Summary
Cholangiocytes, the cells lining bile ducts, are a heterogenous, highly dynamic population of epithelial cells. While these cells comprise a small fraction of the total cellular component of the liver, they perform the essential role of bile modification and transport of biliary and blood constituents. From a pathophysiological standpoint, cholangiocytes are the target of a diverse group of biliary disorders, collectively referred to as the cholangiopathies. To date, the cause of most cholangiopathies remains obscure. It is known, however, that cholangiocytes exist in an environment rich in potential mediators of cellular injury, express receptors that recognize potential injurious insults, and participate in portal tract repair processes following hepatic injury. As such, cholangiocytes may not be only a passive target, but are likely directly and actively involved in the pathogenesis of cholangiopathies. Here, we briefly summarize the characteristics of the reactive cholangiocyte and cholangiocyte responses to potentially injurious endogenous and exogenous molecules, and in addition, present emerging concepts in our understanding of the etiopathogenesis of several cholangiopathies.
Keywords: Cholangiocytes, Cholangiopathy, Biliary, Reactivity, Repair
Introduction
The intrahepatic bile ducts form a complex three-dimensional network of conduits lined by a single layer of specialized epithelial cells, the cholangiocytes. In addition to their role in bile modification and transport, cholangiocytes actively sense and respond to the inflammatory environment associated with liver injury, as well as bioactive molecules present in bile, including endogenous (bile acids, lipids, nucleotides) and exogenous (microbially-derived, xenobiotic) molecules that modify cholangiocyte function and/or phenotype. These molecules, some potentially injurious, can induce cellular responses affecting repair and remodeling of the biliary epithelium.
Cholangiocytes vary in size, shape, and function along the biliary tree (i.e., are heterogeneous [1]), and animal models of biliary injury show that the nature of the biliary insult can determine which subpopulation of cholangiocytes is affected [2,3]. For example, in response to acute injury, large cholangiocytes (i.e., those from larger ducts) proliferate and maintain normal biliary homeostasis. In contrast, chronic injury promotes increased proliferation of both large and small ducts and induces a reparative process associated with ductular changes that may include not only expansion of existing ducts, but also induction of liver progenitor cells (i.e., ductular reaction) (reviewed in [4]). This heterogeneity of response is consistent with and may account for the differential distribution of cholangiopathies along the biliary tree.
Cholangiocytes are the target of a diverse group of biliary disorders of different etiologies, collectively referred to as cholangiopathies [5]. Here we present a pathogenic framework for a subset of these disorders, namely idiopathic (i.e., primary sclerosing cholangitis-PSC) and immune-mediated (i.e., primary biliary cirrhosis-PBC) cholangiopathies, focusing primarily on cholangiocyte responses to constituents of bile. At the center of this conceptual framework is the concept of the “activated” or “reactive” cholangiocyte, characterized by an enhanced proinflammatory response including the secretion of chemokines, cytokines, growth factors, and morphogens that promote increased cholangiocyte proliferation, and biliary remodeling. These cholangiocytes participate in an intricate cross-talk between a variety of resident and recruited cells, including hepatocytes, progenitor cells, hepatic stellate cells, portal fibroblasts, and leukocytes (primarily macrophages, neutrophils, and lymphocytes). In yet to be fully characterized genetically susceptible individuals, the exaggeration or derangement of cholangiocyte responses to bioactive molecules and the ensuing reparative processes are likely an essential component of disease evolution. Identification of the nature and origin of the insults and a more comprehensive understanding of the molecular pathways regulating reactivity and repair processes throughout the biliary tree, are important and active areas of research that will likely identify new therapeutic targets and approaches.
The cholangiopathies
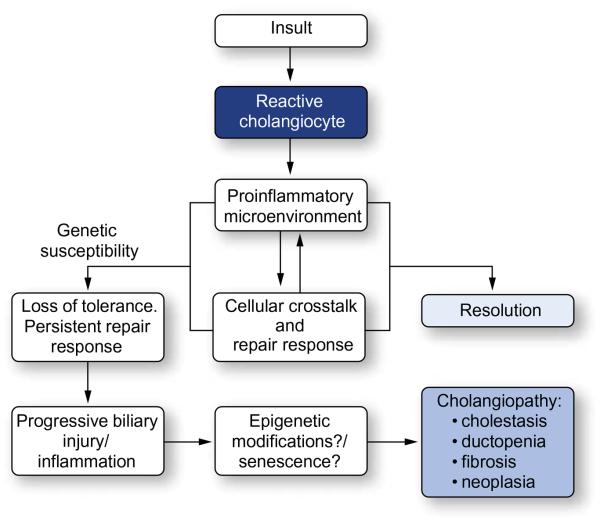
The cholangiopathies are a group of disorders that can be organized into several broad categories: immune-mediated, drug- or toxin-induced, infectious, genetic, ischemic and idiopathic. However, these categories overlap and more than one pathogenic mechanism may be operative in a given cholangiopathy. Despite their heterogeneity, the cholangiopathies have in common a number of cholangiocyte-associated processes that may contribute to their pathogenesis, including pro-inflammatory signaling, innate-immune responses, proliferation, and differentiation. These cholangiocyte processes, particularly in genetically susceptible individuals, may become disrupted and set the stage for other phenomena including aberrant biliary remodeling, cholestasis, and fibrosis (Fig. 1).
Fig. 1. The role of cholangiocytes in cholangiopathies.
