult to objectify, due to their dependence on the patients’ individual character and demographic features (e.g. education level, intelligence, and dialect). A few techniques have been devised to investigate the sensory roots by having conduction paths through these structures, such as H reflex, SEP, and dSEPs. dSEPs may be more convenient for the evaluation of spinal disorders involving several segments, since in this method the afferent volley enters into the spinal cord by way of fewer roots than the mixed nerve stimulation in SEP studies, which send action potential volleys via a higher number of nerve roots and may mask the effects of pathological process in one segment by the normal conduction in the others.
Objectifying the presence and degree of sensory root involvement would be of help in diagnosis and therapeutic decision making and may also prevent misdiagnosis and mistreatment of the patients. The purpose of this study was to encourage colleagues dealing with spinal disorders to call for dSEPs in selected cases, because dSEPs could be valuable for patients with multiple root involvement and/or spinal cord damage, by providing objective clues about the presence, localization, and nature of the lesions.
References
- 1.Assessment: dermatomal somatosensory evoked potentials Report of the American Academy of Neurology's Therapeutics and Technology Assessments Subcommittee. Neurology 1997;49(4):1127–30 [PubMed] [Google Scholar]
- 2.Liverson LA, Ma DM. Laboratory reference for clinical neurophysiology. New York: Oxford University Press Inc; 1992. p. 320 [Google Scholar]
- 3.Aminoff MJ, Goodin DS. Matter arising: dermatomal somatosensory evoked potentials in lumbosacral root compression. J Neurol Neurosurg Psychiatry 1988;51(5):740–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pape E. Sensory nerve somatosensory evoked potentials (SEP) in the evaluation of patients with sciatica: false P1 latency prolongation may be due to admixture of dermatomal SEP. Electromyogr Clin Neurophysiol 2001;41(6):337–44 [PubMed] [Google Scholar]
- 5.Albeck MJ, Taher G, Lauritzen M, Trojaborg W. Diagnostic value of electrophysiological tests in patients with sciatica. Acta Neurol Scand 2000;101(4):249–54 [DOI] [PubMed] [Google Scholar]
- 6.Kafiti HA, Sedwick EM. Somatosensory evoked potentials from posterior tibial nerve and lumbosacral dermatomes. Electroencephalogr Clin Neurophysiol 1986;65(4):249–59 [DOI] [PubMed] [Google Scholar]
- 7.Tullberg T, Svanborg E, Isaacsson J, Grane P. A preoperative and postoperative study of the accuracy and value of electrodiagnosis in patients with lumbosacral disc herniation. Spine (Phila Pa 1976) 1993;18(7):837–42 [DOI] [PubMed] [Google Scholar]
- 8.Kafiti HA, Sedwick EM. Evaluation of dermatomal somatosensory evoked potential in the diagnosis of lumbo-sacral root compression. J Neurol Neurosurg Psychiatry 1987;50(9):1204–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsonidis C, Tsitsopoulos P, Sitzoglu K, Hadjiioannou P, Fotiou F, Anagnostopoulos I. Comparison of dermatomal SEPs and operative findings in lumbar disc disease. Int J Psychophysiol 1996;24(3):267–70 [DOI] [PubMed] [Google Scholar]
- 10.Righetti CA, Tosi L, Zanette G. Dermatomal somatosensory evoked potentials in the diagnosis of lumbosacral radiculopathies. Ital J Neurol Sci 1996;17(3):193–9 [DOI] [PubMed] [Google Scholar]
- 11.Machida M, Asai T, Sato K, Toriyama S, Yamada T. New approach for diagnosis in herniated lumbosacral disc. Dermatomal somatosensory evoked potentials (DSSEPs). Spine 1986;11(4):380–4 [DOI] [PubMed] [Google Scholar]
- 12.Dvonch V, Scarff T, Bunch WH, Smith D, Boscardin J. Dermatomal somatosensory evoked potentials: Their use in lumbar radiculopathy. Spine 1985;9(3):291–3 [DOI] [PubMed] [Google Scholar]
