Abstract
Context
Referred pain has been observed in some patients after cordotomy, wherein noxious stimulus applied to a region rendered analgesic by cordotomy produces pain at a spot different from the one where the noxious stimulus is applied. We report a patient who had intramedullary spinal cord metastasis of extraskeletal small cell osteosarcoma, a rare form of metastatic disease, and experienced contralateral referred pain.
Findings
Initially, the patient had a mass in the left posterior neck region and later developed a large extradural mass at the C3–C7 level. The masses were excised, and the histological findings led to a diagnosis of small cell osteosarcoma. He underwent chemotherapy and radiation therapy. He experienced numbness in his left leg; subsequently, the numbness slowly spread up the thigh to the left side of the abdomen. When pinched in the numb area on the left side of the body, he felt as though he had been pinched in both that area and the corresponding area on the right side. A magnetic resonance imaging scan showed an enhancing lesion in the right side of the cord at the C6–C7 level.
Conclusion/clinical relevance
An intramedullary spinal cord metastasis can arise from primary extraskeletal small cell osteosarcoma and cause contralateral referred pain, especially in a mirror-image location. Contralateral referred pain may be caused by a subsidiary pathway comprising ascending chains of short neurons that link the dorsal horn neurons longitudinally and latitudinally.
Keywords: Cervical vertebrae, Neoplastic metastasis, Osteosarcoma, Referred pain, Spinal cord neoplasms, Intramedullary cordotomy
Introduction
Central nervous system metastases are being observed with higher incidence as better therapies have extended survival in patients with systemic cancer.1 Central nervous system metastases are an important cause of morbidity and mortality in patients with systemic cancers because they affect speech, coordination, cognitive function, behavior, quality of life, and survival.2 Most central nervous system metastases involve the brain parenchyma, leptomeninges, or dura.3–5 Less commonly, metastases involve the cranial nerves or spinal cord.3,5 Among them, intramedullary spinal cord metastasis is a rare clinical entity.6,7 Intramedullary spinal cord metastasis clinically affects only 0.1–0.4% of all cancer patients and accounts for only 0.8–3.9% of symptomatic metastatic tumors affecting the spinal cord.6,7 By comparison, extradural spinal cord compression due to metastasis occurs in 10% of all cancer patients.7 Lung cancer accounts for about half of all cases of intramedullary spinal cord metastasis.6,7 Breast cancer, melanoma, lymphoma, and renal cell carcinoma are also common sources of intramedullary spinal cord metastasis.6,7 However, intramedullary spinal cord metastases originating from mesenchymal tumors are rare.8,9
Referred pain is pain perceived at a spot other than the one where a noxious stimulus is applied and may be observed in many different clinical situations.10–13 However, this phenomenon occurs distinctly and frequently after cordotomy.11,12
Pain and thermal sensations are mediated by the lateral spinothalamic tract.14 The sensory afferent input to the spinal cord enters via the dorsal roots.14 The first-order neuron with its cell body in the dorsal root ganglion enters the spinal cord via the dorsal root.14 The first-order neuron synapses with the second-order neuron in the dorsal horn of the spinal cord.14 The second-order neuron then decussates in the anterior spinal commissure and ascends in the lateral funiculus as the lateral spinothalamic tract, and synapses with the ventral posterolateral nucleus of the thalamus.14 Spinal cord lesions can cause loss of pain and thermal sensation on the contralateral trunk and limbs.11,12,15 A cordotomy that involves sectioning of the anterior half of the spinal cord on one side eliminates input from the lateral spinothalamic tract to the contralateral side of the body.16 Historically, cordotomy was mostly used for treating unilateral cancer pain.11,12,16 Cordotomy can be performed either percutaneously or as an open procedure.16
In a small proportion of patients, although an analgesic effect is achieved, painful stimuli applied to parts of the body caudal to the lesion can cause pain sensation that is not felt at the point of stimulation but is felt in a normally innervated part of the body.11,12 Therefore, for instance, a pin prick on the left leg, where an analgesic effect is achieved, may cause referred pain in the normally innervated right leg. Various patterns of referred pain are observed. Any single form or any combination of the forms of reference may occur in any single patient.11,12 Pain may be referred (1) from the analgesic side where an analgesic effect is achieved to the contralateral analogous part of the body; (2) cranially or caudally to the normally innervated parts of the body; or (3) at one constant region of the body for every stimulus that is applied to the parts where an analgesic effect has been achieved. The patient may also experience sensation that seems to emanate from the site stimulated.11,12
We report the case of a patient who had intramedullary spinal cord metastasis of primary extraskeletal small cell osteosarcoma and developed contralateral referred pain.
