Abstract
Objective
Women with systemic lupus erythematosus (SLE) have an increased risk of cardiovascular disease (CVD). Traditional CVD and SLE-disease related risk factors do not fully account for this increased risk. Perivascular adipose tissue (PVAT) is a visceral adipose depot in close proximity to blood vessels possibly influencing CVD. We hypothesized that women with SLE have an increased volume of descending thoracic aortic PVAT (aPVAT) associated with increased vascular calcification.
Methods
Using electron beam computed tomography, we quantified the aPVAT in clinically CVD-free SLE women (n=135) and age-/race-matched healthy controls (HC, n=152). Coronary artery calcification (CAC) and aortic calcification (AC) were quantified using Agatston scores and the aPVAT was quantified using standard Hounsfield Units (HU) for adipose tissue.
Results
Women with SLE had greater median aPVAT (32.2 cm3 vs. HC aPVAT 28.6 cm3, p=0.0071) and greater median AC (26.0 vs HC AC 6.0, p=0.0013) than the healthy control women. Total aPVAT (per 25 cm3) remained significantly associated with SLE after adjusting for CVD risk factors (Odds Ratio 1.74 [95% Confidence Interval: 1.04-2.9], p=0.034), but was attenuated when adjusting for circulating inflammatory markers (p=0.34). In a logistic regression analysis, SLE aPVAT (per 25 cm3) was associated with AC (6.78 [2.0-23], p=0.0019), which remained significant after adjusting for circulating inflammatory markers (p=0.0074), and CAC (2.66 [1.4-5.0], p=0.0028).
Conclusions
Total aPVAT is greater in clinically CVD-free SLE women than in age-/race-matched controls and is associated with calcification in different vascular beds.
Keywords: atherosclerosis, adipose, systemic lupus erythematosus, calcification
INTRODUCTION
Systemic lupus erythematosus (SLE, lupus) is an autoimmune disease characterized by chronic systemic inflammation. Populations with SLE exhibit a bimodal mortality pattern, with the latter peak being attributed to atherosclerotic cardiovascular disease (CVD) which is markedly elevated in women with SLE.1 The amplified risk of CVD-related events in relatively young SLE women is only partially explained through traditional CVD risk factors 2, and may be related to factors associated with chronic systemic inflammation such as circulating high sensitivity C-reactive protein (hsCRP) and complement protein levels.3, 4
Atherosclerosis and CVD are regarded as inflammatory diseases.5 Many of the circulating pro-inflammatory markers present in the development of atherosclerosis are upregulated in the SLE patient and have been linked to premature subclinical CVD in the SLE population including vascular calcification of the coronary arteries and aorta.6, 7 These same pro-inflammatory markers link obesity and visceral adipose tissue to atherosclerosis and inflammation.8, 9
Adipose tissue has its own anti- and pro-atherogenic cytokines that can initiate many of the mechanisms linking obesity to CVD, including systemic inflammation.10 Visceral adipose volume is more strongly related with CVD than is subcutaneous adipose volume.11 In particular, perivascular adipose tissue (PVAT), a small, visceral adipose depot in close proximity to blood vessels, may influence vascular function and progression of CVD more directly than general visceral adiposity.12 Epicardial and pericardial adipose are other examples of small PVAT depots having both cardioprotective and pathological pro-inflammatory properties.13, 14 Although the association of general visceral adipose tissue with CVD has been well documented, little is known about the distribution of small visceral adipose tissue depots in SLE patients, who are predisposed to premature CVD.
The purpose of this study was two-fold: first, to determine if SLE women had an increased volume of descending thoracic aorta perivascular adipose tissue (aPVAT) when compared to healthy control participants, and second, to determine if aPVAT was associated with subclinical cardiovascular disease measures of calcification, including coronary artery calcification (CAC) and aortic calcification (AC).
METHODS
Inclusion / Exclusion Criteria
This retrospective case-control study included SLE participants and their respective age- and race-matched controls (1:1) in methods previously described.6 Women who fulfilled the 1987 revised American College of Rheumatology criteria for SLE but with no prior history of cardiovascular event were non-selectively recruited from the Pittsburgh Lupus Registry to participate in this study, which was designed to compare the prevalence and risk factors of coronary artery and aortic calcification in SLE women and healthy controls. These women were participants of the “Heart Effects on Atherosclerosis and Risk of Thrombosis in SLE” (HEARTS) study funded by the National Institutes of Health as previously described.6
SLE women had at least two years of disease duration at study entry. The Pittsburgh Lupus Registry includes women diagnosed with SLE who have been seen either at the University of Pittsburgh Medical Center inpatient and outpatient facilities or by practicing rheumatologists in the Pittsburgh metropolitan area. In this study, the healthy non-SLE women (controls) were matched to the SLE women by age (+/− 5 years) and race. Healthy control subjects were recruited based on: 1) Voters registration list or Motor Vehicle License list depending on where the case (SLE woman) was found in these lists; or 2) direct sample neighborhood control if the case was not in the previous lists. (SLE, n=161; Control, n=161)
After the exclusion of participants with diabetes mellitus, as defined by a history of diabetes, fasting glucose ≥7 mmol/L (≥126 mg/dL), or hypoglycemic therapy, the study population contained 153 SLE patients and 158 Controls.
