Summary
Exosomes are small (30–100 nm in diameter) extracellular membrane-enclosed vesicles released by different cell types into the extracellular space or into biological fluids by exocytosis as a result of fusion of intracellular multivesicular bodies with the plasma membrane. The primary function of exosomes is intercellular communication with both beneficial (physiological) and harmful (pathological) potential outcomes. Liver cells are exosome-releasing cells as well as targets for endogenous exosomes and exosomes derived from cells of other organs. Despite limited studies on liver exosomes, initial observations suggest that these vesicles are important in liver physiology and pathophysiology. In this review, we briefly summarize the recent findings on liver exosomes, their functions and significance for novel diagnostic and therapeutic approaches.
Keywords: Exosomes, Liver diseases, Biomarkers, Therapeutics
Introduction
A wide variety of cells release small (30–100 nm in diameter) membrane-enclosed vesicles termed exosomes into the extracellular milieu. Over the past several years, there has been increasing interest in the biological properties and pathophysiological relevance of these extracellular vesicles derived from liver cells, principally hepatocytes and cholangiocytes. In this review, we briefly summarize the recent findings on liver exosomes, their functions and significance for novel diagnostic and therapeutic approaches.
Formation, composition, and functions of exosomes
Exosomes are derived from the internal vesicles of multivesicular bodies (MVBs). While some MVBs are degraded in lysosomes, other MVBs fuse with the plasma membrane and release their content of microvesicles in an exocytic manner into biological fluids such as blood, urine, bile, saliva in vivo, or into culture medium in vitro [1–3] (Fig. 1). The number of exosomes present in biological fluids is high; in serum, it is estimated to be about 3 million exosomes per microliter [3].
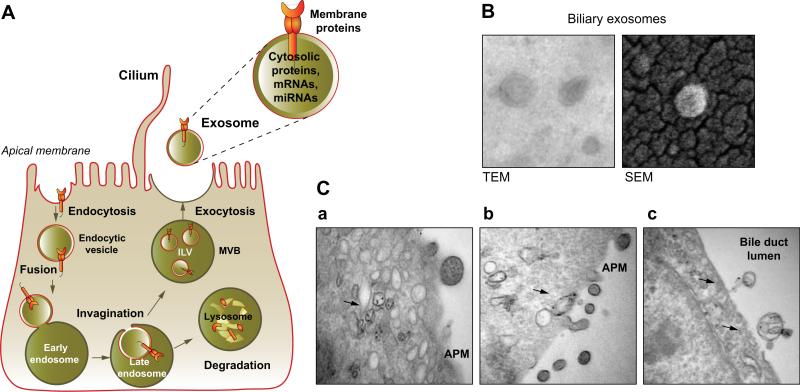
Fig. 1. Exosome release.
(A) Exosomes containing membrane and cytosolic proteins, mRNAs, and miRNAs, are derived from the multivesicular body (MVB) sorting pathway. Membrane proteins are oriented in a fashion (extracellular region out) that permits profound biological autocrine and paracrine effects. (B) Exosomes isolated from rat bile have a cup- or “deflated football”-shaped morphology by transmission electron microscopy (TEM), but they have a perfectly round shape by scanning electron microscopy (SEM). (C) In cholangiocytes of mouse liver, MVBs containing exosomes (arrows) (a) move to the apical plasma membrane (APM) (b), and release exosomes into the bile duct lumen by exocytosis (c). (B and C adapted from [6], with permission).
Morphologically by electron microscopy, exosomes typically are vesicles with distinctive cup- or “deflated football”-shaped morphology (Fig. 1); the shape may reflect a collapse of vesicles during sample preparation, because cryo-electron and scanning electron microscopy show a perfectly rounded shape of isolated exosomes (Fig. 1) [4]. Biochemically, exosomes contain common “marker” proteins (e.g., tetraspanins such as CD9, CD10, CD26, CD53, CD63, CD81, CD82; endosome-associated proteins that are involved in MVB biogenesis, Alix and TSG101; cytoplasmic heat shock proteins, Hsc70 and Hsp90), and cell-type specific proteins and nucleic acids including mRNAs, microRNAs (miRNAs) and other non-coding RNAs, the composition of which depends on the functional state of the cells (e.g., rested, stimulated, stressed, transformed, etc.) [1,3]. The estimated total cargo per exosome is about 100 proteins and 10,000 net nucleotides [3]. As of January 2012, 11,261 proteins, 2375 mRNAs and 764 miRNAs have been identified in association with exosomes suggesting their great heterogeneity and uniqueness [5].