Case report
A 16-year-old boy detected a mass in the left posterior neck region, which gradually increased in size. He visited a university-affiliated hospital six months later. Magnetic resonance imaging (MRI) examination showed the presence of an ill-defined mass that was surrounded by the trapezius, splenius, and levator scapulae muscles. The mass was isointense to the muscles on T1-weighted images and hyperintense to the muscles on T2-weighted images. T1-weighted gadolinium-enhanced images showed heterogeneous enhancement of the lesion (Fig. 1). The nuchal mass was excised, and the histological examination showed diffuse growth of round-to-oval tumor cells, with a round nucleus and small amounts of cytoplasm (Fig. 2). Small spindle cells were infrequently observed, and the mass had a focal hemangiopericytoma-like vascular pattern. Mitotic figures were frequently observed (up to 7 per high-power field) in cellular areas. The tumor cells were separated by pink matrix. Furthermore, areas of metaplastic bone formation were observed (Fig. 2). Glycogen was present in the cytoplasm of the some cells as evidenced by periodic acid-Schiff reactivity without diastase staining. Immunohistochemical studies showed that the tumor cells were diffusely positive for vimentin, focally positive for S-100, and weakly positive for epithelial membrane antigen but were negative for cluster of differentiation (CD)99. The initial interpretation of the lesion was synovial sarcoma and was based on its presentation as a soft tissue mass; therefore, the patient received adjuvant chemotherapy.
Figure 1.
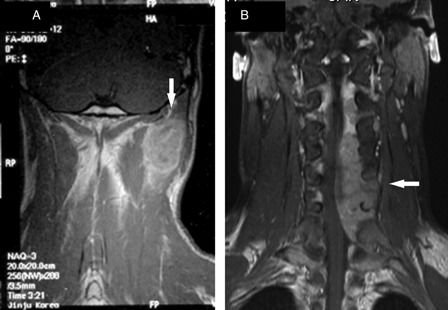
Coronal T1-weighted post-contrast magnetic resonance images of the cervical spine. Magnetic resonance imaging scan of the cervical spine showing a large enhancing mass (arrow) surrounded by the trapezius, splenius, and levator scapulae muscles (A). Magnetic resonance imaging scan of the cervical spine obtained 1 year later showing a large extradural enhancing lesion (arrow), which is displacing the spinal cord to the right (B).
Figure 2.
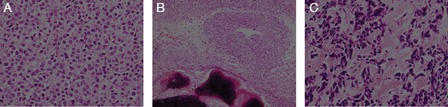
Histologic findings of the operative specimen. Tumor showing proliferation of undifferentiated small round cells with small amounts of cytoplasm (original magnification, ×400) (A). Formation of metaplastic bone among the tumor cells (original magnification, ×100) (B). The tumor cells are separated by pink matrix (original magnification, ×400) (C).
Ten months later, he developed numbness, pain, and weakness in the left arm. He was admitted to the neurosurgery ward in our hospital. Physical examination showed weakness and hypalgesia in the C4–C7 root region on the left side. An MRI scan of his cervical spine showed a large extradural uniformly enhancing lesion at the C3–C7 (Fig. 1). Subtotal surgical excision of the lesion was performed via a C2–C7 laminectomy on the left side. The microscopic findings of the extradural spinal tumor were similar to those of the previous nuchal mass, except for the absence of bone formation. Immunohistochemical studies showed that the tumor cells were diffusely positive for vimentin and focally positive for CD99 and S-100. The tumor cells were negative for cytokeratin, epithelial membrane antigen, CD34, desmin, and leukocyte common antigen. Cytogenetic studies did not show reciprocal translocation of the long arms of chromosomes 11 and 22. The histological findings for the nuchal mass and the extradural spinal metastasis made small cell osteosarcoma a much more likely diagnosis than synovial sarcoma.
After surgery, pulmonary metastasis was detected. The patient received chemotherapy and radiation therapy, resulting in considerable resolution of his neurological symptoms but little or no resolution of the remaining cervical lesion. Only sensory disturbances remained in the left lateral arm.
Two years later, he felt numbness in the left leg that slowly spread up to his thigh and the left side of the abdomen. When pinched in the numb area on the left side of the body, he felt as though he had been pinched in both that area and the corresponding area on the right side. An MRI scan showed a bright enhancing lesion (diameter, 7 mm) in the right side of the cord at the C6–C7 level (Fig. 3) consistent with metastatic disease. Two months later, neurological examination showed a sensory level to pain from C4 to C6, a spared area from C6 to T7, and impairment of pain sensation below T7 on the left side. Postural and vibratory senses were completely intact. However, the patient did not complain about the contralateral referred pain to the neurologist because he could hardly believe his senses at that time. He subsequently experienced numbness in the right arm, which slowly spread down the trunk to the right leg. Re-examination by the same neurologist showed a sensory level to pain and temperature from C5 to C6, a spared area from C6 to T5, and impairment of pain sensation below T5 on the right side. Sensory alteration of pain and temperature from T1 downwards on the left was detected. When painful pressure was applied to the hypalgesic area extending from T5 to L3 on the left side, the patient felt as though the pressure was being applied to both that area and the corresponding area on the right side. Eight months after the diagnosis of spinal metastasis, he expired while he was still undergoing chemotherapy.