Cholangiocytes recognize potential insults (endogenous or exogenous) and respond through the upregulation of proinflammatory mediators. These features are a component of the intricate crosstalk between a variety of resident and recruited cells, including hepatocytes, progenitor cells, fibroblasts and leukocytes, in an attempt to repair the epithelium. In most cases, the proinflammatory response and injury/damage to the biliary tree is resolved. However, in the genetically susceptible individual, perpetuation of the repair response results in progressive liver injury and a persistent inflammatory response, leading to chronic inflammation of the bile ducts and ultimately, disease.
Two of the cholangiopathies, PSC and PBC, are rare but important causes of chronic liver disease. Both are characterized by chronic inflammation of the bile ducts and follow a course that generally progresses to biliary cirrhosis, portal hypertension, and liver failure. From an epidemiological perspective, PBC, as with most autoimmune diseases, primarily affects women (>90% of cases) and is associated with a variety of extrahepatic autoimmune conditions including sicca syndrome, connective tissue disease (e.g., systemic sclerosis), and autoimmune thyroid disorders, among others. In contrast, PSC, which is considered idiopathic, likely immune-mediated but not autoimmune, is more common in males, has not been shown to respond to immuno-suppression and is frequently associated with inflammatory bowel disease (IBD). Indeed, IBD (most often ulcerative colitis) is found in approximately 75% of PSC patients [6,7]. The relationship between IBD and PSC remains obscure, yet forms the basis for several hypotheses, including the “leaky gut” and “aberrant gut lymphocyte homing” hypotheses. The “leaky gut” hypothesis suggests that PSC is caused, in susceptible individuals, by exposure of the biliary tree to microbes or metabolites of enteric microbiota (e.g., endotoxins, metabolites), while the “aberrant gut lymphocyte homing” hypothesis suggests that mucosal lymphocytes from the diseased gut are recruited to the hepatobiliary tract in response to adhesion molecules and chemokines that are normally restricted to the gut [8].
As with most cholangiopathies, particularly in the early stages, PBC and PSC generally affect distinct regions of the biliary tree. Bile duct destruction in PBC is limited to small interlobular and septal bile ducts, while in PSC, the larger intra- and extrahepatic bile ducts are preferentially affected. Although both PSC and PBC increase the risk of hepatocellular carcinoma, unlike PBC, PSC is also a major risk factor for cholangiocarcinoma, occurring in approximately 0.5% per year, and does not require a background of cirrhosis [9,10]. Optimal cancer surveillance in PSC patients is an area of ongoing investigation.
With respect to pharmacotherapy, the bile acid, ursodeoxycholic acid (UDCA), has been shown to improve biochemical parameters, histology, and survival in PBC and is widely recommended [11,12]. In contrast, there is no effective pharmacotherapy for PSC, although there is growing interest in the use of intermediate dose UDCA (or its analogs) and antibiotics, at least in a subset of patients [13]. Although their epidemiology, natural history, and associated diseases are distinct, both PBC and PSC have in common the ultimate progression to cholestasis and fibrosis, suggesting commonality in pathways and cell types involved in the initiation and progression of disease.
Pathogenic framework
The dynamic biliary epithelium
The concept of a dynamic proliferative epithelium was originally termed “ductular reaction” over 50 years ago [14]. With respect to proliferative characteristics at the ductular level, a number of morphologically distinct proliferative responses have been described both in animal models as well as human disease. While historically the classification of and terminology surrounding ductular reaction has been variable and inconsistent, four distinct ductular reactions are currently recognized (reviewed in [15]), and a clearer categorization of proliferating cholangiocytes has emerged. In general, the ductular reaction encompasses the proliferation or expansion of existing ducts, or an expanded population of differentiating hepatic progenitor cells that reside at the interface of terminal cholangioles (the finest branches of the biliary tree) and Canals of Hering. These bipotential hepatic progenitor cells can differentiate towards hepatocytes or cholangiocytes [25].
Our current understanding of the histological characteristics of the ductular reaction [16-18], molecular mechanism regulating this process [19,20], and the pathophysiological consequences of cholangiocyte proliferation [21-23] have grown greatly over the last years. This expansion of knowledge is due primarily to advances in the development of both in vivo and in vitro experimental models, the recognition of the heterogeneity of this diverse population of cells, and the recognition of a hepatic progenitor cell population that can recapitulate embryogenesis of the biliary tree. Indeed, the ductular reaction involving hepatic progenitor cells shares a number of morphological and molecular properties (i.e., expression of morphogens, growth factors, and transcription factors) with ductal plate cells during embryonic development of bile ducts (reviewed in [4]). Similar to their role in embryogenesis, these morphogens contribute to cellular crosstalk in the mature liver and regulate progenitor cell fate, and, importantly, contribute to biliary repair processes (reviewed in [4,26]).