- 13.Borrego CJ, Trujillo JM, Ospina OL, Midina MP. Dermatomal somatosensory evoked potentials normative study and usefulness for the evaluation of radicular injury. J Electrophysiol Technol 1987;13:63–80 [Google Scholar]
- 14.Feinsod M, Blau D, Findler G, Hadani M, Beller AJ. Somatosensory evoked potentials to peroneal nerve stimulation in patients with herniated lumbar disc. Neurosurg 1982;11(4):506–11 [DOI] [PubMed] [Google Scholar]
- 15.Aminoff MJ. Segmentally specific somatosensory evoked potentials. Neurol Cin 1991;9(3):663–9 [PubMed] [Google Scholar]
- 16.Eisen A, Hoirch M, Moll A. Evaluation of radiculopathies by segmental stimulation and Somatosensory Evoked Potentials. Can J Neurol Sci 1983;10(3):178–82 [DOI] [PubMed] [Google Scholar]
- 17.Green J, Gildemeister R, Hazelwood C. Dermatomally stimulated somatosensory evoked potentials in the clinical diagnosis of lumbar disc disease. Clin Electroencephalogr 1983;14(3):152–60 [DOI] [PubMed] [Google Scholar]
- 18.Yazıcıoglu K, Ozgul A, Kalyon TA, Gündüz S, Arpacıoğlu O, Bilgiç F. The diagnostic value of dermatomal somatosensory evoked potentials in lumbosacral disc herniations: a critical approach. Electromyogr Clin Neurophysiol. 1999;39(3):175–81 [PubMed] [Google Scholar]
- 19.Tokuhaski Y, Satoh K, Funami S. A quantitative evaluation of sensory dysfunction in lumbosacral radiculopathy. Spine 1991;16(11):1321–8 [DOI] [PubMed] [Google Scholar]
- 20.Scarff TB, Dallman DE, Toleikis JR, Bunch WH. Dermatomal somatosensory evoked potentials in the diagnosis of lumbar root entrapment. Surg Forum 1981;32:489–491 [Google Scholar]
- 21.Seyal M, Sandhu LS, Mack YP. Spinal segmental somatosensory evoked potentials in lumbosacral radiculopathies. Neurology 1989;39(6):801–5 [DOI] [PubMed] [Google Scholar]
- 22.Dumitru D, Dreyfuss P. Dermatomal/segmental somatosensory evoked potential evaluation of L5/S1 unilateral/unilevel radiculopathies. Muscle Nerve 1996;19(4):442–9 [DOI] [PubMed] [Google Scholar]
- 23.Linguori R, Krarup C, Trojaborg W. Determination of the segmental sensory and motor innervation of the lumbosacral spinal nerves. An electrophysiological study. Brain 1992;115(Pt 3):915–34 [DOI] [PubMed] [Google Scholar]
- 24.Dykes RW, Terzis JK. Spinal nerve distributions in the upper limb: the organization of the dermatome and afferent myotome. Philos Trans R Soc Lond B Biol Sci 1981;293(1070):509–54 [DOI] [PubMed] [Google Scholar]
- 25.Aminoff MJ. Electrodiagnosis in clinical neurology: expert consult. New York: Churchill Livingstone; 1980 [Google Scholar]
- 26.Kraft GH. Dermatomal somatosensory evoked potentials in evaluation of lumbosacral spinal stenosis. Phys Med Rehabil Clin N Am 2003;14(1):71–5 [DOI] [PubMed] [Google Scholar]
- 27.Storm SA, Kraft GH. The clinical use of dermatomal somatosensory evoked potentials in lumbosacral spinal stenosis. Phys Med Rehabil Clin N Am 2004;15(1):107–15 [DOI] [PubMed] [Google Scholar]
- 28.Shields CB, Ping, Zhang Y, Shields LB, Burke DA, Glassman SD. Objective assessment of cervical spinal cord injury levels by transcranial magnetic motor-evoked potentials. Surg Neurol 2006;66(5):475–83 [DOI] [PubMed] [Google Scholar]
- 29.Kramer JK, Taylor P, Steeves JD, Curt A. Dermatomal somatosensory evoked potentials and electrical perception thresholds during recovery from cervical spinal cord injury. Rehabil Neural Repair 2010;24(4):309–17 doi: 10.1177/1545968309348312 [DOI] [PubMed] [Google Scholar]
- 30.Kramer JK, Moss A, Steeves JD, Curt A. Assessment of posterior spinal cord function with electrical perception threshold in spinal cord injury. J Neurotrauma 2008;25(8):1019–26 doi: 10.1089/neu.2007.0503 [DOI] [PubMed] [Google Scholar]
- 31.Katz RT, Toleikis RJ, Knuth AE. Somatosensory-evoked and dermatomal-evoked potentials are not clinically useful in the prognostication of acute spinal cord injury. Spine (Phila Pa 1976) 1991;16(7):730–5 [DOI] [PubMed] [Google Scholar]