Figure 3.
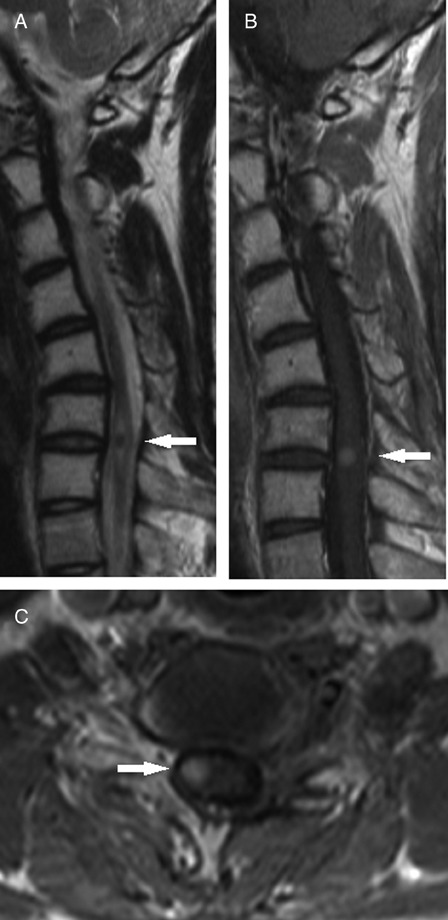
Magnetic resonance imaging scan of the intramedullary mass causing expansion of the cord and edema over several segments. T2-weighted image showing a spinal cord tumor (arrow) that is surrounded by perifocal edema and has an isointense signal (A). Sagittal (B) and axial (C) T1-weighted post-contrast images showing intense and uniform enhancement of a well-circumscribed intramedullary mass (arrow).
Discussion
This patient is of interest for two reasons. First, he developed primary small cell osteosarcoma in the soft tissue and then an intramedullary spinal cord metastasis in the cervical spine. Second, an intramedullary spinal cord mass caused contralateral referred pain.
Small cell osteosarcomas, which are a variant of osteosarcomas, are composed of small cells and varying amounts of osteoid.17 This rare tumor accounts for approximately 1–1.5% of osteosarcomas and has similar distribution with respect to sex, age, and skeletal aspects as conventional osteosarcoma.17 Most of these tumors occur in the metaphysis of long bones.17 However, primary extraskeletal small cell osteosarcomas that arise in tissues other than bone are rare.18,19 Small cell osteosarcomas tend to disseminate hematogenously to the lungs and other parenchymal organs.20 Initially, our patient had a soft-tissue mass in the posterior neck region and later developed an extradural metastasis in the cervical spine. Three years after diagnosis, he developed an intramedullary spinal cord metastasis. The underlying pathophysiology of intramedullary spinal cord metastasis can include hematogenous spread via the arterial system or Batson's venous plexus, direct extension through dura mater or nerve roots, lymphatic spread from contiguous vertebral metastases, or leptomeningeal invasion from embolic dissemination occurring through the subarachnoid space.7,21,22
Referred pain is observed in some patients after cordotomy, wherein a noxious stimulus applied to a region rendered analgesic by cordotomy produces pain at a spot different from the one where the noxious stimulus is applied.11,12 Researchers suggest that the referred pain experienced after cordotomy is caused by a subsidiary pathway comprising ascending chains of short neurons that link the dorsal horn neurons longitudinally and latitudinally.12 This subsidiary pathway is suppressed by feedback inhibition from second-order neurons and/or more central neurons of the nociceptive pathway.12 Under normal circumstances, the noxious impulses go through the usual nociceptive pathway and exert reflected and/or local feedback inhibition on the subsidiary pathway.12 Cordotomy inhibits the noxious impulses from following the nociceptive pathway and simultaneously releases the inhibitory control of the subsidiary pathway such that the impulses are redirected to the subsidiary pathway.12 The referred pain experienced after cordotomy and the referred pain associated with intramedullary tumors can share a common underlying neural mechanism.
Conclusion
In conclusion, an intramedullary spinal cord metastasis from primary extraskeletal small cell osteosarcoma can cause contralateral referred pain, especially in a mirror-image location like cordotomy.