Data Collection/Measures
Information on patient demographics and potential risk factors was collected at the time of Electron Beam Computed Tomography (EBT) scan as previously described.6 The University of Pittsburgh Institutional Review Board approved these studies, and all patients gave written informed consent. The study visit included anthropomorphic measurements (height, weight, and waist and hip circumferences), two consecutive blood pressure readings (with patients seated), and a blood draw after a required fast, which was uniform between groups. Blood samples were used to measure total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol at the Lipid Laboratory in the University of Pittsburgh, Graduate School of Public Health, which has been certified by the Centers for Disease Control and Prevention. Low-density lipoprotein (LDL) cholesterol was calculated from measured total cholesterol, HDL and triglycerides (Friedewald equation). Metabolic syndrome was defined by the National Cholesterol Education Program Adults Treatment Panel III guidelines. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by [insulin (mU/liter) × glucose (mmoles/Liter)] ÷22.5. Hypertension was defined by physician diagnosis, measured mean blood pressure ≥140/90 mm Hg, or antihypertensive medication use. Other information was collected on family history of atherosclerotic cardiovascular disease (ASCVD) (first-degree relative having myocardial infarction or stroke before age 60), cigarette smoking (ever or never), and menopausal status. Information was recorded on corticosteroid treatment (current use, ever used, and daily dosage) and current use of hydroxychloroquine, immunosuppressants, non-steroidal anti-inflammatory drugs, antihypertensives, hormonal therapy, and lipid-lowering medications.
Fibrinogen was measured using a modified clot-rate assay. An enzyme-linked immunosorbent assay was used for determination of high sensitivity CRP (hsCRP). Soluble intercellular adhesion molecule (sICAM-1) and soluble eSelectin (eSelectin) were measured using commercial assays (Parameter Human sICAM-1 Immunoassay and Parameter Human soluble eSelectin Immunoassay, respectively; R&D Systems, Minneapolis, MN) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
EBT scans
Coronary and aortic calcifications were detected by EBT, a non-invasive measure of subclinical CVD as previously described.6 The EBT scans were performed using an Imatron C-150 scanner (Imatron, San Francisco, CA) using standard imaging procedures for all participants. The same scanner and methodology was used in the SLE and healthy controls. For CAC, 3-mm slices were scanned at the same point in diastole (at 80% of the patient's RR interval in electrocardiogram) during a single breath hold, starting at the aortic root to the apex of the heart. Following completion of the coronary scan, aortic scans (6mm transaxial images, 300 ms exposure time) were obtained from the aortic arch to the iliac bifurcation. All scan data were saved to optical disc and scoring of calcification (Agatston and volumetric) was performed using a DICOM work station and software by AcuImage, Inc (San Francisco, CA). The individual region of interest scores were then summed for a total Agatston calcium score.
Descending Thoracic Aorta PVAT Volume
The 6mm transaxial EBT images were used to quantify aPVAT using the commercially available software Slice-O-Matic (Tomovision, Montreal, Canada). Several EBT scans for aPVAT quantification were not included due to missing files on the optical disk or bad optical disks (missing: nSLE=18, nControl=6).
The aPVAT was distinguished from the remaining tissue using physiological landmarks. The posterior border is the anterior portion of the vertebral foramen. The anterior border and left/right lateral borders change as the images progress distally from the carina and include the left bronchus, esophagus, and crus of diaphragm. The first proximal image analyzed includes the carina, also known as the pulmonary bifurcation, and each 6mm image is quantified through vertebral body T12. Analysis stops at the pedicles of L1. Adipose tissue areas were calculated by the range of attenuation values for adipose tissue (–190 to –30 HU). A volume (mm3) was calculated by summing the areas and multiplying by the EBT slice thickness (6mm). Two readers (KS and MG) quantified the aPVAT volume in a random subset of participants (n=11) for the reproducibility analysis. Excellent intra- and inter-reader reproducibility for the measurement of aPVAT (intra-class correlation coefficient 0.999 and 0.998, respectively) was demonstrated for the designated protocol. Only one reader (KS) completed the CT readings for this study.
Statistical Analysis
Univariate comparison of demographic variables, cardio-metabolic factors, subclinical CVD measures including CAC and AC, and aPVAT volume between SLE and control were conducted using t-tests or Mann-Whitney U tests for continuous variables and chi square or Fisher's exact test for categorical variables. Spearman correlations were used to assess associations between continuous cardio-metabolic factors and aPVAT volume.
Univariate linear regression was used to determine the CVD risk factors and circulating inflammatory factors significantly associated with log transformed aPVAT per 25cm3. In order to prevent model over-adjustment, prior to any multivariate analysis, collinearity between similar covariates was assessed by variance of inflation (VIC <10) and principal component analysis (Eigenvalues). In addition, interactions between associated covariates were assessed in the models. Based on these analyses, along with supporting evidence of interaction from Kao et al,6 the final multivariate regression models were created using either traditional CVD risk factors, including smoking history (ever versus never), average systolic blood pressure (SBP), waist-to-hip ratio, postmenopausal status, log transformed HOMA-IR, cholesterol ratio (total cholesterol/HDL), and homocysteine serum levels, or circulating inflammatory factors including log hsCRP, log PAI, fibrinogen, sICAM and eSelectin. Two best-fit multivariate linear regressions were used to determine the CVD risk factors and also circulating inflammatory markers which were associated with aPVAT using a forward selection and an inclusion criteria of p<0.25.
Univariate logistic regression analyses were used to determine the association between aPVAT, CAC, and AC with the likelihood of SLE and to determine the association between aPVAT and either any CAC or any AC. Multivariate logistic regression analyses were separately adjusted for the same predetermined CVD risk factors and inflammatory factors as the linear regression analyses. In addition, two best-fit multivariate logistic regressions were used to determine CVD risk factors and circulating inflammatory markers associated with any CAC and any AC using a forward selection and an inclusion criteria of p<0.25. All analyses were performed using SAS (version 9.2, SAS Institute, Cary, North Carolina). The level of statistical significance was set at a 2-sided p-value of <0.05.
