Abstract
Background and aims
Epidemiological data clearly indicate that cigarette smoking is associated with an increased risk for developing chronic pancreatitis and pancreatic cancer. Despite of this clear epidemiological correlation, cigarette smoke-induced pancreatic damage has only been investigated in a small number of experimental studies.
Methods
Experimental studies examining the effect of cigarette smoke or cigarette smoke constituents on the pancreas were reviewed.
Results
Recent data indicate that smoking also induces chronic pancreatic inflammation in rodents within a period of 12 weeks upon exposure with environmental cigarette smoke. Supported by the finding that morphologic pancreatic damage is also induced by nicotine treatment, cigarette smoke-induced pancreatic damage is likely to be caused by a disturbance of regulation of exocrine pancreas. The morphological alterations, however, induced by nicotine, are less pronounced and therefore, other substances and pathophysiologic mechanisms, such as carcinogen action or cigarette smoke-induced reduction of anti-protease activity, are likely to aggravate pancreatic damage upon cigarette smoke inhalation.
Conclusion
These data indicate that several constituents of cigarette smoke induce a disturbance of pancreatic function. This multifactorial event induces morphologic pancreatic damage upon cigarette smoke exposure in rodents.
Keywords: Cigarette smoking, Nicotine, Carcinogen, Pancreatic cancer, Chronic pancreatitis
Introduction
The decrease in popularity of cigarette smoking in many countries put a hold on the rise in pancreatic cancer mortality observed over decades [1-2]. This correlation between cigarette smoking and pancreatic cancer has been observed in many epidemiological studies, thus confirming cigarette smoking as the only avoidable risk factor for the development of pancreatic cancer subsequently allowing the speculation that an instant smoking cessation could lead to a 15% reduction of pancreatic cancer cases [3-5].
Despite this strong epidemiological correlation between pancreatic cancer incidence and cigarette smoking, the molecular mechanisms responsible for this observation only have been investigated in a small number of experimental studies or clinical trials which are related to substantial technical difficulties arising when attempting to study cigarette smoke-related pancreatic alterations. In clinical studies, environmental carcinogens inducing detectable carcinogen levels also in non-smokers together with low concentrations of carcinogen or carcinogen adducts require a large number of patients to be examined, leaving most studies with non-significant results [6]. In spite of unlimited amounts of specimens, experimental studies are hampered by two facts. First, experiments in rodents lack a clinical parallel because of the fact that patients usually are exposed to cigarette smoke over a period of many years, mostly over many decades. This type of exposure cannot be simulated in rodents with a life expectancy of approximately 2 years and consequently the doses of cigarette smoke applied experimentally are higher than in humans, suggesting that acute toxicity of cigarette smoke prevails over chronic carcinogenic events. A second difficulty in experimental studies is the fact that even though somewhat standardized by the use of standard cigarettes, cigarette smoke has to be generated immediately before use and is a mixture of thousands of different chemicals interacting in their actions [7]. Therefore, it is almost impossible to identify one substance responsible for one specific action because of the likely interactions of different chemicals, an uncomfortable situation for basic scientists. As a consequence, most studies either investigated the biological effect of tobacco smoke-derived substances with carcinogenic potential on the pancreas or investigated pancreatic alterations induced by pharmacologically active substances such as nicotine [8-10]. This strict separation of carcinogenic events from interference with regulatory events however, has to be undertaken with caution because recent studies indicate that carcinogenic substances as for instance 4-(methylnitrosamino)-1-(3-pyridil)-1-butanone (NNK) also exert widespread pharmacological actions [11]. Nevertheless, by treating animals either with nicotine or tobacco-derived carcinogens, adverse effects of both components of cigarette smoke on the pancreatic gland have been clearly documented.