Cholangiocytes respond to insult or injury through the expression of a repertoire of proinflammatory cytokines/chemokines (e.g., tumor necrosis factor alpha (TNF-α), interleukin-6 (IL6), and interleukin-8 (IL8)) [14-17], growth factors (e.g., platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGFβ) [18-20]), and morphogens (Hedgehog and Notch [24,25]). These molecules function in an autocrine and/or paracrine manner to mediate cellular events, including proliferation, apoptosis, vasculogenesis, and fibrosis, associated with the biliary repair response. Furthermore, secretion of these factors modifies the periductular micro-environment and mediates the recruitment of innate and adaptive immune cells to protect against biliary insult or infection, and resident and recruited mesenchymal and endothelial cells to repair and remodel the biliary tree. With respect to the ductular reaction, a clearer picture is emerging of the molecular events required for repair and remodeling. It is now recognized that in an inflammatory environment, optimal viability and growth of epithelial progenitors require hedgehog signaling [24,26,27], while Notch signaling is necessary for specification of cholangiocyte fate [28] in a process mediated by marcophage-derived Wnt signaling [19]. It is likely that these properties of the reactive cholangiocyte, while necessary for repair and remodeling, contribute to the pathogenesis of cholangiopathies. Of further interest, recent data support the existence of stem/progenitor cells residing in peribiliary glands [29]. This repository of multipotent stem cells show phenotypic characteristics of the hepatic progenitors found in the Canals of Hering. Interestingly, the peribiliary glands are first found at the level of the septal ducts and continue to the extrahepatic ducts [29], suggesting that, in addition to providing a source of multipotent cells for extrahepatic bile ducts, they are likely sources of biliary epithelial cells involved in intrahepatic biliary remodeling processes. Whether these cells are activated and follow a differentiation pattern similar to the progenitor cells at the terminal branches of the biliary tree remains to be determined.
An area that has also received considerable attention is the neuroendocrine transdifferentiation of cholangiocytes in response to cholestasis or injury. Indeed, several manuscripts have characterized a cholangiocyte neuroendocrine phenotype, that is, a compartment of ductal cells that secrete and respond to a number of hormones, neuropeptides, and neurotransmitters (reviewed in [30]). This property of reactive cholangiocytes contributes to their increased proliferative capacity, and is likely involved in the pathogenesis of cholangiopathies. Hence, the initiation of cholangiocyte reactivity, the induction of progenitor cells and the resultant increase in intercellular communication drive hepatobiliary repair mechanisms and, when dysregulated, likely lie at the center of the pathogenic pathways of at least some of the cholangiopathies. Ongoing investigations analyzing the molecular mechanisms by which cytokines/chemokines, growth factors, hormones, and morphogens function collectively to initiate and perpetuate biliary repair and remodeling under normal and pathological conditions are essential to understand the etiology and pathogenesis of several cholangiopathies.
The gut-liver axis
Bile is a source of potential mediators of inflammation. Hepatic bile is directly emptied into the duodenum and the bile components are modified by the resident intestinal microflora. Selective intestinal uptake of primary and bacterially modified bile acids (secondary bile acids) results in their recycling back to the liver through the portal circulation in a process known as enterohepatic circulation. Moreover, a healthy intestine prevents the translocation of large amounts of microbes and microbially derived products. However, disruption of the intestinal barrier function results in the translocation of large amounts of microbes and microbially derived products. Through this route, the liver is exposed to bacterially-derived products and xenobiotics, including products that can induce inflammatory responses in multiple hepatic cell types and contribute to chronic liver disease [31]. Therefore, bile composition is modified not only by canalicular secretion from hepatocytes and cholehepatic shunting of bile acids, but by the enterohepatic circulation of microbially-derived products, modified endogenous molecules (e.g., secondary bile acids) and xenobiotics. The potential exists, therefore, for the composition of bile, including the presence of exogenous and endogenous mediators of inflammation, to reflect, at least in part, the diversity of resident enteric microbes, or the enteric microbiome. Conversely, the composition of bile may directly influence the diversity of the gut microbiota. Indeed, it was recently demonstrated that an increase in the bile acid, taurocholic acid, induced by a high milk-derived-fat diet, promoted the growth of the sulphite-reducing pathobiont, Bilophila wadsworthia. Moreover, this expansion was associated with the increased incidence of colitis in IL10-deficient mice [32]. Hence, metagenomics, or the study of genetic material extracted from environmental samples, coupled with advances in high-throughput DNA sequencing and bioinformatics tools, has allowed the sequencing of microbial communities, including those that reside on or in humans. Using these techniques, it is now recognized that bacterial dysbiosis, or a change in the normal bacterial composition, is found in the gut of patients with cirrhosis [33] and inflammatory bowel disease (reviewed in [34]). It seems likely, given the intimate anatomic and physiologic relation between the gut and the liver, that the composition and potential dysbiosis of the microbiome influence hepatobiliary physiology, and likely contribute to the etiopatho-genesis of cholangiopathies; yet definitive cause-effect relationships in humans are lacking.