RESULTS
The characteristics of the study groups are summarized in Table 1. The SLE and control groups were comparable among several covariates including age, BMI, ethnicity, smoking status, metabolic syndrome (MS), systolic and diastolic blood pressures, insulin and HOMA-IR. Women with SLE were slightly more likely to be postmenopausal (p=0.058), were more often hypertensive (p<0.0001) and had greater waist-to-hip ratios (p=0.001) compared to healthy controls. The SLE group had lower pulse pressures (p=0.0049) partially due to blood pressure (BP) lowering medications to control hypertension. In addition, the SLE group had lower serum glucose levels (p<0.0001), total cholesterol (p=0.0027) and LDLc (p=0.0009) than did the control group.
Table 1.
Demographic, clinical, and laboratory characteristics of SLE participants and controls.
| Covariates | SLE (n=153) | Control (n=158) | p-value |
|---|---|---|---|
| Age (yr) | 49.5 (9.7) | 51.1 (9.7) | 0.14 |
| Caucasian | 134 (87.6%) | 143 (90.5%) | 0.41 |
| Current Smoker | 16 (10.5%) | 20 (12.7%) | 0.54 |
| Post menopausal Status | 90 (58.5%) | 76 (48.1%) | 0.058 |
| BMI (kg/m2)+ | 27.0(22.6 - 31.9) | 26.9 (23.8 - 32.1) | 0.26 |
| Metabolic Syndrome | 39(25%) | 29(31%) | 0.17 |
| Waist Circumference (cm) | 83(75-94.5) | 81.5 (73 -93) | 0.46 |
| Waist to Hip Ratio | 0.833(0.79-0.88) | 0.797(0.77-0.84) | 0.001 |
| SBP (mm Hg) | 119(106-132) | 120 (110-135) | 0.087 |
| DBP (mm Hg) | 76.6(10.3) | 76.2 (9.84) | 0.71 |
| pulse pressure | 41(35-50) | 45.8(37.5-59) | 0.005 |
| Hypertensive | 82(54%) | 48(31%) | <0.0001 |
| Glucose (mmol/L) | 89 (82-94) | 93 (88-101) | <0.0001 |
| Insulin (pmol/L) | 12.1 (9.1-17.6) | 11.4 (9.2-14.8) | 0.29 |
| HOMA-IR (mmol/L × μU/mL) | 7.01(4.9-10) | 6.66(5.4-9.4) | 0.88 |
| LIPID PANEL | |||
| Triglycerides (mmol/L) | 111 (76-153) | 103 (74-140) | 0.14 |
| Total Cholesterol (mmol/L) | 185 (162-215) | 203 (175-222) | 0.003 |
| HDLc (mmol/L) | 54.2 (43.1-62) | 56 (46.4-63.8) | 0.12 |
| LDLc (mmol/L) | 107 (87-107) | 121 (101-141) | 0.001 |
| Cholesterol Ratio (tot cho/hdl) | 1.23 (1.1-1.4) | 1.27 (1.1-1.5) | 0.85 |
| CIRCULATING INFLAMMATORY MARKERS | |||
| High sensitivity CRP (mg/L) | 2.34 (0.93-5.5) | 1.61 (0.61-3.6) | 0.032 |
| Fibrinogen (mg/dL) | 337.4 (86.1) | 344.7 (63.0) | 0.4 |
| PAI (ng/ml) | 15.7 (9.6-28) | 11.8 (6.0-25.4) | 0.016 |
| sICAM (ng/ml) | 265 (233-319) | 245 (212-276) | <0.0001 |
| CD40 ligand (pg/ml) | 5543 (3759-7822) | 6205 (4804-8136 | 0.012 |
| eSelectin (ng/ml) | 47.1 (31.2-62.8) | 36.6 (26.4-53.2) | 0.001 |
| Albumin (mg/dL) | 4.6 (4.2-4.9) | 4.3 (4-4.6) | <0.0001 |
| Homocysteine (μmol/L) | 9.7 (8-11.8) | 8.9 (7.6-10.3) | 0.009 |
| C3(mg/dL) | 96 (82-118) | N/A | --- |
| C4(mg/dL) | 19 (15-25) | N/A | --- |
| MEDICATIONS | |||
| NSAID | 59 (38.6%) | 21 (13.3%) | <0.0001 |
| Aspirin | 20 (13.1%) | 13 (8.23%) | 0.17 |
| Hormone Replacement Therapy | 20 (13.1%) | 19 (12.0%) | 0.78 |
| Lipid Lowering Medication | 13 (8.5%) | 5 (3.2%) | 0.044 |
| Corticosteroids | 63 (41.2%) | 0 | <0.0001 |
| years on prednisone (n=59) | 12(7-18) | N/A | --- |
| Immunosuppressant | 27 (17.7%) | 0 | <0.0001 |
| Hydroxychloroquine use | 71 (46.4%) | 0 | <0.0001 |
| Family History MI <60yo | 62(41%) | 48 (31%) | 0.071 |
| Family History Stroke <60yo | 35 (23%) | 18 (12.0%) | 0.007 |
Mean with (Standard Deviation) are presented for normally distributed variables, median with (Inter-Quartile Range) are presented for non-normally distributed variables, and % are presented for binary variables.
The SLE group was more likely to be on lipid lowering medications (p=0.044), although this did not fully explain the observed differences between groups in lipid panel results. As expected, several circulating inflammatory factors including hsCRP, PAI, sICAM, eSelectin, albumin, and homocysteine were significantly higher in the SLE group than in the control group (all p<0.05, Table 1).