The carcinogenic potential of single tobacco-derived carcinogens has been shown in a number of rodent models. NNK, a nicotine dependent carcinogen which is formed during the curing process of tobacco, increases the rate of pancreatic ductal adenocarcinoma development in F344 rats [12-13]. BOP, a second tobacco smoke-related carcinogens also induce pancreatic adenocarcinomas of ductal phenotype in Syrian gold hamsters and the implantation of dimethylbenzanthracene (DMBA) crystals in the pancreatic head region of mice [14]. In the case of DMBA, crystal implantation time-course studies throughout pancreatic carcinogenesis even demonstrated that the initial steps in pancreatic carcinogenesis are induced by a rapid trans-differentiation of pancreatic acinar, ductal, and islet cells to tubular complexes resembling possible precursor lesions for the subsequently developing malignancy [15]. Experimentally, tobacco-derived carcinogens have therefore demonstrated their carcinogenic effects on the pancreas, the doses applied and necessary for cancer induction, however, are unlikely to be reached by cigarette smoke inhalation in humans.
In addition to these carcinogenic events, the pharmacologically active substance nicotine contained in cigarette smoke has shown to induce morphologic pancreatic alterations as well [10, 16]. The mechanism by which nicotine relays its effects on the pancreatic gland is believed to be mediated by nicotinergic acetylcholine receptors, which are ubiquitously expressed and are also involved physiologically in the regulation of pancreatic enzyme secretion [17]. As a consequence, treatment of animals with high doses of nicotine induced significant disturbances of pancreatic enzyme secretion and even induced acinar cell damage independent of the route of application [18-20]. In spite of convincing data concerning pancreatic damage, treating animals with nicotine or with tobacco-derived carcinogens, however, did not resemble a situation comparable with the clinical situation where a subject is exposed to thousands of chemicals contained in cigarette smoke and where these substances enter the organism by inhalation.
Cigarette smoke-induced pancreatic inflammation
To gain a more realistic view on cigarette smoke-induced pancreatic alterations, pancreatic pathology was investigated after cigarette smoke exposure of rats to environmental tobacco smoke [21-22]. Environmental tobacco smoke is a defined mixture of side- and mainstream smoke, two types of tobacco smoke that differ substantially in their composition mostly because of different burning temperatures. Sprague Dawley rats were treated in groups of 12 animals with smoke from either one or two 1R4F filtered reference cigarettes for 70 min twice a day over a period of 3 months. The smoke exposure was monitored by quantifying the total suspended particulate manner (TSP) of the aerosol by an air sampler. The average doses applied were 100 mg/m3 TSP for animals treated with the smoke from one cigarette and 160 mg/m3 TSP for animals treated with the smoke of two filter cigarettes. An additional set of animals was sham treated without smoke exposure. The exposure to cigarette smoke was further monitored by blood cotinine determinations, a metabolite of nicotine exhibiting a longer half-life than nicotine itself. Serum cotinine concentrations were 145±7.88 ng/ml in animals treated with the smoke of one cigarette and was further increased to 210.00±17.3 ng/ml in animals exposed to the smoke of two 1R4F cigarettes. Cotinine could also be detected in the pancreas of animals treated with 160 mg/m3 TSP cigarette smoke, while because of the short half-life of nicotine and the 12-h time span between the final smoke exposure and the termination of the experiment, nicotine itself was not detected.
