Abstract
The pumping innate to collecting lymphatic vessels routinely exposes the endothelium to oscillatory wall shear stress and other dynamic forces. However, studying the mechanical sensitivity of the lymphatic endothelium remains a difficult task due to limitations of commercial or custom systems to apply a variety of time-varying stresses in vitro. Current biomechanical in vitro testing devices are very expensive, limited in capability, or highly complex; rendering them largely inaccessible to the endothelial cell biology community. To address these short-comings, the authors propose a reliable, low-cost platform for augmenting the capabilities of commercially available pumps to produce a wide variety of flow rate waveforms. In particular, the Arduino Uno, a microcontroller development board, is used to provide open-loop control of a digital peristaltic pump using precisely-timed serial commands. In addition, the flexibility of this platform is further demonstrated through its support of a custom-built cell-straining device capable of producing oscillatory strains with varying amplitudes and frequencies. Hence, this microcontroller development board is shown to be an inexpensive, precise, and easy-to-use tool for supplementing in vitro assays to quantify the effects of biomechanical forces on lymphatic endothelial cells.
Keywords: wall shear stress, peristaltic pump, lymphatic endothelial cells, Arduino, microcontroller platform
1. Introduction
Most tissues in the body are supported by the lymphatic system for a variety of functions, including the regulation of fluid balance, the removal of particulate matter from the interstitium, as well as the transport of fat from the intestine to the blood, among others. Collecting lymphatic vessels contract much like the heart to promote flow, with transport occurring through the coordinated intrinsic pumping of individual vessel segments known as lymphangions (Zawieja, 2009; Nipper and Dixon, 2011). These contractions continuously expose luminal lymphatic endothelial cells (LECs) to oscillatory wall shear stress (with frequencies typically below 1 Hz) (Dixon et al., 2006) as well as other forces, all of which have been shown to affect vessel pumping (Hargens and Zweifach, 1977; Gashev et al., 2002; Davis et al., 2009). In addition, several extrinsic factors also work to promote lymph flow such as skeletal muscle contraction, respiration, and interstitial fluid formation. Because of the numerous mechanisms that affect lymph transport in vivo, flow rates can vary quite drastically under physiologic conditions; accordingly, the intrinsic lymphatic pump must actively respond to these varying in vivo loads. Many of these responses are, in part, endothelium mediated, and utilize similar mechanisms that have been known to regulate vasoactive responses in the blood vasculature (Gashev et al., 2002; Kawai et al., 2010; Bohlen et al., 2009). However, the magnitudes of these forces experienced by LECs in vivo are significantly less than those in similar-sized blood microvessels [Table 1] (Hargens and Zweifach, 1977; Lipowsky, 2005; Dixon et al., 2006), and recent evidence suggests that their unique biomechanical environment is essential in guiding lymphatic development (Chen et al., 2012; Sabine et al., 2012). Moreover, the ability to apply custom time-varying shear stresses is useful in the context of elucidating the functional response of LECs, which experience rapid and often irregular changes in the mechanical environment in vivo.
Table 1.
Typical pressure and wall shear stress (WSS) values in the microvessels of the body.
| Arterioles | Venules | Capillaries | Collecting Lymphatics | |
|---|---|---|---|---|
| Pressure (mmHg) | 75–100 | 20–30 | 30–40 | 3–4 |
| Average WSS (dynes/cm2) | 50–60 | 10–30 | 20–50 | < 1 |
To study the specific effects of fluid shear stress on LECs in vitro, investigators have developed an assortment of devices for exposing endothelial cells (ECs) to dynamic shear stress waveforms (Helmlinger et al., 1991; Peng et al., 2000; Estrada et al., 2011). However, these systems are custom-built and fairly complex, rendering them largely inaccessible to the broader EC community. Commercial systems are available for applying fluid shear stress waveforms (e.g. Flexcell®, Ibidi, Vacu-Cell™); however, these commercial systems are expensive and have limited flexibility for applying various dynamic flow rates. In particular, these systems are incapable of producing anything other than small subset of pulsatile or oscillatory waveforms (e.g. a square wave or sine wave). Thus, the authors report a technique for enhancing the capabilities of commercially-available pumps to generate a wide variety of flow waveforms, including those seen in lymphatics in vivo. The proposed system is a reliable, inexpensive, and easy-to-use platform for augmenting a digital peristaltic pump with an open-loop control system to produce time-varying flow rates. Additionally, the authors further demonstrate the flexibility of this platform by using it to support a custom-built cell-straining device capable of producing oscillatory strains with a multitude of amplitudes and frequencies.