Calcification: AC and CAC
Aortic calcification (AC) was detected in more SLE women (n=118/153, 77%) than in control women (n=94/158, 59%) (p=0.0008). The two groups were comparable when evaluating coronary arterial calcification (CAC: SLE (n=69/153, 45%); control (n=60/158, 38%)), p=0.20). The SLE women had significantly higher AC scores (median 26.0 (interquartile range 25-75%, (IQR)): (3.0-233)) when compared to the control women (6.0 (IQR: 0.0-71)) (log transformed, p=0.0013), while there was no significance detected between the CAC score of the SLE women (0.0 (IQR: 0.0-14)) and control women (0.0 (IQR: 0.0-3.3), (log transformed, p=0.086).
Association with SLE
In a univariate logistic regression analysis, the presence of AC alone was associated with SLE with an odds ratio of 2.30 ([95% confidence interval (95% CI): 1.4-3.8], p=0.001). For the multivariate analysis, AC remained significantly associated with SLE after adjusting for either CVD risk factors (OR 2.94, [95% CI: 1.7- 5.2], p=0.0002) or circulating inflammatory markers (OR 1.83, [95% CI:1.1-3.1], p=0.027). The presence of CAC did not show any statistical preference between SLE and the control group regardless of the CVD risk factors (p=0.22) or circulating inflammatory markers (p=0.78).
Total aPVAT Volume
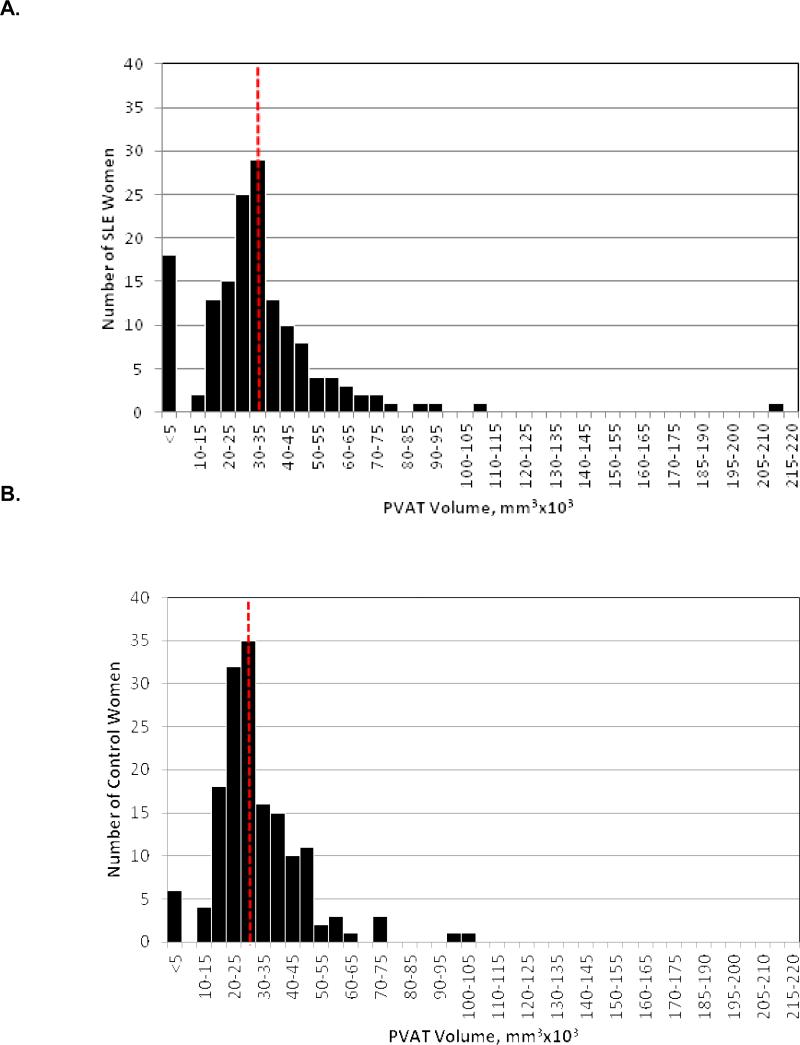
The SLE group had significantly greater aPVAT (median 32.2 (IQR: 25-42) cm3) than the control group (28.6 (IQR: 22-37) cm3) (p=0.0071).(Figure 1) There were no differences between SLE patients and controls in overall height or the length of the aorta. We found no statistical difference in total aPVAT volume between SLE subjects who had never used steroids (n=15) and those who had ever used steroids (n=120) with aPVAT median (IQR) values of 35 (30-47) vs. 31.6 (25-41), respectively (p=0.29). Interestingly, SLE subjects who had never used steroids (n=15) had significantly greater aPVAT volume when compared to the healthy controls (n=148) who had never used steroids with aPVAT median (IQR) values of 35 (30-47) vs. 28.4(22-37), respectively (p=0.03).
Figure 1.
Total aPVAT (mm3×103) distribution for A) SLE Median (25% – 75%): 32.2 (25-42) mm3×103; B) controls, 28.6 (22-37) mm3×103. (p=0.0071). Dash indicates median values.
Overall aPVAT was significantly correlated with several traditional CVD risk factors and circulating inflammatory markers associated with both SLE and CVD development.(Table 2) In the univariate linear regression analysis, waist-to-hip ratio, postmenopausal status, smoking history, average SBP, log HOMA-IR, cholesterol ratio, homocysteine, log hsCRP, fibrinogen, sICAM, log PAI, and eSelectin were found to be significantly associated with log aPVAT (all, p<0.05).(Table 3) Separate multivariable analyses were conducted by grouping either the traditional CVD risk factors or the circulating inflammatory for both SLE patients and controls.(Table 3)
Table 2.