In 58% of the animals treated with two filter cigarettes twice a day over a period of 12 weeks, histomorphologic pancreatic damage was detected, while in animals subjected to 100 TSP twice daily or sham-treated animals, pancreatic histomorphological lesions were not detected. Cigarette smoke exposure induced two types of pancreatic lesions. The most frequently seen pancreatic lesion was characterized by a focal increase in extracellular matrix with subsequently decreased acinar structures. These lesions were typically confined by sharp borders frequently following the anatomical lobular structure. In addition to the increase in extracellular matrix, infiltrating cells were identified as immune cells by CD45 positive staining. These changes were of focal nature, while adjacent pancreatic tissue was morphologically unaffected. The occurrence of these lesions was low, and less than 5% of the overall pancreatic tissue was affected. Apart from these lesions, less conspicuous lesions characterized by a predominance of infiltrating TGF beta positive immune cells were observed and were interpreted as precursor lesions of inflammatory origin. The reorganization of acinar cells to connective tissue was verified by an up-regulation of procollagen type I RNA in animals displaying histomorphologic alterations when compared to animals treated with 160 TSP cigarette smoke with normal pancreatic histology. Along with pancreatic acinar cell damage, an induction of inflammatory mediators was observed. Macrophage inflammatory protein 1 alpha, a protein involved in chemotraction of macrophages, was induced in animals with morphologic pancreatic lesions, while expression was low or not detectable in the pancreata of animals treated with two cigarettes twice a day not showing altered histology [23]. Similar expression profiles were detected for interleukin 1 beta, a molecule expressed by acinar cells and immune cells which is involved in the first line of immune response [24] and transforming growth fact beta 1 indicating the involvement of TGF beta in reorganization of the pancreatic acinar tissue [25]. These data indicated that the inhalation of cigarette smoke induces an ongoing pancreatic inflammatory process related to transforming growth fact beta leading to acinar cell destruction with reorganization of circumscribed areas of the exocrine pancreas to connective tissue.
Cigarette smoke-induced alterations of digestive enzymes
Cigarette smoke-induced alterations and destruction of acinar cells was supported by the up-regulation of pancreatitis-associated protein 1 (PAP1) in animals treated with 160 TSP cigarette smoke. This gene product resembles a stress-induced protein exerting anti-inflammatory properties in acute pancreatitis, and PAP1 is aberrantly expressed by acinar cells upon cellular stress [26]. Furthermore, PAP1 is believed to form protein plugs when interacting with active digestive enzymes potentially sealing leaks of smaller pancreatic ducts in acute pancreatitis [27]. For cigarette smoke-induced pancreatic damage, the formation of protein precipitates, however, could explain the focal nature of the histological alterations observed in cigarette smoke-induced pancreatic damage, especially when PAP1 is secreted by damaged acinar cells and subsequently protein precipitates form in smaller duct interrupting the downstream drainage of pancreatic juice. By this mechanism, a vicious cycle of reduced drainage induced by already damaged acinar cells would be entered, therewith aggravating the acinar cell damage of the affected pancreatic segment.
The initial acinar cell stress induced by cigarette smoke inhalation was most likely based on a disturbance in the regulation of pancreatic digestive enzymes in relation to their secreted anti-proteases exerting protective properties [21]. Especially profound were alterations of trypsinogen regulation because trypsinogen is the key enzyme secreted by the pancreas and because trypsin has the ability to activate all other pro-enzymes after being activated by enterokinase or after auto-activation. Subsequently, the already high expression of trypsinogen in the pancreas, which is because of its nature of being a secreted protein of an exocrine gland, was further up-regulated in rats treated with 160 TSP cigarette smoke over a period of 12 weeks. A likewise observation was made for chymotrypsinogen even though for chymotrypsinogen, the up-regulation was less pronounced. Interestingly, the up-regulation of trypsinogen and chymotrypsinogen was less pronounced in animals showing histomorphological inflammatory pancreatic lesions, which parallels the observation already made in acute pancreatitis where the initiation of acinar cell damage rapidly decreases the release of digestive enzymes from isolated acini [28].
In addition to the increased expression of digestive enzymes, the expression of pancreas-specific trypsin inhibitor (PSTI), a protein preventing premature auto-activation of trypsinogen, remained unaltered upon cigarette smoke exposure [21]. This induced an imbalance of trypsinogen and PSTI expression in the pancreata of animals treated with cigarette smoke, inducing a shift toward increased expression of proteases. Furthermore, pancreatic trypsin and chymotrypsin activity of pancreatic cell lysates was decreased, indicating increased secretion of digestive enzymes in animals exposed to cigarette smoke [22]. These data indicate that cigarette smoke inhalation induces an increased turnover of pancreatic digestive enzymes, however, simultaneously, does not induce an up-regulation of protective anti-proteases, suggesting an increased vulnerability of the pancreas toward auto-digestion.