2. Methods and Results
2.1. Arduino Uno Development Board
The Arduino Uno development board is based on the Atmel ATmega328, an 8-bit, 16 MHz microcontroller with 14 digital input/output (I/O) pins, 6 of which are capable of pulse-width modulation (PWM), as well as a 6-channel, 10-bit analog-to-digital converter. Digital communication capabilities include UART TTL serial, SPI serial, and two-wire interface serial (I2C). The Arduino development platform features a cross-platform, Java-based IDE as well as a C/C++ library which offers high-level access to hardware functions.
2.2. Programmable Fluid Shear Stress Via Peristaltic Pump
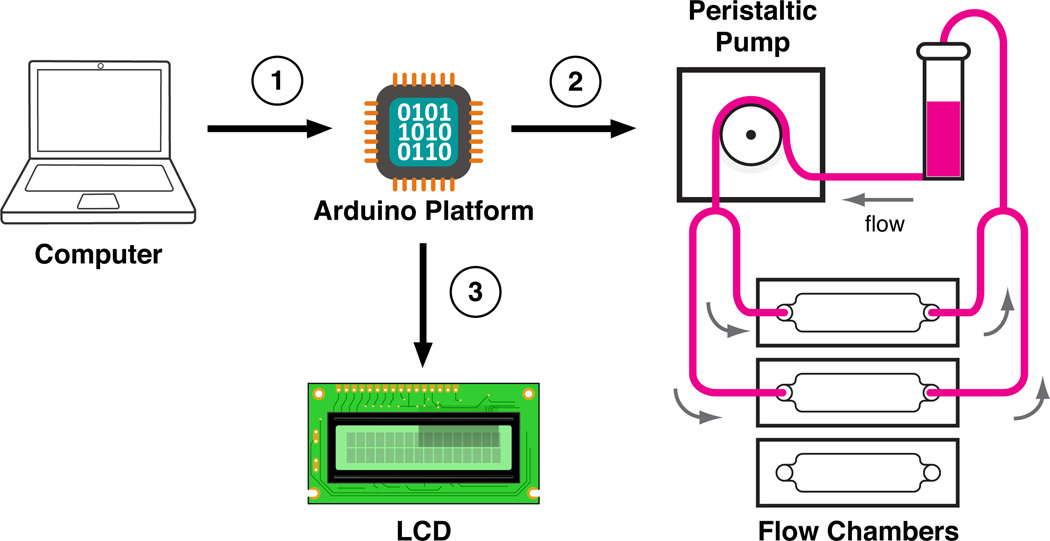
Figure 1 shows the flow system used to generate time-varying fluid shear stresses on an LEC monolayer grown in a parallel-plate flow chamber using an Ismatec REGLO Digital MS-4/12 peristaltic pump (IDEX Health and Science, Glattbrugg, Switzerland). After entering a desired periodic shear stress function and experimental duration via a custom Python script (Supplemental Appendix), the required peristaltic pump speed commands are sent serially to the Arduino Uno. The Arduino Uno stores these commands on its local EEPROM, which allows experiments to be conducted entirely with the Arduino Uno development board. Once running, the Arduino Uno recreates the desired waveform by sending each of the locally-stored pump commands at specific time increments to the peristaltic pump via a TTL to RS232 level shifter (SparkFun Electronics, Boulder, CO). The experiment progress is displayed on an LCD, which is operated with Arduino’s LiquidCrystal library.
Figure 1.
Outline of the flow system: (1) the Arduino platform is programmed by a personal computer (PC) over USB to create a desired fluid shear stress (flow rate) waveform for a certain duration of time; (2) once programmed, the Arduino can independently control the peristaltic pump via an RS232 serial connection with precise timing in an open-loop fashion; (3) the experimental progress is displayed on a connected LCD screen for the experimenter to monitor.