Cholangiocyte response to endogenous and exogenous insults
Several families of proteins are involved in recognition of potentially injurious microbial components and products released from injured or dying cells. The best characterized of these pathogen recognition receptors (PRRs) are Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain proteins (NODs) [28-30]. PAMPs, the microbial molecular signatures that activate PRRs (e.g., Gram-negative bacterially-derived lipopolysaccharide, LPS) induce TLR oligomerization and conformational changes in the cytoplasmic portion of these receptors, the so-called Toll/IL-1 receptor (TIR) domain. The TIR domains recruit signaling adaptor proteins (i.e., myeloid differentiation protein 88 [MyD88], interleukin 1 receptor-associated kinases (IRAKs), and TIR domain-containing adapter-inducing interferon-β (TRIF)). Signal cascades are transduced via either MyD88 dependent or independent (TRIF) pathways, culminating in the activation of transcription factors, including MyD88 dependent nuclear factor kappa-B (NF-κB) or TRIF dependent interferon regulatory transcription factor 3 (IRF3). Enterohepatic circulation brings potential mediators of inflammation from the gut to the hepatic sinusoids. A variety of hepatic cell types, including the sinusoidal endothelial cells, Kupffer cells, hepatocytes, hepatic stellate cells, dendritic cells, and recruited bone marrow derived monocytes express TLRs and, hence, recognize and respond to PAMPS through TLR dependent upregulation of proinflammatory mediators and contribute to repair processes [31]. Moreover, as others and we have previously shown, cholangiocytes express multiple TLRs (and associated molecular machinery) that upon pathogen recognition, initiate signaling cascades altering cholangiocyte physiology and the extracellular milieu [15,31,32]. Hence, cholangiocytes are capable of initiating or perpetuating an innate immune response against potentially pathogenic insults (for a recent review see [35]). Indeed, cholangiocyte TLR and subsequent NF-κB activation have been demonstrated in a variety of cell culture and animal models of cholangiocyte responses to pathogens [36-43].We have also recently demonstrated that stimulation of cultured cholangiocytes with PAMPs induce the activation of not only NF-κB, but also the rapid activation of the Ras/MAPK pathway, both contributing to the expression of proinflammatory mediators and cholangiocyte proliferation [43].
In response to pathogen recognition, cholangiocytes produce and secrete a number of pro-inflammatory cytokines/chemokines (e.g., TNF-α, interleukin-6, interleukin-8) [40,41]. Human cholangiocyte expression of these proinflammatory mediators functions as part of the biliary innate immune and repair responses and alters the periductal cytokine networks mediating recruitment and activation of T cells, macrophages, neutrophils, NK cells and resident and recruited mesenchymal cells. Hence, the cholangiocyte proinflammatory signaling cascade, in response to lumenal insults, is likely an essential event initiating biliary repair responses. Importantly, to avoid excessive innate immune responses, many innate immune and epithelial cells initiate a protective process of endotoxin tolerance. Endotoxin tolerance is a phenomenon in which, following a robust initial response to endotoxin insult (e.g., LPS) cells enter a transient state where they exhibit reduced responsiveness to subsequent endotoxin insult. Cholangiocytes exhibit endotoxin tolerance in culture [38]. It was recently determined that following an initial endotoxin insult, using either the TLR4 ligand LPS, or the TLR1/2 ligand PAM3CSK9, cultured cholangiocytes were less responsive to a secondary treatment with LPS, as demonstrated by NFkB activation [38]. In this instance, endotoxin tolerance was regulated through IRAK-M, a member of the interleukin-1 receptor-associated kinase family that functions as a negative regulator of TLR signaling. The hypothesis that impaired endotoxin tolerance may be a contributing factor in chronic inflammatory cholangiopathies (i.e., PSC) was also investigated [44]. Freshly isolated primary cholangiocytes, isolated from the PSC liver, exhibited increased TLR expression compared to cholangiocytes isolated from the non-diseased control liver, and did not develop endotoxin tolerance after repeated endotoxin exposure. Further evidence supported that this lack of endotoxin tolerance in isolated cholangiocytes was secondary to excessive pro-inflammatory mediators (i.e., IFNα and TNFβ) present in the diseased livers, which was maintained in short-term culture. Importantly, cholangiocytes isolated from PSC livers, when maintained in prolonged culture in the absence of inflammatory mediators, reverted to a phenotype capable of initiating endotoxin tolerance. These results are consistent with the hypothesis that the inflammatory milieu of the diseased liver (i.e., cellular crosstalk in an inflammatory microenvironment), coupled with persistent exposure and response to PAMPs, promotes a persistent inflammatory phenotype of cholangiocytes.
The potential role of enteric microbes, cholangiocyte TLRs and associated endotoxin tolerance, in the background of genetic susceptibility in hepatobiliary pathophysiology, was recently exemplified in a model of sclerosing cholangits using cystic fibrosis transductance regulator (CFTR) deficient mice [45]. In the liver, CFTR is expressed exclusively by cholangiocytes and is essential for alkalinization of bile and choleresis. In humans, the disease cystic fibrosis (CF) involves many organs, including the liver, where a subset of patients develops hepatobiliary complications including sclerosing cholangitis-like architecture. In the CFTR deficient animal, a “leaky gut”, induced by dextran sodium sulfate treatment, and hence exposure of the liver and biliary tree to enteric microbial products, contributed to hepatobiliary pathology via exaggerated TLR4 signaling and reduced endotoxin tolerance. Hence, using this model, Fiorotto et al. demonstrated support for a “two-hit” hypothesis of exogenous insults (microbially derived) inducing hepatobiliary disease (sclerosing cholangitis) in genetically susceptible (CFTR deficient) animals. It seems likely, therefore, that PRRs initiate an activation cascade that if exaggerated, altered, or chronic, could contribute to disease initiation or progression. For example, in extreme cases (e.g., secondary sclerosing cholangitis induced by Cryptosporidium parvum), cholangiocyte response to microbial insult results in cholestasis and periductal fibrosis [46].