The primary function of exosomes is intercellular communication; i.e., to shuttle various signaling molecules between neighboring and distant cells [1–3]. Exosomes can interact with target cells via unknown receptors, including receptors localized to primary cilia, and activate downstream intracellular signaling pathways [1–3,6]. They can also directly fuse with the cell membrane integrating exosomal membrane proteins into the plasma membrane or be endocytosed delivering their cargo into the cytoplasm of the recipient cells [1,3,4] (Fig. 2).
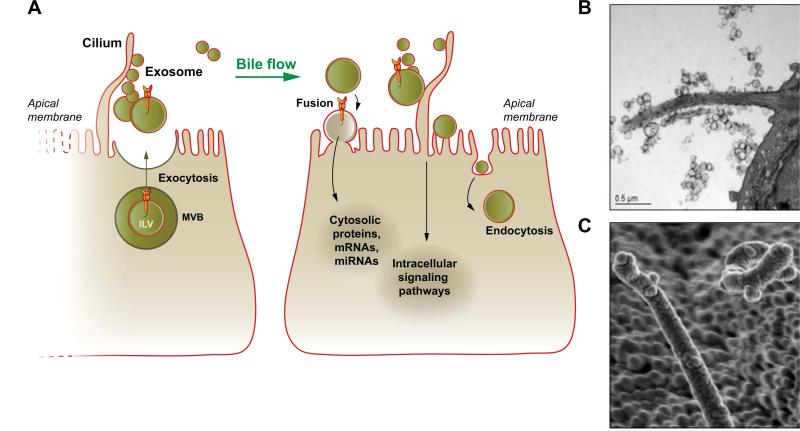
Fig. 2. Exosomes in intercellular signaling.
(A) In the liver, exosomes derived from hepatocytes and cholangiocytes are transported by bile flow to target cholangiocytes with which they may interact via several mechanisms depending on their cargo and biological properties. They can fuse with the plasma membrane and deliver their content into the cytoplasm of a target cell; interact with receptors on the apical plasma and ciliary membrane inducing intracellular signaling; and endocytosed for recycling. (B and C) Biliary exosomes surround and attach to cholangiocyte cilia in mouse liver as viewed by TEM (B) and SEM (C), supporting the involvement of exosomes and cilia in mechanisms of intercellular signaling. (B and C adapted from [6], with permission).
Exosomes are heterogenous in size and content and demonstrate different biological effects and targeting specificities. They can influence the immune system, act as signaling complexes, transfer receptors from one cell to another, convey specific mRNAs, miRNAs, and proteins into the cytoplasm of recipient cells, and, as a result, impact numerous physiological processes [2–4]. Exosomes also facilitate viral transport, spread cell damage, and stimulate malignant transformation [4,7].
Exosomes in liver physiology and disease
Both types of liver epithelia (i.e., hepatocytes and cholangiocytes), natural killer T (NKT) cells, hepatic stellate cells, adult liver stem cells, and hepatic sinusoidal endothelial cells are exosome-releasing and/or exosome-targeting cells [6,8–12]. Hepatocyte- and cholangiocyte-derived exosomes are involved in hepatocyte-to-cholangiocyte, cholangiocyte-to-cholangiocyte and cholangiocyte-to-hepatic sinusoidal endothelial cell communication [6,10]. Biliary exosomes affect the ERK signaling pathway and miR-15a expression in cholangiocytes and inhibit their proliferation [6]. Hepatic stellate cells and rat cholangiocytes release exosomes containing biologically active Hedgehog ligands and alter gene expression in target hepatic sinusoidal endothelial cells [10]. Exosomes derived from mouse hepatocytes contain several members of the cytochrome P450, UDP-glucuronosyl-transferase and glutathione S-transferase drug-metabolizing protein families and are presumably involved in detoxification of drugs and endogenous toxic substances in target cells [9].
Liver cells are also targets for exosomes derived from the cells of other organs and tissues. For example, exosomes derived from the intestinal epithelial cells carry prostaglandin E2 and induce liver immune tolerance by communicating directly with the liver NTK cells via the Wnt/β-catenin signaling pathway [8].