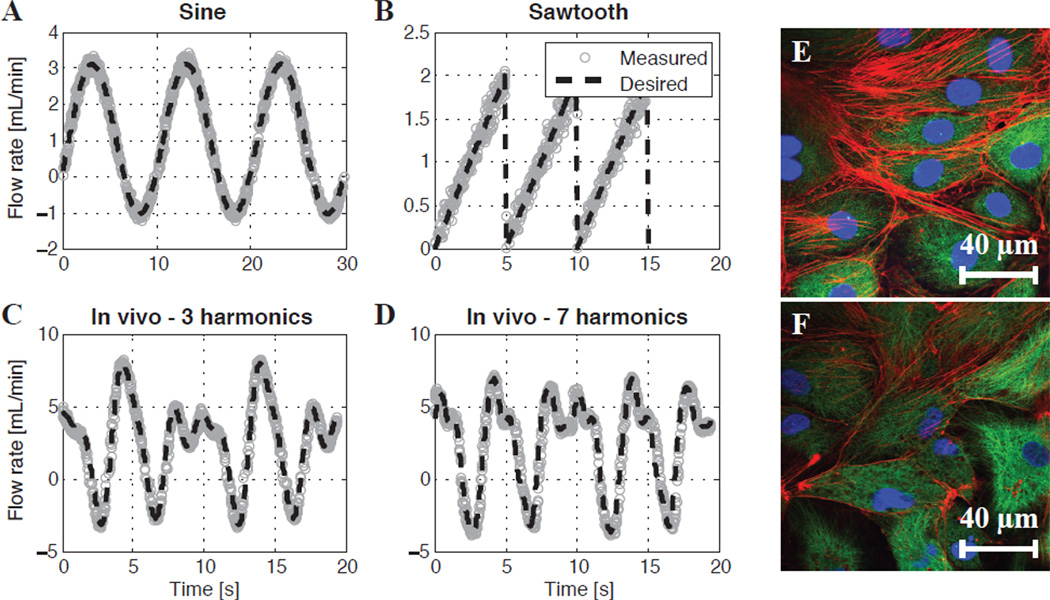
In order to mimic the user-desired waveform, the Python script generates a certain number of discrete pump commands based on a suitable time delay for the pump. In this way, the Arduino Uno will avoid sending commands too fast for the peristaltic pump to implement. Upon initialization of the experiment, the Arduino Uno will automatically refine this delay time using a self-calibration routine that takes extraneous pump commands (e.g. start and change direction) into account (Supplemental Appendix). In this way, the overall period of the desired waveform is ensured to be accurate. Figure 2a–d shows several examples of desired flow rate waveforms compared with the measured flow rate waveforms on the peristaltic pump. The measured flow rate is obtained by acquiring the speed of the pump rollers via an internal pump encoder which returns a square wave whose frequency is proportional to the speed. Accordingly, because a peristaltic pump is a positive displacement pump, the velocity of the rollers is linearly proportional the mean flow rate through the corresponding tube (according to its internal diameter). Overall, good agreement is achieved between the desired and measured flow rates.
Figure 2.
Measured vs. desired flow rates for the digital peristaltic pump being controlled by the Arduino Uno (a–d). The pump system accurately produces several desired waveforms: (a) a sine wave, (b) a sawtooth wave, (c) a waveform with the top three harmonics of a rat lymphatic waveform measured in vivo, and (d) a waveform with the top seven harmonics of a rat lymphatic waveform measured in vivo (Kassis et al., 2012). (e) Controlled LECs cultured under a static, no-flow condition. (f) LECs exposed to the sine wave found in (a) for 24 hours. For the false-colored images: the red channel is F-actin (phalloidin), the green channel is tubulin (α-β tubulin), and the blue channel is the cell nucleus (DAPI).