Aortic PVAT Spearman correlations with traditional CVD risk factors and circulating inflammatory markers
| SLE aPVAT | Control aPVAT | Overall aPVAT | |
|---|---|---|---|
| ρ | ρ | ρ | |
| Traditional CVD Risk Factors | |||
| Age | 0.384* | 0.415* | 0.388* |
| HOMA-IR | 0.384* | 0.422* | 0.401* |
| Cholesterol Ratio | 0.178+ | 0.216+ | 0.191+ |
| Homocysteine | 0.164 | 0.17+ | 0.192+ |
| SBP | 0.300* | 0.427* | 0.342* |
| BMI | 0.518* | 0.572* | 0.531* |
| Waist-to-Hip ratio | 0.327* | 0.228+ | 0.303* |
| Circulating Inflammatory Markers | |||
| CRP | 0.382* | 0.373* | 0.404* |
| Fibrinogen | 0.191+ | 0.221+ | 0.197* |
| sICAM | 0.130 | 0.354* | 0.269* |
| PAI | 0.285* | 0.360* | 0.348* |
| eSelectin | 0.154 | 0.276+ | 0.230* |
p<0.001
p<0.05
Table 3.
Multivariate (MV) linear regression analysis log transformed aPVAT per 25cm3.
| SLE aPVAT | Control aPVAT | |||||
|---|---|---|---|---|---|---|
| Traditional CVD Risk Factors | Univariate | Multivariate CVD-adjusted | Best Fit, p<0.25 | Univariate | Multivariate CVD-adjusted | Best Fit, p<0.25 |
| Waist-to-Hip Ratio | 0.91(0.38)^ | 0.59(0.37) | 0.62(0.37) | 0.69(0.29)^ | 0.36(0.25) | 0.36(0.25) |
| Postmenopausal Status | 0.23(0.074)+ | 0.18(0.075)^ | 0.18(0.073)^ | 0.27(0.059)* | 0.15(0.058)+ | 0.16(0.057)+ |
| History of Smoking | 0.044(0.077) | 0.017(0.073) | -- | 0.15(0.063)^ | 0.099(0.054) | 0.10(0.053) |
| Average SBP | 0.063(0.019)* | 0.038(0.019) | 0.037(0.019) | 0.091(0.017)* | 0.047(0.017)+ | 0.047(0.017)+ |
| log HOMA-IR | 0.23(0.062)* | 0.17(0.063)+ | 0.18(0.062) | 0.36(0.060)* | 0.24(0.061)* | 0.25(0.059)* |
| cholesterol ratio | 0.061(0.035) | 0.037(0.033) | -- | 0.065(0.029)^ | 0.010(0.026) | -- |
| Homocysteine | 0.01(0.009) | −0.001(0.008) | -- | 0.011(0.007) | 0.002(0.006) | -- |
| Circulating Inflammatory Factors | Univariate | Multivariate cInflam-adjusted | Best Fit, p<0.25 | Univariate | Multivariate cInflam-adjusted | Best Fit,p<0.25 |
|---|---|---|---|---|---|---|
| log CRP | 0.12(0.029)* | 0.11(0.034)+ | 0.12(0.031)* | 0.12(0.024)* | 0.087(0.029)+ | 0.087(0.025)* |
| Fibrinogen | 0.007(0.004) | 0.001(0.005) | -- | 0.012(0.005)^ | 0.0001(0.006) | -- |
| sICAM | 0.004(0.004) | −0.006(0.005) | -- | 0.012(0.004)+ | 0.004(0.005) | 0.006(0.004) |
| log PAI | 0.11(0.042)+ | 0.062(0.045) | 0.058(0.044) | 0.12(0.027)* | 0.10(0.034)+ | 0.11(0.033)+ |
| eSelectin | 0.003(0.002) | 0.002(0.002) | -- | 0.005(0.002)+ | 0.002(0.002) | -- |
For the adjusted models, traditional CVD covariates (CVD): Waist-to-Hip ratio, Postmenopausal Status, Smoking history (ever vs never), Average SBP (per 10mmHg), log HOMA-IR, Cholesterol Ratio (HDL/Total), Homocysteine levels. Circulating inflammatory covariates (cInflam): log hsCRP, Fibrinogen (per 10 mg/dL), sICAM (per 10 ng/ml), log PAI-1, eSelectin. Data presented include the β-coefficient (SE)
p<0.001
p<0.01
p<0.05
p>0.25
Association with SLE
In a univariate logistic regression model, aPVAT (per 25 cm3) was significantly associated with SLE (OR 1.59 [95%CI: 1.1-2.3],p=0.02). When traditional CVD risk factors were added to the model, the aPVAT association with SLE remained significant (OR 1.70 [95%CI: 1.0-2.7], p=0.032). Conversely, the aPVAT association with SLE was attenuated when circulating inflammatory markers were added (OR 1.20 [95%CI: 0.83-1.7], p=0.34).
Aortic PVAT and Calcification
AC and aPVAT
In a univariate logistic regression analysis, there was a significant association between aPVAT and any AC for both SLE women (p=0.002) and control women (p=0.001).(Table 4) For women with SLE, adjustment for traditional CVD risk factors attenuated the relationship between aPVAT and any AC, while adjustment for circulating inflammatory markers preserved the significance (p=0.007). For the control women, adjustment of traditional CVD risk factors did not affect the significant relationship between aPVAT and any AC. Again, adjustment for circulating inflammatory markers maintained significance. (Table 4)
Table 4.