To date, the information on the potential involvement of exosomes in liver diseases is limited to the pathogenesis of hepatocellular carcinoma (HCC) [13], viral hepatitis (HCV) [14] and liver inflammation [15]. It has been reported that the human hepatoma cell line, Hep3B, releases exosomes containing a specific subset of miRNAs that are taken up by an hepatocellular carcinoma cell line, HepG2, resulting in inhibition of transforming growth factor β activated kinase-1 (TAK1), loss of which has been implicated in hepatocarcinogenesis [13]. A potential role for exosomes in viral hepatitis is supported by observations that the exosomal pathway is required for release of HCV from infected cells. Also, HCV envelope proteins are associated with exosomes, and exosome-associated viral RNA is present in plasma of HCV-infected patients [14]. The role of exosomes in liver inflammation is suggested from a study demonstrating that repeated injection of exosomes isolated from peripheral blood of mice fed a high-fat diet to mice fed a regular diet resulted in accumulation in the liver of activated immature myeloid cells, CD11b+Ly6ChiLy6G−, that caused chronic inflammation and promoted obesity-related disorders, such as fatty liver disease [15].
Liver exosomes as diagnostic and prognostic biomarkers
Currently, exosomes from urine and blood are considered as noninvasive (urine) and minimally invasive (blood) sources of molecular biomarkers for early detection and prognosis of various diseases, including diseases of the liver [3]. This approach is based on the observations that exosomes present in blood and urine contain specific proteins, mRNAs and miRNAs derived from multiple organs, including liver, and that the exosomal cargo may be associated with a given disease [16,17]. For example, in animal models of liver injury that resemble liver damage in viral hepatitis, changes in the abundance and content of liver-derived exosomes were observed in both blood and urine. In particular, in D-galactosamine (DGAL)-treated rats (an animal model for acute liver injury), the number of CD10-, CD26-, and CD81-positive uri-nary exosomes was dramatically reduced [17]. In the same DGAL model and in acetaminophen-treated rats, the level of exosome-associated liver-specific mRNA in serum was increased and this increase correlated with evidence of liver injury [i.e., alanine (ALT) and aspartate (AST) aminotransferase levels]. The comparative analysis of exosomal liver-specific mRNAs with serum transaminases indicated that mRNAs were more specific and more sensitive biomarkers of liver injury [16]. The serum levels of hepatocyte-derived exosome-associated miR-122, miR-148a, and miR-194 were elevated in patients with liver injury due to acute rejection after liver transplantation; importantly, their levels appeared to rise earlier than aminotransferase levels [18].
Another example of the potential usefulness of exosomes as biomarkers of disease is assessing exosomal miRNAs as an indication of hepatocyte injury in alcoholic, drug-induced, and inflammatory liver diseases [19]. An increase in exosomal miR-122, which is abundant in hepatocytes, and miR-155, which regulates inflammation in immune cells, was observed in the serum of mouse models of alcoholic liver disease, drug-induced liver injury, and Toll-like receptor ligand-induced inflammatory cell-mediated liver damage. A characteristic expression of these two miRNAs allowed differentiation between hepatocyte injury and inflammation [19]. The miR-17-92 cluster, especially miR-92a, is markedly overexpressed in HCC, and is associated with cancer development and progression. On the other hand, the relative amount of exosomal miR-92a is significantly decreased in the plasma of HCC patients compared with healthy donors. Although, the pathophysiological significance of the decrease of miR-92a in plasma is still unknown, deregulation of miR-92a expression in cells and plasma is implicated in the development of HCC, and the level of exosomal miR-92a in plasma is considered as a potential diagnostic marker of HCC [7].
An increase in the level of CD10 protein was observed in uri-nary exosomes obtained from glycine N-methyltransferase knockout mice, an animal model of chronic liver injury associated with steatosis, fibrosis, and HCC. The increased level of CD10 protein was detected in very early disease stages and was consistently observed over time course of disease progression [17]. An increase of CD81 protein in serum exosomes of patients with chronic HCV was presumably associated with inflammation and severity of fibrosis [20]. The summary of recent observations on exosomes as promising diagnostic and prognostic biomarkers in liver diseases is shown in Table 1.
Table 1.