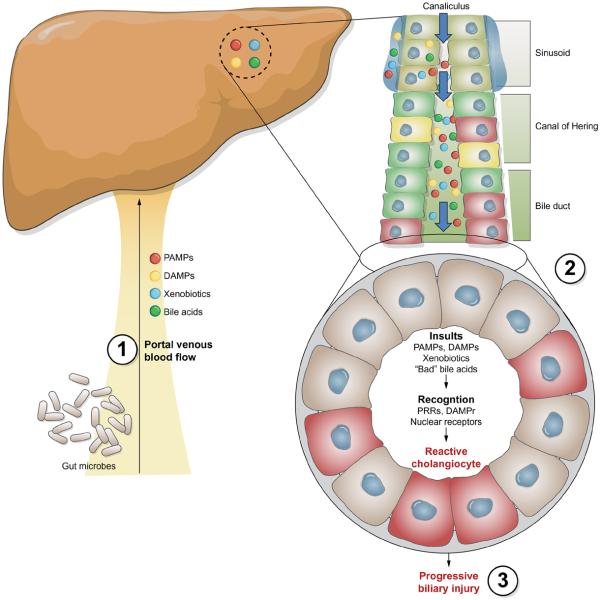
In addition to microorganisms or microbially derived molecules, several endogenous molecules, including several present in bile, have been reported to activate TLRs [28]. Indeed, products released from injured or dying cells (damage-associated molecular patterns, DAMPs), including HMGB1, S100A8/S100A9, and heat shock proteins, activate a variety of TLRs and/or DAMP receptors [47]. To date, the levels of DAMPs in human bile under normal and pathophysiological conditions, as well as the cellular expression of DAMP receptors in the liver, the signaling cascades initiated by these receptors, and the physiological consequences of DAMPs on hepatic function have not been fully investigated. Furthermore, other components of bile have been demonstrated to mediate inflammatory processes including oxysterols, oxygenated derivatives of cholesterol. These molecules can be non-enzymatically produced in inflammatory environments and have been identified in bile obtained from patients with inflammatory biliary disorders [47]. One of these oxysterols, 22(R)-hydroxycholesterol, was demonstrated to increase the levels of cyclooxygenase-2 in cholangiocarcinoma cells via a p38 MAPK dependent mechanism of mRNA stabilization [48]. Furthermore, treatment of cultured cholangiocytes with selected oxysterols rapidly activates both cholangiocyte NF-κB and MAPK pathways and induces cholangiocyte expression of interleukin-6 and interleukin-8 (O’Hara and LaRusso, unpublished observations). Thus, the role of DAMPS, endogenous components of bile such as bile acids, nucleotides and oxysterols in mediating signaling cascades in cholangiocytes, while a promising area of investigation, remains ill-defined, as does the role of these endogenous mediators of inflammation in hepatobiliary pathophysiology in general and cholangiopathies in particular (Fig. 2).
Fig. 2. Cholangiocytes exist in an environment rich of potential mediators of cellular injury.
Through enterohepatic circulation: (1) the liver is exposed to bacterially derived products (PAMPs), xenobiotics and endogenous mediators of inflammation (DAMPs). Portal blood flows into hepatic sinusoids, where a variety of resident and recruited cells can recognize and respond to these potential insults. Ultimately, hepatocytes take up, modify, and excrete these molecules into the canaliculus. Canalicular bile percolates through the biliary tree where cholangiocytes recognize and react to these potential insults through the increased release and response to repair associated chemokines/cytokines, growth factors, and morphogens, initiating both autocrine and paracrine signaling cascades (2). A more comprehensive understanding of the role of the gut in hepatic function and the molecular networks mediating injury induced growth and repair, as well as the cholangiocyte epigenetic phenomena, and the genetic variation (3) that contribute to the perpetuation of this phenotype and sequelae, including fibrogenesis and carcinogenesis, will undoubtedly open avenues for potential therapeutic approaches.
Hence, cholangiocytes exist in an environment rich in potential mediators of inflammation and are in constant communication with resident and recruited cells as well as the intestinal tract. How cholangiocytes, throughout the biliary tree, respond to exogenous and endogenous insults, including the precise signaling cascades initiated by these insults, is an active area of research that continues to provide insight into the innate immune/proinflammatory function of this epithelium. A more comprehensive understanding of (i) how the gut influences hepatic function or dysfunction and the molecular mechanisms by which putative injurious molecules stimulate cholangiocyte reactivity; (ii) how the induction of cholangiocyte reactivity influences the epithelia, resident and recruited mesenchymal and immune cells, and (iii) the molecular mechanisms and genetic variation that contribute to pathogenic perpetuation of cholangiocyte reactivity and the ductular reaction, will undoubtedly improve our management of many cholangiopathies and open avenues for potential therapeutics. This in turn will contribute to our understanding of how cholangiocytes (and bile) function as central players in enterohepatic physiology, yet may become targets for disease and destruction.
Regulatory mechanisms involved in cholangiocyte activation
Protein coding genes perform the essential physiological functions of the cell; however, only 2% of the human genome encodes proteins, while the vast majority is transcribed largely as long and short non-coding RNAs (ncRNAs) [48,49]. It has become increasingly clear that one class of these ncRNAs, miRNAs, play an essential role in cholangiocyte function. Indeed, recent studies indicate that miRNA-mediated post-transcriptional pathways are critical to host cell regulatory responses in a variety of cholangiocyte processes including proliferation [50], apoptosis [51,52], and, particularly germane to this review, response to microbial insult [53,54]. For instance, cultured human cholangiocytes express let-7 miRNA family members, which under normal physiological conditions, post-transcriptionally downregulate TLR4 expression. Following exposure of cultured human cholangiocyte to microbes (i.e., C. parvum) or microbial products (i.e., LPS), the expression of let-7 decreased resulting in the increased expression of TLR4 in an NF-kB-dependent manner [53,54]. Hence, expression of cholangiocyte innate immune-associated genes is a highly regulated process, occurring through both transcriptional and post-transcriptional mechanisms, involving miRNAs, that assures that the epithelium recognizes and responds to insults, but does not induce injury through an inappropriate and/or hyper-reactive response.