Influence of cigarette smoke on proteases and anti-protease activity
In addition to the altered ratio of protease to anti-protease secretion, other mechanisms could account for the induction of acinar cell damage and focal pancreatic inflammation by cigarette smoke inhalation. A cigarette smoke-induced increase in the rate of trypsin auto-activation or a reduction in anti-protease activity in pancreatic juice is also likely. The importance of the equilibrium of trypsin activity to anti-protease activity has been underlined by the understanding of genetic mutations in hereditary pancreatitis where both mutations of cationic trypsinogen gene (PRSS1) and mutations of its anti-protease serine protease inhibitor, Kazal type 1 (SPINK1), result in increased pancreatic trypsin activity, and where both mutations are associated with disease [29-30]. Biochemical analysis of PRSS1 mutations revealed, for the two most common mutations, an increased trypsin activity. For the R122H mutation, increased trypsin activity is mediated by an increased stability, preventing physiologic autolysis, and for the N29I, mutation is related to enhanced trypsin auto-activation [31-33]. A similar clinical appearance is observed with SPINK1 mutations, which are also associated with hereditary chronic pancreatitis [34], even though the most frequently found N34S mutation does not influence anti-protease activity of SPINK1 [35]. While to date the mechanism of the N34S mutation has not been resolved, less-frequent SPINK1 mutations seem to be related to chronic pancreatitis by inducing an altered secretion with cellular retention of this anti-protease within acinar cells [36-39]. In mutation analysis studies of hereditary pancreatitis, the importance of the ratio between tryptic activity and anti-protease activity is further underlined by observations that the G191R mutation of anionic trypsinogen (PRSS2), which introduces an additional tryptic cleavage site with complete loss of enzymatic activity of PRSS2, induces a protective effect for developing chronic pancreatitis [40].
Similar to hereditary chronic pancreatitis, the balance of protease and anti-protease activity is altered chronically by cigarette smoke inhalation [21, 41], which is most likely induced by several components of cigarette smoke. For one, an altered regulation of pancreatic enzyme synthesis and secretion but also direct interactions of cigarette smoke constituents with anti-proteases, are probable mechanisms. Even though so far uninvestigated for pancreatic anti-proteases, studies undertaken with leukocytary protease activity and pulmonary protease activity indicate that a direct inhibition of anti-protease activity might play a pivotal role also in the occurrence of pancreatic damage. Upon cigarette smoke inhalation, alpha-1 antitrypsin and alpha 1 proteinase inhibitor activity in tissue homogenates has been shown to be decreased by cigarette smoke components [42-43]. The inhibition of anti-protease activity was induced by decreased association rates of anti-proteases with proteases such as neutrophil elastase. Subsequent biochemical studies revealed that cigarette smoke-induced oxidative damage contributes substantially to anti-protease inactivation, because radical scavenges, such as glutathione and ascorbic acid, reduced these effects [44-45]. Experimentally, cigarette smoke-induced damages to anti-proteases increased the susceptibility of anti-proteases to be cleaved by proteases, further promoting a dysbalance between proteolytic and anti-proteolytic activity, because leukocyte elastase, the proteolytic counterpart, was less susceptible to inactivation by cigarette smoke components [46-47]. These mechanism could account for the decreased serum tryptic inhibitory capacity found in smokers and animals exposed to cigarette smoke and are likely to contribute to pancreatic damage [41, 48-49].