2.3. Adjustable Cell Monolayer Straining
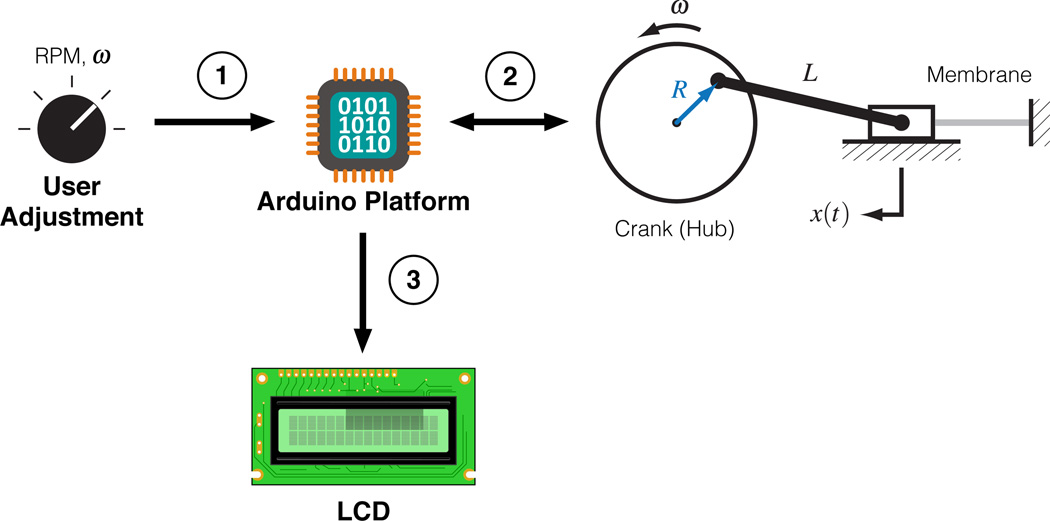
The authors have also developed a cell-straining device [Fig. S1a] capable of imposing a static or oscillatory strain of varying magnitude and frequency [Fig. 3]. The amplitude of strain may be adjusted by adjoining one end of the connecting rod to the hub at points of varying radii, while the frequency of strain may be adjusted by regulating the speed of the DC motor powering the hub. The details of this displacement may be seen through Eq. (1),
| (1) |
where x(t) is the displacement of the membrane, R is the radius of the crank, ω is the rotational velocity of the crank, and L is the length of the connecting rod. Velocity feedback of the hub (crank) is acquired with an infrared photo interrupter (SparkFun Electronics, Boulder, CO), which outputs a rising voltage edge upon sensing one of the twelve slots located around the outer perimeter of the hub. Time between rising edges is measured by the Arduino Uno, which can then calculate the speed for display in revolutions per minute (rpm) on the attached LCD screen (Supplemental Appendix).
Figure 3.
Outline of the cell-straining device: (1) the user adjusts a potentiometer knob that controls the PWM frequency output of a motor driver board; (2) the PWM frequency from the motor driver board controls the speed of the crank, which dictates the frequency of strain, and this measurement is fed back to the Arduino Uno via a photo interrupter; (3) the Arduino Uno uses the photo interrupter as an encoder to calculate the speed of the crank then displays it on an attached LCD.
3. Discussion
In order to create time-varying waveforms on a digital peristaltic pump, such as the one used in this study, one could use a personal computer (PC) to send out serial (flow rate) commands at sufficiently-fast intervals to approximate the desired waveform. However, typical PC operating systems are not adept at precise timing, and PCs can be difficult to operate near or inside of cell culture incubators. Conversely, another option would be a conventional microcontroller: the inherent simplicity of these embedded systems give them very desirable timing characteristics and thus have been useful in a wide variety of biomedical applications (Sun et al., 1992; Bhaskaran et al., 1993; Frijns et al., 1994). However, this raw simplicity in hardware comes at the expense of writing the required software, which is typically written in either assembly or C/C++ and often requires intimate knowledge of the particular hardware architecture. This complexity in writing the software makes it difficult for non-experts to pick up and use these systems without a considerable time investment. Due to these limitations, the authors have chosen to use the Arduino Uno development board ($30, http://arduino.cc), which is an open-source electronics prototyping platform based on user-friendly hardware and software. Although based on an 8-bit Atmel microcontroller, the Arduino Uno offers high-level libraries that allows programming to be more intuitive and does not require familiarity with the low-level hardware registers usually required for I/O. In fact, its ease-of-use has made the Arduino platform recently popular with artists, hobbyists, and students interested in electronic automation (Kushner, 2011).
The peristaltic pump system demonstrated in this study is an example of a successful augmentation with the Arduino Uno. Although the minimum delay time between commands (~30–50 ms) required by this pump prohibits waveforms with high temporal resolutions to be used, this system can adequately reproduce the oscillatory flow functions seen in the lymphatics [Fig. 2c–d]. However, this limitation is unique to digital pumps such as these; pumps with an analog input would be limited only by the speed of their electromechanical dynamics, which could allow various vascular waveforms to be produced. Analog-capable pumps could make use of the Arduino Uno’s PWM output and an inexpensive H-bridge circuit to produce time-varying flows.