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
References
- 1.Deangelis LM. Tumors of the central nervous system and intracranial hypertension and hypotension. In: Goldman L, Schafer AI, (eds.) Goldman's Cecil medicine. Philadelphia, PA: Elsevier/Saunders; 2011. p. 1247–57 [Google Scholar]
- 2.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res 2007;13(6):1644–7 [DOI] [PubMed] [Google Scholar]
- 3.Sneed PK, Kased N, Huang K, Rubenstein JL. Brain metastases and neoplastic meningitis. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG, (eds.) Abeloff's clinical oncology. Philadelphia: Churchill Livingstone/Elsevier; 2008. p. 827–44 [Google Scholar]
- 4.Kang SJ, Kim KS, Ha YS, Huh SY, Lee JH, Kim JK, et al. Diagnostic value of cerebrospinal fluid level of carcinoembryonic antigen in patients with leptomeningeal carcinomatous metastasis. J Clin Neurol 2010;6(1):33–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer 1963;16:781–7 [DOI] [PubMed] [Google Scholar]
- 6.Potti A, Abdel-Raheem M, Levitt R, Schell DA, Mehdi SA. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): clinical patterns, diagnosis and therapeutic considerations. Lung Cancer 2001;31(2–3):319–23 [DOI] [PubMed] [Google Scholar]
- 7.Connolly ES, Jr., Winfree CJ, McCormick PC, Cruz M, Stein BM. Intramedullary spinal cord metastasis: report of three cases and review of the literature. Surg Neurol 1996;46(4):329–37 [DOI] [PubMed] [Google Scholar]
- 8.Gasser TG, Pospiech J, Stolke D, Schwechheimer K. Spinal intramedullary metastases. Report of two cases and review of the literature. Neurosurg Rev 2001;24(2–3):88–92 [DOI] [PubMed] [Google Scholar]
- 9.Scollato A, Buccoliero AM, Di Rita A, Gallina P, Di Lorenzo N. Intramedullary spinal cord metastasis from synovial sarcoma. Case illustration. J Neurosurg Spine 2008;8(4):400. [DOI] [PubMed] [Google Scholar]
- 10.Katz J, Melzack R. Referred sensations in chronic pain patients. Pain 1987;28:51–9 [DOI] [PubMed] [Google Scholar]
- 11.Nathan PW. Reference of sensation at the spinal level. J Neurol Neurosurg Psychiatry 1956;19(2):88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaro T, Amakawa K, Kimura S, Arai T. Reference of pain following percutaneous cervical cordotomy. Pain 1993;53(2):205–11 [DOI] [PubMed] [Google Scholar]
- 13.Ray BS, Wolff HG. Studies on pain: “spread of pain”; evidence on site of spread within the neuraxis of effects of painful stimulation. Arch Neurol Psychiatry 1945;53(4):257–61 [Google Scholar]
- 14.Montgomery EB, Wall M, Henderson VW. Principles of neurologic diagnosis. Boston, MA: Little, Brown and Company; 1986 [Google Scholar]
- 15.Brazis PW, Masdeu JC, Biller J. Spinal cord. Localization in clinical neurology. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. p. 99–126 [Google Scholar]
- 16.Patterson JT, Hanbali F, Franklin RL, Nauta HJW. Neurosurgery. In: Sabiston DC, Townsend CM, (eds.) Sabiston textbook of surgery: the biological basis of modern surgical practice. Philadelphia, PA: Saunders/Elsevier; 2008. p. 2090–130 [Google Scholar]
- 17.Hameed M. Small round cell tumors of bone. Arch Pathol Lab Med 2007;131(2):192–204 [DOI] [PubMed] [Google Scholar]
- 18.Sipos EP, Tamargo RJ, Epstein JI, North RB. Primary intracerebral small-cell osteosarcoma in an adolescent girl: report of a case. J Neurooncol 1997;32(2):169–74 [DOI] [PubMed] [Google Scholar]
- 19.Robinson LH, Pitt MJ, Jaffe KA, Siegal GP. Small cell osteosarcoma of the soft tissue. Skeletal Radiol 1995;24(6):462–5 [DOI] [PubMed] [Google Scholar]
- 20.Ayala AG, Ro JY, Raymond AK, Jaffe N, Chawla S, Carrasco H, et al. Small cell osteosarcoma. A clinicopathologic study of 27 cases. Cancer 1989;64(10):2162–73 [DOI] [PubMed] [Google Scholar]
- 21.Guppy KH, Wagner F. Metastasis to the conus medullaris: case report. Neurosurgery 2006;59(5):E1148. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain MC, Eaton KD, Fink JR, Tredway T. Intradural intramedullary spinal cord metastasis due to mesothelioma. J Neurooncol 2010;97(1):133–6 [DOI] [PubMed] [Google Scholar]