Logistic regression of aPVAT association with any (0 or any) vascular calcification (AC or CAC).
| SLE | Control | ||||
|---|---|---|---|---|---|
| Presence of AC | Model Adjustments | OR (95%CI) | p-value | OR (95%CI) | p-value |
| aPVAT | 6.78(2.0-23) | 0.002 | 4.61(1.9-11) | 0.001 | |
| aPVAT, CVD | 3.08(0.85-11) | 0.09 | 3.46(1.3-9.5) | 0.016 | |
| aPVAT, cInflam | 5.70(1.6-20) | 0.007 | 4.04(1.5-11) | 0.006 | |
| Best Fit:aPVAT,CVD, cInflam | 4.52(1.3-15) | 0.016 | 4.66(1.8-12) | 0.002 | |
| Presence of CAC | Model Adjustments | OR (95%CI) | p-value | OR (95%CI) | p-value |
| aPVAT | 2.66(1.4-5.0) | 0.003 | 4.85(2.2-11) | <0.0001 | |
| aPVAT, CVD | 1.43(0.81-2.5) | 0.22 | 1.90(0.78-4.6) | 0.16 | |
| aPVAT, cInflam | 1.74(0.93-3.2) | 0.084 | 2.68(1.1-6.2) | 0.023 | |
| Best Fit: aPVAT, CVD, cInflam | --- | p>0.25 | --- | p>0.25 | |
For the adjusted models, traditional CVD covariates (CVD): Waist-to-Hip ratio, Postmenopausal Status, Smoking history (ever vs never), Average SBP (per 10mmHg), log HOMA-IR, Cholesterol Ratio (HDL/Total), Homocysteine levels. Circulating inflammatory covariates (cInflam): log hsCRP, Fibrinogen (per 10 mg/dL), sICAM (per 10 ng/ml), log PAI-1, eSelectin. Data presented includes OR (95% CI) and p-value.
A best fit multivariate logistic regression including aPVAT, the traditional CVD risk factors and the circulating inflammatory markers was conducted to determine the most significant contributions of these covariates to AC for both SLE and health control women (p<0.25 for inclusion). The aPVAT remained significantly associated with AC for the SLE women (OR 4.52 [95%CI: 1.3-15], p=0.016) along with average SBP (per 10mmHg, p=0.016), sICAM (per 10ng/mL, p=0.038) and eSelectin (p=0.034). For the healthy control women, the aPVAT also remained significantly associated with AC (OR 4.66 [95% CI: 1.8-12], p=0.002) along with cholesterol ratio (p=0.053), log hsCRP (p=0.078) and fibrinogen (per 10mg/dL, p=0.17).
CAC and aPVAT
In a univariate logistic regression analysis, the aPVAT was significantly associated with any CAC for both SLE women (p=0.0028) and control women (p<0.0001). (Table 4) Adjustment for traditional CVD risk factors and measures of adiposity failed to maintain the significant association between aPVAT and CAC for each group of women. However, when adjusting only for the circulating inflammatory markers, the aPVAT of control women regained a significant association with CAC (p=0.023). (Table 4)
Increasing aPVAT with Calcification
In separate GLM analyses for SLE and HC, we found a significant pattern of increasing aPVAT with greater degree of vascular calcification (p<0.0001 for both SLE and HC groups). In other words, those with no vascular calcification had the lowest aPVAT volume, followed by higher aPVAT volume in those with either AC or CAC, and the highest aPVAT volume in those with both AC and CAC.
DISCUSSION
This is the first study to compare the unique PVAT surrounding the descending thoracic aorta in clinically CVD-free SLE women and their age- and race-matched healthy controls. In this study, we found that women with SLE have more aPVAT than do their healthy counterparts. Although the volume of aPVAT is approximately two orders of magnitude less than total visceral adipose measures16, we have shown that this small visceral adipose depot significantly correlates with cardio-metabolic risk factors, has a significant association with SLE, and is significantly associated with vascular calcification even after adjustment of CVD risk factors and circulating inflammatory markers.
There is a gap in understanding the exceptionally high risk of CVD in SLE patients, which is not fully accounted for with traditional CVD risk factors or with SLE-related risk factors.2 A cumulative inflammatory burden due to SLE and increased visceral adiposity may explain exacerbated CVD in this population. Similar to other studies, we found no significant difference in BMI or metabolic syndrome status between SLE women and controls.17 We did detect a difference in waist-to-hip ratio, which is a surrogate measure for adipose distribution. SLE women are known to be at risk for a premature state of sarcopenic obesity.18 The fact that SLE women carry adipose differently and may have a lower lean mass may support an environment that allows for increased volumes of small visceral adipose depots, including perivascular adipose, to develop at an accelerated rate.
The proximity of relatively small visceral adipose depots, which includes epicardial, pericardial, and perivascular adipose, to the vasculature suggests that they may have the potential to modulate CVD development and atherosclerotic lesion formation when compared to broadly defined regions of adipose.16, 19, 20In vitro work with human perivascular coronary adipocytes demonstrates an exacerbated pro-inflammatory condition and reduced differentiation when compared with subcutaneous and other visceral adipocytes.14 Athero-prone mouse models have displayed an exacerbated inflammatory state extending beyond the atherosclerotic plaque to the periadventitial and visceral adipose. 21 We have shown a significant association between aPVAT and SLE despite adjusting for CVD risk factors. This association is attenuated when considering the circulating inflammatory markers underscoring the cumulative inflammatory burden in women with SLE.