In addition to transcriptional and post-transcriptional events that regulate the cholangiocyte response to insult and repair processes, the genetic background of the individual (as well as epigenetic modifications to cholangiocyte chromatin) is essential for biliary homeostasis. Genome wide association studies (GWAS) have identified allelic variants associated with both PBC and PSC including both human leukocyte antigen (HLA) and non-HLA associations [55,56]. For example, GWAS analysis of Canadian and U.S. subjects revealed strong association between PBC and multiple loci across the HLA class II region, with the strongest association at the HLA-DQB1 loci. Furthermore, strong associations were observed at non-HLA loci including IL12A and IL12RB2 (encoding the cytokine interleukin-12A and the interleukin-12 receptor β2 loci, respectively) [55]. GWAS analysis of PSC has reproducibly identified association at the HLA-B loci [58,59], as well as non-HLA associations at the MST1 loci (encoding macrophage stimulating-1) [58,59]. While this approach has identified several disease associated loci, these variants account for only a portion of the heritable disease risk. While the genetic basis of the host certainly plays a principle role in disease initiation and progression, we are just now beginning to understand the complex molecular regulatory networks involved in carrying out the genetic program and epigenetic modifications of DNA that heritably alter cellular phenotype through meiotic and mitotic events. Among the relatively unexplored regulatory mechanisms that function in the cholangiocyte, and may be involved in cholangiocyte activation, are these epigenetic phenomena. Besides gene regulatory proteins (transcription factors), epigenetic mechanisms play an essential role in the transcriptional control of gene expression. These epigenetic mechanisms change the accessibility of chromatin through DNA methylation and histone modifications, and epigenetic modulators are likely critically involved in the regulation of inflammatory processes associated with cholangiopathies [57]. Epigenetic modifications in the heritability of these idiopathic diseases (i.e., parent-of-origin effects) should be considered; however, epigenetic modifications can result in altered chromatin accessibility transmitted through mitosis, and hence cell progeny. These modifications may be involved in the persistence of a proinflammatory phenotype, and perhaps contribute to the etiopathogenesis of both PSC and PBC. Genetic predispositions, as well as epigenetic, transcriptional, and post-transcriptional regulation of gene expression all contribute to cellular phenotype and each works as a component of the gene expression circuitry and hence must be considered when attempting to understand cellular function and dysfunction.
While the dynamic nature of the biliary epithelia has received considerable attention over the last two decades, a direct causal link between these processes and the etiopathogenesis of cholangiopathies has yet to been established. In addition to proliferation and repair, damaged cholangiocytes initiate processes resulting in their removal. One of these processes, apoptosis (i.e., programmed cell death), has been described as a potential contributing factor in the pathogenesis of cholangiopathies (reviewed in [5]). Similar to apoptosis and in many instances a precursor to the extrinsic apoptotic pathway (i.e., targeted destruction by lymphocytes), permanent withdrawal from the cell cycle (i.e., senescence) functions as a protective mechanism to remove damaged cells from the population. The accumulation of senescent cholangiocytes has been identified in both PBC and PSC [58-62]. We have recently confirmed the existence of senescent cholangiocytes in PSC tissue (unpublished data). The induction of senescence holds promise as a potential link between persistent inflammation, cholangiocyte proliferation, and the etiopathogenesis of select cholangiopathies. Most cells, in both culture and in vivo, have a finite capacity to proliferate, a process intimately associated with telomere shortening, and linked to the morbidities associated with aging (reviewed in [63,64]). Cholangiocytes from normal livers maintain telomere length with increased age, likely reflecting an increased capacity of proliferation and a resistance to age-associated senescence [65]. However, upon recognition of persistent and/or aberrant proliferative signals (i.e., oncogene-induced) and/or recognition of the accumulation of persistent DNA damage (i.e., DNA strand breaks), cells can initiate the senescence program through the activation of one or both of the strong tumor suppressor pathways (p16INK4A/Retinoblastoma or p53/p21WAF) (reviewed in [66]) and permanent silencing of cell cycle progression-related genes (i.e., epigenetic silencing of E2F-responsive genes), that likely involves microRNAs [67]. Intriguingly, senescent cells frequently transition to a senescence-associated secretory phenotype (SASP), characterized by the robust secretion of chemokines, cytokines, growth factors, and matrix metalloproteases (MMPs) that function in repair/remodeling and recruiting of immune cells (reviewed in [64,68]). It seems likely that prolonged proliferative signals, coupled with the potential accumulation of DNA damage induced from the inflammatory milieu, promote the induction of a mechanism to remove the damaged cells from the population. The potential roles of senescent cholangiocytes in repair/remodeling and inflammation, remain to be explored in detail, particularly as a potential contributing factor in the pathogenesis of cholangiopathies.