Exosomes as biomarkers of liver diseases.
| Liver disease | Source of exosomes | Exosomal cargo | Observation | Reference |
|---|---|---|---|---|
| Liver injury induced by DGAL | Urine | CD10, CD26, CD81 | Decreased number of exosomes | [17] |
| Liver injury associated with steatosis, fibrosis, HCC | Urine | CD10 | Increased number of exosomes | [17] |
| HCV | Serum | CD81 | Increased number of exosomes | [20] |
| Liver injury induced by DGAL/APAP | Serum | Liver-specific mRNAs | Increased number of exosomes | [16] |
| Liver injury/rejection | Serum | miR-122, miR-148a, miR-194 | Increased number of exosomes | [18] |
| Alcoholic, drug-induced, inflammatory liver diseases | Serum, plasma | miR-122, miR-155 | Increased number of exosomes | [19] |
| HCC | Plasma | miR-92a | Decreased number of exosomes | [7] |
DGAL, D-galactosamine; APAP, acetaminophen; HCC, hepatocellular carcinoma; HCV, viral hepatitis.
Exosomes as novel therapeutics for liver diseases
Recently, progress has been made in developing novel, exosome-based therapeutic approaches for treating different types of cancer [3], including liver tumors. Based on observations that exosomes released by HCC cells under stress condition contain heat shock proteins (HSPs) that can improve tumor immunogenicity and induce NKT cell antitumor responses, the potential use of HSP-bearing exosomes for immunotherapy of HCC was proposed [21]. Exosomes derived from human adult liver stem cells (HLSC) inhibited the growth and survival of HepG2 and primary HCC cells in vitro by delivering antitumor miRNAs [12]. HLSC-derived exosomes also induced proliferation and apoptosis resistance of human and rat hepatocytes in vitro and accelerated the morphological and functional recovery of the rat liver in a model of 70% hepatectomy in vivo, by delivering a specific subset of 35 mRNAs that are associated with the regulation of transcription, translation, proliferation, and apoptosis of target cells [22].
Exosomes derived from human umbilical cord mesenchymal stem cells relieved liver fibrosis induced by carbon tetrachloride (CCL4) when they were administered into the mouse fibrotic liver for 4 hours. As a result of such treatment, the surface fibrous capsules were reduced, their textures softened, hepatic inflammation and collagen deposition were reduced, and collagen type I and III decreased. These changes and the recovered levels of serum AST suggested a positive therapeutic effect of exosomes on fibrotic liver [23].
Human hepatoma cells HuH7, stably transfected with small interfering RNA (siRNA) against viral entry receptor CD81, release siRNA-containing exosomes which in cell co-culture experiments shuttle this siRNA to non-transfected HuH7 cells inhibiting CD81 expression in target cells. The shuttling of exosomes containing exogenous siRNA between liver cells in vivo was demonstrated in immunodeficient NOD/SCID mice engrafted with human hepatoma cells producing CD81 siRNA. Exosomes containing this siRNA were released by human hepatoma cells and caused suppression of CD81 expression in target hepatocytes. This observation suggests the potential use of exosomes containing siRNAs in RNA interference-based therapies against HCV and other liver diseases [11].
It has been also demonstrated that murine bone marrow stromal cells pulsed with homologous tumor-derived exosomes inhibit proliferation of liver cancer cells, suggesting the potential use of exosomes as a novel antitumor treatment [24]. The summary of recent observations on exosomes as potential novel therapeutics in liver diseases is presented in Table 2.
Table 2.
Exosomes as therapeutics in liver diseases.
| Liver disease | Donor cells | Exosomal cargo | Target cells | Application | Outcome | [Ref.] |
|---|---|---|---|---|---|---|
| HCC | HCC cells | Heat shock proteins | NKT cells | HCC | Improve tumor immunogenicity, induce NKT cell antitumor response | [21] |
| HCC | Human adult liver stem cells | Antitumor miRNAs | HepG2, primary HCC cells, hepatocytes | HCC | Inhibit the growth and survival of HCC cells, induce proliferation and apoptosis of human and rat hepatocytes | [12, 22] |
| CCL4-induced fibrotic liver | Human umbilical cord mesenchymal stem cells | Unknown | Hepatocytes | Hepatic inflammation and collagen deposition | Alleviate hepatic inflammation and collagen deposition | [23] |
| HCV | HuH7, HuH6, HepG2, hepatocytes | miRNA, siRNAs | HuH7, HuH6, HepG2, hepatocytes | HCV | Exchange exosomes with potential therapeutic effects | [11] |
| Liver cancer | Murine bone marrow stromal cells pulsed with homologous tumor-derived exosomes | Unknown | Liver cancer cells | Liver cancer | Antitumor effect | [2] |
There are several other potential approaches for the use of exosomes in therapeutic applications for liver diseases including: (i) engineering exosomes containing specific biomolecules or small molecule drugs and delivering them to the target cells; (ii) monitoring the efficacy of treatment by using exosomes as molecular biomarkers of disease development, progression and cure; and (iii) stimulating the release of exosomes containing unneeded or harmful molecules.