Increased volumes of adipose within smaller visceral depots may further contribute to the chronic inflammatory state in a disease like SLE. Also of interest is the composition of the small visceral adipose depots including white versus brown or beige adipose tissue (WAT, BAT, BRITE, respectively). While WAT has been associated with the production of adipokines, BAT and BRITE have higher metabolic rates, are more vascularized and have increased volumes in lean versus obese humans.22, 23 Perhaps increased amounts of BAT or BRITE may help to maintain a WAT homeostasis allowing the production of anti-atherogenic adipokines. However, adverse visceral adipose remodeling of cellular composition and adipocyte size contribute to the production of many of the circulating pro-inflammatory and atherogenic factors linking excessive adiposity and CVD with SLE inflammation.24, 25 The pre-existing inflammation observed in the SLE women may disrupt adipose equilibrium potentially altering the composition (i.e. WAT versus BRITE) allowing the aPVAT to increase in volume and readily progress to a dysfunctional state.
Women with SLE also have a higher risk for subclinical CVD than do healthy controls as measured by CAC 6 AC 26 and pulse wave velocity.7, 27 As shown in this study, SLE women had more extensive vascular calcification than did controls. Pericardial adipose tissue is associated with CAC 13, 28, and AC of the aortic arch has been independently related to coronary heart disease and ischemic stroke in women.29 In the Framingham Offspring study, perivascular adipose of the descending thoracic aorta was significantly associated with visceral abdominal adipose tissue and CVD risk factors, along with both CAC and abdominal AC.16 Given the systemic nature of CVD, descending thoracic AC has been found to be a strong predictor of CAC independent of CVD risk factors and is also an important subclinical CVD measure.30
Aortic calcification generally precedes CAC, which is an indicator of more advanced systemic vascular calcification.31 This study evaluated a cohort of clinically CVD-free women and supports previous findings that AC precedes CAC. In addition, these findings also suggest that the development of excessive aPVAT volume may occur within the same time frame as AC progression. Within this population of women, any AC was associated with SLE independent of traditional CVD and circulating inflammatory factors while any CAC was not significantly associated, which may in part be due to the high prevalence in the healthy control women. For those with more advanced CAC, the volume of aPVAT may already be significantly developed at this point of CVD progression. The only significant difference in surrogate adiposity measures between the two groups was the waist-to-hip ratio and the aPVAT volume. In our current study, it is unknown whether the small volume of visceral aPVAT increases prior to significant increases in abdominal visceral or subcutaneous adipose volumes. The small visceral aPVAT and other perivascular adipose depots may develop a clinically meaningful volume prior to a significant difference in waist-to-hip ratios. The significant difference in adipose distribution may indicate not only increased volumes of abdominal visceral adipose, but also increased volumes of small visceral adipose depots including aPVAT. The premature AC and the significantly higher volume of aPVAT in SLE women may reflect an increased pre-clinical CVD burden for women with SLE.
In addition, this early calcification of the aorta may account for a portion of the observed premature increase in vascular stiffness occurring in SLE women 7 leading to a higher risk of CV events in this population.2 Elevated circulating complement proteins have also been associated with increased vascular stiffness in the SLE population.7 We have previously investigated possible mechanisms for vascular stiffening in a mouse model and found complement proteins bound to the ultrastructural collagen and elastin fibers of the aorta vascular wall and aPVAT prior to athero-lesion development.32,33 The aPVAT may be a source of complement, which possibly compromises the structural integrity of the vascular wall. This same ‘outside-in’ mechanism for CVD development may be at work in the human. The aPVAT of the SLE group may be contributing to this increased vascular stiffness by following a similar pattern of adipose dysfunction generally associated with that of a chronic low grade inflammatory state observed in atherosclerotic or obese individuals. Comparisons with other subclinical CVD measures such as vascular stiffness, carotid intima-media thickness, and carotid plaque development along with analysis using sustained cardiovascular events will help to identify clinically meaningful aPVAT volumes.
Several limitations within this study exist including the retrospective cross-sectional study design. No prognostic evaluations of PVAT can be made at this time; however, the influence of aPVAT, AC, and CAC on CVD events based on collected longitudinal data within this SLE population is in progress. The majority of this population is Caucasian and any extrapolation of the findings to other ethnicities must be done with caution. Interestingly, the metabolic profiles of both the SLE women and the healthy control women were comparable and may explain the high prevalence of AC and the early onset of CAC in the healthy control group as noted by Kao et al.6 Certainly, medications such as corticosteroids often prescribed for extended periods of time may affect the function of adipose tissue. A constraint of our study is the limited number of SLE patients not on any medications, with most patients on many medications. Regardless of the small sample size, our study found a trend towards greater volumes of aPVAT in women with SLE who never took corticosteroids. This suggests that the anti-inflammatory properties of corticosteroids may play a role in adipose function and a more detailed analysis evaluating the role of anti-inflammatory medications, such as corticosteroids and immunosuppressants, would be valuable.
There may be other CVD risk factors not included in the multivariate analyses that need to be considered when evaluating adipose tissue, such as adipokines. Serum metabolic profiles including adipokine levels such as, leptin, resistin, visfatin, and adiponectin would be salient additions in future work. Elevated circulating leptin levels have been associated with MI and stroke independent of CVD risk factors 34, while adiponectin may modulate SLE activity and levels may be increased with CVD progression.35
CONCLUSIONS
In SLE as in other high risk CVD populations, early detection of subclinical atherosclerosis is highly important so that appropriate preventative strategies, including treatment of the modifiable CVD risk factors, lifestyle modification, and appropriate medication can be administered. PVAT is a viable, metabolically active visceral adipose depot with optimal location for influencing the surrounding arterial structures. Descending thoracic aPVAT is significantly greater in CVD free SLE women than in healthy controls despite similar anthropomorphic measures. Therefore, characterization of distinct visceral adipose depots and their association with proven subclinical CVD measures may allow us to infer the degree of adipose dysfunction. Early detection of adipose dysfunction using small PVAT depots in conjunction with preventive treatment plans may aid in delaying the progression of adverse arterial structural changes ultimately leading to improved cardiovascular outcomes.