Conclusions and perspectives
Over the past three decades, our appreciation of cholangiocytes as a heterogenous, highly dynamic population of cells, has contributed to the tremendous growth in our knowledge of cholangiocyte function and dysfunction. Most of these advances have required the development and implementation of novel and innovative experimental models, and the evolution of concepts that allow the generation of hypotheses leading to relevant questions. It is now generally accepted that the cholangiocyte is an active and important participant in normal liver function and, rather than being only a target cell of cholangiopathies, actively contributes to the pathogenesis of disease. Although the mechanisms underlying the development of acquired cholangiopathies are still emerging, common pathogenic pathways, genetic susceptibilities, and epigenetic phenomena continue to emerge. Future research to understand the interaction of environmental elements, including the direct communication between gut microbes and hepatic physiology, with the genetically and epigenetically regulated host responses of cholangiocytes, will almost certainly shed light on the pathogenesis of cholangiopathies. Such knowledge will provide the framework within which to develop preventive and therapeutic strategies for cholangiopathies.
Key Points.
Cholangiocytes, the cells lining bile ducts, are a heterogenous, highly dynamic population of epithelial cells that recognize and respond to insult and/or injury (i.e., cholangiocyte reactivity)
The molecular mechanisms regulating the cholangiocyte response to exogenous and endogenous insults is an active area of research that continues to provide insight into the innate immune, proinflammatory and reparative function of this epithelium
Cholangiocytes are the target of a diverse group of biliary disorders of different etiopathogenesis, collectively referred to as cholangiopathies
In a subset of cholangiopathies, the cholangiocyte response to insult or injury and the resultant communication with resident and recruited cells, implicates the cholangiocyte as not only a target, but also likely a central and active candidate in the initiation and propagation of disease
Acknowledgments
Financial support
This work was supported by NIH grants R01 DK057993 and DK024031 (to NF LaRusso), R01 AI089713 (to SP O’Hara), and P30DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology).
Abbreviations
- PSC
primary sclerosing cholangitis
- PBC
primary biliary cirrhosis
- IBD
inflammatory bowel disease
- UCDA
ursodeoxycholic acid
- PRR
pathogen recognition receptor
- TLR
Toll-like receptor
- NOD
nucleotide-binding oligomerization domain
- PAMP
pathogen-associated molecular pattern
- LPS
lipopolysaccharide
- CFTR
cystic fibrosis transductance regulator
- DAMP
damage-associated molecular pattern
- ncRNA
non-coding RNA
- miRNA
microRNA
- NF-kB
nuclear factor kappa B
- GWAS
genome-wide association studies
- HLA
human leukocyte antigen
Footnotes
Conflict of interest
The underlying research reported in the study was funded by the NIH Institutes of Health.
References
- 1.Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227–240. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- 2.Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, et al. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–876. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- 3.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–G190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 4.Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol. 2012;56:1159–1170. doi: 10.1016/j.jhep.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Ngu JH, Gearry RB, Wright AJ, Stedman CA. Inflammatory bowel disease is associated with poor outcomes of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2011;9:1092–1097. doi: 10.1016/j.cgh.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–206. [PubMed] [Google Scholar]
- 8.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 9.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 10.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 11.Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J Pediatr Gastroenterol Nutr. 2008;47:61–67. doi: 10.1097/MPG.0b013e31816fee95. [DOI] [PubMed] [Google Scholar]
- 12.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 13.Lindor KD. New treatment strategies for primary sclerosing cholangitis. Dig Dis. 2011;29:113–116. doi: 10.1159/000324145. [DOI] [PubMed] [Google Scholar]
- 14.Popper H, Kent G, Stein R. Ductular cell reaction in the liver in hepatic injury. J Mt Sinai Hosp N Y. 1957;24:551–556. [PubMed] [Google Scholar]
- 15.Priester S, Wise C, Glaser SS. Involvement of cholangiocyte proliferation in biliary fibrosis. World J Gastrointest Pathophysiol. 2010;1:30–37. doi: 10.4291/wjgp.v1.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. III. Implications for liver pathology. Virchows Arch. 2011;458:271–279. doi: 10.1007/s00428-011-1050-9. [DOI] [PubMed] [Google Scholar]
- 17.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011;458:261–270. doi: 10.1007/s00428-011-1049-2. [DOI] [PubMed] [Google Scholar]
- 18.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011;458:251–259. doi: 10.1007/s00428-011-1048-3. [DOI] [PubMed] [Google Scholar]
- 19.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, et al. Macrophage-derived Wnt opposes notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omenetti A, Diehl AM. Hedgehog signaling in cholangiocytes. Curr Opin Gastroenterol. 2011;27:268–275. doi: 10.1097/MOG.0b013e32834550b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser SS, Onori P, Wise C, Yang F, Marzioni M, Alvaro D, et al. Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis. Dig Liver Dis. 2010;42:245–252. doi: 10.1016/j.dld.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 25.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 26.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–G870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 30.Munshi MK, Priester S, Gaudio E, Yang F, Alpini G, Mancinelli R, et al. Regulation of biliary proliferation by neuroendocrine factors: implications for the pathogenesis of cholestatic liver diseases. Am J Pathol. 2011;178:472–484. doi: 10.1016/j.ajpath.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol. 2012;3:138. doi: 10.3389/fphys.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10–/– mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 34.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012. doi: 10.1038/nrgastro.2012.152. http://dx.doi.org/10.1038/nrgastro.2012.152. [DOI] [PubMed]