In addition to augmenting pump-based systems, the authors further demonstrate the flexibility of this platform by supporting a custom-built cell-straining device for mimicking the strain amplitudes and frequencies experienced by LECs within collecting lymphatics. The device can produce strains between 10–50% and frequencies exceeding 1 Hz, which is within the observed range for the lymphatics (Hargens and Zweifach, 1977; Dixon et al., 2006; Davis et al., 2009). The Arduino Uno’s precise timing capabilities (D’Ausilio, 2011), coupled with its ability to use hardware interrupts, allows the system to measure the strain frequency and display it on an attached LCD for user feedback. Thus, the authors propose the Arduino Uno as an inexpensive, flexible, and adaptable platform for the development of in vitro assays to quantify the effects of biomechanical forces on the lymphatic endothelium.
Supplementary Material
Acknowledgment
This material is based upon work supported under a National Science Foundation Graduate Research Fellowship. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This study was also funded by the National Institutes of Health (R00HL091133).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None.
References
- Bhaskaran A, Ferris CD, Sandige RS. A sampling and storage system for arbitrary biomedical waveforms. Biomedical Sciences Instrumentation. 1993;29:393–399. [PubMed] [Google Scholar]
- Bohlen HG, Wang W, Gashev AA, Gasheva OY, Zawieja DC. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H1319–H1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vincente A, Mericko P, Levy RJ, Makinen T, Oliver G, Kahn ML. Blood flow reprograms lymphatic vessels to blood vessels. The Journal of Clinical Investigation. 2012;122:2006–2017. doi: 10.1172/JCI57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ausilio A. Arduino: A low-cost multipurpose lab equipment. Behavior Research Methods. 2011;44:305–313. doi: 10.3758/s13428-011-0163-z. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. The Journal of Physiology. 2009;587:165–182. doi: 10.1113/jphysiol.2008.162438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, Moore JE, Jr, Cote G, Gashev AA, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- Estrada R, Giridharan GA, Nguyen MD, Roussel TJ, Shakeri M, Parichehreh V, Prabhu SD, Sethu P. Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Analytical Chemistry. 2011;83:3170–3177. doi: 10.1021/ac2002998. [DOI] [PubMed] [Google Scholar]
- Frijns JH, van Wijngaarden A, Peeters S. A multi-channel simultaneous data acquisition and waveform generator system designed for medical applications. Journal of Medical Engineering and Technology. 1994;18:54–60. doi: 10.3109/03091909409030229. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. Journal of Physiology. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. The American Journal of Physiology. 1977;233:H57–H65. doi: 10.1152/ajpheart.1977.233.1.H57. [DOI] [PubMed] [Google Scholar]
- Helmlinger G, Geiger RV, Schreck S, Nerem RM. Effects of pulsatile flow on cultured vascular endothelial cell morphology. Journal of Biomechanical Engineering. 1991;113:123–131. doi: 10.1115/1.2891226. [DOI] [PubMed] [Google Scholar]
- Kassis T, Kohan AB, Weiler MJ, Nipper ME, Cornelius R, Tso P, Dixon JB. A dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. Journal of Biomedical Optics. 2012 doi: 10.1117/1.JBO.17.8.086005. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Yokoyama Y, Kaidoh M, Ohhashi T. Shear stress-induced ATP-mediated endothelial constitutive nitric oxide synthase expression in human lymphatic endothelial cells. American Journal of Physiology - Cell Physiology. 2010;298:C647–C655. doi: 10.1152/ajpcell.00249.2009. [DOI] [PubMed] [Google Scholar]
- Kushner D. The making of Arduino. IEEE Spectrum. 2011 http://spectrum.ieee.org/geek-life/hands-on/the-making-of-arduino/ [Google Scholar]
- Lipowsky H. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- Nipper M, Dixon JB. Engineering the lymphatic system. Cardiovascular Engineering and Technology. 2011;2:296–308. doi: 10.1007/s13239-011-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Recchia FA, Byrne BJ, Wittstein IS, Ziegelstein RC, Kass DA. In vitro system to study realistic pulsatile flow and stretch signaling in cultured vascular cells. American Journal of Physiology - Cell Physiology. 2000;279:C797–C805. doi: 10.1152/ajpcell.2000.279.3.C797. [DOI] [PubMed] [Google Scholar]
- Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes JM, Adams RH, Makinen T, Kiefer F, Kwak BR, Petrova TV. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Developmental Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Sun Y, Suppappola S, Wrublewski TA. Microcontroller-based real-time QRS detection. Biomedical Instrumentation and Technology. 1992;26:477–484. [PubMed] [Google Scholar]
- Zawieja DC. Contractile physiology of lymphatics. Lymphatic Research and Biology. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.