Conclusions
As this review outlines, exosomes are important molecular entities involved in cell-to-cell communication within the liver, contributing to liver physiology and pathophysiology. Exosomes also hold great potential as novel diagnostic and prognostic molecular biomarkers of liver diseases and as novel therapeutic modalities. However, although significant advances have been made, many questions remain. Very little is known about the precise molecular mechanisms of exosome biogenesis, release, targeting, and interactions with target cells within the liver. The existing data also suggest that liver exosomes are heterogenous vesicles derived from different hepatic cell types and, because of their heterogeneity, likely perform specific functions in liver health and disease that remain to be clarified.
Key Points.
Exosomes are small membrane-enclosed vesicles released by cells into the extracellular space or into biological fluids
Exosomes containing cell-type specific proteins, mRNAs, and miRNAs are involved in intercellular communication by delivering their cargo to the recipient cells
Liver cells are exosome-releasing and exosome-targeting cells
Exosomes contribute to the pathogenesis of hepatocellular carcinoma, viral hepatitis, and hepatic inflammation
Exosomes hold great potential as diagnostic and prognostic molecular biomarkers of liver diseases and as novel therapeutic modalities
Acknowledgments
Financial support
This work was supported by the National Institutes of Health (DK24031 and DK57993 for NFL), by the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), and by the Mayo Foundation.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/authorsrights
Conflict of interest
The authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65:342–347. doi: 10.1016/j.addr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastroin-test Liver Physiol. 2010;299:G990–G999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohno SI, Ishikawa A, Kuroda M. Roles of exosomes and microvesicles in disease pathogenesis. Adv Drug Deliv Rev. 2013;65:398–401. doi: 10.1016/j.addr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, Wang Q, et al. Intestinal mucus-derived nanoparticles mediate activation of Wnt/beta-catenin signaling plays a role ininduction of liver NKT cell anergy. Hepatology. 2013;57:1250–1261. doi: 10.1002/hep.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:e322. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut. 2012;61:1330–1339. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 12.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamai K, Shiina M, Tanaka N, Nakano T, Yamamoto A, Kondo Y, et al. Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway. Virology. 2012;422:377–385. doi: 10.1016/j.virol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Deng ZB, Liu Y, Liu C, Xiang X, Wang J, Cheng Z, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412–1420. doi: 10.1002/hep.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wetmore BA, Brees DJ, Singh R, Watkins PB, Andersen ME, Loy J, et al. Quantitative analyses and transcriptomic profiling of circulating messenger RNAs as biomarkers of rat liver injury. Hepatology. 2010;51:2127–2139. doi: 10.1002/hep.23574. [DOI] [PubMed] [Google Scholar]
- 17.Conde-Vancells J, Rodriguez-Suarez E, Gonzalez E, Berisa A, Gil D, Embade N, et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteomics Clin Appl. 2010;4:416–425. doi: 10.1002/prca.200900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farid WR, Pan Q, van der Meer AJ, de Ruiter PE, Ramakrishnaiah V, de Jonge J, et al. Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl. 2012;18:290–297. doi: 10.1002/lt.22438. [DOI] [PubMed] [Google Scholar]
- 19.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welker MW, Reichert D, Susser S, Sarrazin C, Martinez Y, Herrmann E, et al. Soluble serum CD81 is elevated in patients with chronic hepatitis C and correlates with alanine aminotransferase serum activity. PLoS One. 2012;7:e30796. doi: 10.1371/journal.pone.0030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma B, Jiang H, Jia J, Di L, Song G, Yu J, et al. Murine bone marrow stromal cells pulsed with homologous tumor-derived exosomes inhibit proliferation of liver cancer cells. Clin Transl Oncol. 2012;14:764–773. doi: 10.1007/s12094-012-0860-9. [DOI] [PubMed] [Google Scholar]