- 35.Harada K, Nakanuma Y. Cholangiopathy with respect to biliary innate immunity. Int J Hepatol. 2012;2012 doi: 10.1155/2012/793569. Article ID 793569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen XM, O’Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005;175:7447–7456. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- 37.Harada K, Isse K, Nakanuma Y. Interferon gamma accelerates NF-kappaB activation of biliary epithelial cells induced by toll-like receptor and ligand interaction. J Clin Pathol. 2006;59:184–190. doi: 10.1136/jcp.2004.023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada K, Isse K, Sato Y, Ozaki S, Nakanuma Y. Endotoxin tolerance in human intrahepatic biliary epithelial cells is induced by upregulation of IRAK-M. Liver Int. 2006;26:935–942. doi: 10.1111/j.1478-3231.2006.01325.x. [DOI] [PubMed] [Google Scholar]
- 39.Harada K, Ohba K, Ozaki S, Isse K, Hirayama T, Wada A, et al. Peptide antibiotic human beta-defensin-1 and -2 contribute to antimicrobial defense of the intrahepatic biliary tree. Hepatology. 2004;40:925–932. doi: 10.1002/hep.20379. [DOI] [PubMed] [Google Scholar]
- 40.Huang YH, Chou MH, Du YY, Huang CC, Wu CL, Chen CL, et al. Expression of toll-like receptors and type 1 interferon specific protein MxA in biliary atresia. Lab Invest. 2007;87:66–74. doi: 10.1038/labinvest.3700490. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda H, Sasaki M, Ishikawa A, Sato Y, Harada K, Zen Y, et al. Interaction of toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab Invest. 2007;87:559–571. doi: 10.1038/labinvest.3700556. [DOI] [PubMed] [Google Scholar]
- 42.Karrar A, Broome U, Sodergren T, Jaksch M, Bergquist A, Bjornstedt M, et al. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132:1504–1514. doi: 10.1053/j.gastro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 43.O’Hara SP, Splinter PL, Trussoni CE, Gajdos GB, Lineswala PN, Larusso NF. Cholangiocyte N-Ras protein mediates lipopolysaccharide-induced Interleukin 6 secretion and proliferation. J Biol Chem. 2011;286:30352–30360. doi: 10.1074/jbc.M111.269464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller T, Beutler C, Pico AH, Shibolet O, Pratt DS, Pascher A, et al. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int. 2011;31:1574–1588. doi: 10.1111/j.1478-3231.2011.02635.x. [DOI] [PubMed] [Google Scholar]
- 45.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. doi: 10.1053/j.gastro.2011.06.052. 1508 e1491-e1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusuf TE, Baron TH. AIDS cholangiopathy. Curr Treat Options Gastroenterol. 2004;7:111–117. doi: 10.1007/s11938-004-0032-2. [DOI] [PubMed] [Google Scholar]
- 47.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:382–390. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 48.Carninci P. Tagging mammalian transcription complexity. Trends Genet. 2006;22:501–510. doi: 10.1016/j.tig.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 50.Lee SO, Masyuk T, Splinter P, Banales JM, Masyuk A, Stroope A, et al. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J Clin Invest. 2008;118:3714–3724. doi: 10.1172/JCI34922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong AY, Zhou R, Hu G, Liu J, Sosnowska D, Drescher KM, et al. Cryptosporidium parvum induces B7-H1 expression in cholangiocytes by down-regulating microRNA-513. J Infect Dis. 2010;201:160–169. doi: 10.1086/648589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mott JL, Kobayashi S, Bronk SF, Gores GJ. Mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282:28929–28938. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Hara SP, Splinter PL, Gajdos GB, Trussoni CE, Fernandez-Zapico ME, Chen XM, et al. NFkappaB p50-CCAAT/enhancer-binding protein beta (C/EBPbeta)-mediated transcriptional repression of microRNA let-7i following microbial infection. J Biol Chem. 2010;285:216–225. doi: 10.1074/jbc.M109.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melum E, Franke A, Schramm C, Weismuller TJ, Gotthardt DN, Offner FA, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43:17–19. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmer V, Lammert F. Genetics and epigenetics in the fibrogenic evolution of chronic liver diseases. Best Pract Res Clin Gastroenterol. 2011;25:269–280. doi: 10.1016/j.bpg.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki M, Ikeda H, Haga H, Manabe T, Nakanuma Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol. 2005;205:451–459. doi: 10.1002/path.1729. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis. Am J Pathol. 2006;169:831–845. doi: 10.2353/ajpath.2006.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic Res. 2008;42:625–632. doi: 10.1080/10715760802244768. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki M, Ikeda H, Yamaguchi J, Miyakoshi M, Sato Y, Nakanuma Y. Bile ductular cells undergoing cellular senescence increase in chronic liver diseases along with fibrous progression. Am J Clin Pathol. 2010;133:212–223. doi: 10.1309/AJCPWMX47TREYWZG. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki M, Ikeda H, Yamaguchi J, Nakada S, Nakanuma Y. Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology. 2008;48:186–195. doi: 10.1002/hep.22348. [DOI] [PubMed] [Google Scholar]
- 63.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma S, Tachtatzis P, Penrhyn-Lowe S, Scarpini C, Jurk D, Von Zglinicki T, et al. Sustained telomere length in hepatocytes and cholangiocytes with increasing age in normal liver. Hepatology. 2012;56:1510–1520. doi: 10.1002/hep.25787. [DOI] [PubMed] [Google Scholar]
- 66.d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 67.Benhamed M, Herbig U, Ye T, Dejean A, Bischof O. Senescence is an endogenous trigger for microRNA-directed transcriptional gene silencing in human cells. Nat Cell Biol. 2012;14:266–275. doi: 10.1038/ncb2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]