Descending thoracic aorta perivascular adipose (aPVAT) is a small visceral depot.
We quantify aPVAT using EBCT in women with systemic lupus erythematosus (SLE).
Women with SLE have greater aPVAT and vascular calcification than healthy controls.
aPVAT is associated with both aortic and coronary artery calcification.
We have amended and significantly improved the quality of the manuscript based on the reviewers’ thorough analysis and detailed critique.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Janice Sabatine, Avanti Strategies, who received payment from the University of Pittsburgh and Michael Anderson for their editorial assistance.
FUNDING SOURCES
Post-doctoral fellowship through the National Heart, Lung, and Blood Institute (NHLBI Training Grant T32 - 5 T32 HL 83825-3). HEARTS Study supported by the NIH (R01AR046588)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT of INTEREST DISCLOSURES
None
REFERENCES
- 1.Urowitz MB, Bookman AA, Koehler BE, et al. The bimodal mortality pattern of systemic lupus erythematosus. The American journal of medicine. 1976;60:221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. American journal of epidemiology. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 3.Mok CC, Birmingham DJ, Ho LY, et al. High-sensitivity C-reactive protein, disease activity, and cardiovascular risk factors in systemic lupus erythematosus. Arthritis care & research. 2013;65:441–447. doi: 10.1002/acr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu CC, Ahearn JM. The search for lupus biomarkers, Best practice & research. Clinical rheumatology. 2009;23:507–523. doi: 10.1016/j.berh.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Kao AH, Wasko MC, Krishnaswami S, et al. C-reactive protein and coronary artery calcium in asymptomatic women with systemic lupus erythematosus or rheumatoid arthritis. The American journal of cardiology. 2008;102:755–760. doi: 10.1016/j.amjcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selzer F, Sutton-Tyrrell K, Fitzgerald SG, et al. Vascular Stiffness in Women With Systemic Lupus Erythematosus. Hypertension. 2001;37:1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 8.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends in endocrinology and metabolism: TEM. 2011 doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators of inflammation. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantuzzi G. Adipose tissue, adipokines, and inflammation. The Journal of allergy and clinical immunology. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 12.Larsson B. Obesity, fat distribution and cardiovascular disease. International journal of obesity. 1991;15(Suppl 2):53–57. [PubMed] [Google Scholar]
- 13.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 14.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovascular diabetology. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petri M, Perez-Gutthann S, Spence D, et al. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. The American journal of medicine. 1992;93:513–519. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 16.Lehman SJ, Massaro JM, Schlett CL, et al. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE, Nature reviews. Rheumatology. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 18.Santos MJ, Fonseca JE. Metabolic syndrome, inflammation and atherosclerosis the role of adipokines in health and in systemic inflammatory rheumatic diseases. Acta reumatologica portuguesa. 2009;34:590–598. [PubMed] [Google Scholar]
- 19.Konishi M, Sugiyama S, Sugamura K, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010;209:573–578. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulos N, McLean DS, Janik M, et al. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann C, Schafer N, von Lukowicz T, et al. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets. Atherosclerosis. 2009;207:360–367. doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 24.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 25.Bruce IN. Atherogenesis and autoimmune disease: the model of lupus. Lupus. 2005;14:687–690. doi: 10.1191/0961203305lu2201oa. [DOI] [PubMed] [Google Scholar]
- 26.Yiu KH, Wang S, Mok MY, et al. Pattern of arterial calcification in patients with systemic lupus erythematosus. J Rheumatol. 2009;36:2212–2217. doi: 10.3899/jrheum.090312. [DOI] [PubMed] [Google Scholar]
- 27.Roldan CA, Joson J, Qualls CR, et al. Premature aortic stiffness in systemic lupus erythematosus by transesophageal echocardiography. Lupus. 2010;19:1599–1605. doi: 10.1177/0961203310377088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA : the journal of the American Medical Association. 2000;283:2810–2815. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 30.Takasu J, Budoff MJ, O'Brien KD, et al. Relationship between coronary artery and descending thoracic aortic calcification as detected by computed tomography: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204:440–446. doi: 10.1016/j.atherosclerosis.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuller LH, Matthews KA, Sutton-Tyrrell K, et al. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors : the healthy women study. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2189–2198. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- 32.Santelices LC, Rutman SJ, Prantil-Baun R, et al. Relative contributions of age and atherosclerosis to vascular stiffness. Clinical and translational science. 2008;1:62–66. doi: 10.1111/j.1752-8062.2008.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shields KJ, Stolz D, Watkins SC, et al. Complement proteins C3 and C4 bind to collagen and elastin in the vascular wall: a potential role in vascular stiffness and atherosclerosis. Clinical and translational science. 2011;4:146–152. doi: 10.1111/j.1752-8062.2011.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, et al. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. The American journal of cardiology. 2007;100:234–239. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds HR, Buyon J, Kim M, et al. Association of plasma soluble E-selectin and adiponectin with carotid plaque in patients with systemic lupus erythematosus. Atherosclerosis. 2010;210:569–574. doi: 10.1016/j.atherosclerosis.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]