Cigarette smoke-induced regulatory events
Another pathogenetic mechanism where cigarette smoke mediates adverse effects on the pancreatic gland is the disturbance of pancreatic regulation. Cigarette smoke inhalation induced an increased expression and secretion of digestive enzymes. One possible mechanism by which these events could be mediated is the induction of CCK-A receptor expression. This receptor is physiologically responsible for stimulating enzyme synthesis and secretion of pancreatic digestive enzymes. Animals treated with 160 mg/m3 TSP environmental cigarette smoke over a period of 3 months showed an up-regulation of CCK-A by 15% [21]. The mechanisms underlying this observation are somewhat unclear, but nicotine has also been shown to induce pancreatic damage, which is most likely induced by influencing regulatory mechanisms. In these studies, nicotine induces acinar cell damage, which is characterized by cell swelling, vacuolization, and nuclear condensation as signs of morphologic pancreatic damage [19]. Interestingly, the influence of nicotine on the physiologic regulation of pancreatic enzyme secretion depends on the duration of nicotine exposure, indicating long-term effects occurring in addition to acute toxic effects [16, 18]. Short term in vitro nicotine exposure induced an increased amylase release without stimulation, while the secretion upon stimulation with CCK-A receptor ligands was decreased [18, 50]. In contrast, long-term in vivo exposure over a period of 16 weeks followed by in vitro stimulation of isolated acini induced decreased CCK-8 sensitivity and an increased cellular amylase content when compared to isolated acini of untreated animals [20].
However, when compared to these data, cigarette smoke exposure induces more widespread morphologic alterations than nicotine exposure alone, indicating that other substances contained in cigarette smoke must be involved in the induction of morphologic pancreatic damage. Especially, nitrosamines contained in cigarette smoke, such as NNK, are likely to mediate or aggravate pancreatic acinar cell damage by interfering with pancreatic regulation as well. NNK itself, which is formed during the curing process of tobacco, is a known carcinogen with the ability to induce pancreatic and other malignancies experimentally [51-53]. In humans, the pancreas must be exposed to NNK, because NNK has been detected in human pancreatic juice of smokers [6]. In rodents, NNK induces lung cancer and increases the frequency of ductal pancreatic adenocarcinomas in F344 rats [12]. In contrast to many carcinogens where DNA damage is believed to be the mode of action, not all actions of NNK are related to DNA adduct formation, and recent evidence even indicates that NNK has the ability to bind with high affinity to the alpha 7 nicotinic acetylcholine receptor and activates downstream signaling cascades [54]. In addition to the receptor-mediated action of NNK discovered so far in a variety of cell lines, a direct receptor-mediated action of NNK related to pancreatic regulation via acetylcholine receptors seems probable [55-56]. These receptor-mediated events induced by NNK may suggest an involvement of alternative non-DNA-related actions of carcinogens in pancreatic carcinogenesis and explain the carcinogenic properties in spite of the difficulties to clearly correlate DNA adducts of tobacco-related carcinogens in the pancreas to the smoking and cancer history in clinical specimens [57].
Inflammation alters regulation of ductal genes
The presence of cigarette smoke-induced focal inflammation is a likely co-factor for inducing pancreatic cancer in conjunction with DNA-altering effects of carcinogens. So far, alterations in acinar cell gene regulation and acinar cell physiology induced by cigarette smoke can only explain the increased occurrence of chronic pancreatitis in smokers. However, the increased risk for smokers to develop pancreatic cancer cannot be explained by altered regulation in enzyme secretion of acinar cells. Thus, smoking not only exhibits regulatory effects on pancreatic acinar cells but also influences ductal cell function. This has been demonstrated by the examination of pancreatic bicarbonate secretion, where in humans′ bicarbonate secretion was reduced upon cigarette smoke inhalation in a time-dependent manner [58-60]. However, in these cells, carcinogenic events are unlikely to be mediated by a disturbance of the physiologic regulation. In vitro, the impact of polycyclic aromatic hydrocarbons and NNK, two cigarette smoke-associated carcinogens, was investigated in immortalized pancreatic ductal cells [61-62]. In these studies, NNK increased the proliferation of pancreatic ductal cells by beta-adrenergic receptor transactivation of EGFR with downstream ERK 1/2 phosphorylation, indicating one potential pro-carcinogenic effect of NNK [61]. Polycyclic aromatic hydrocarbons, namely 1-methylanthracene with bay-like structures, inhibited gap junction intercellular communications, showing that these compounds are capable to induce epigenetic events in pancreatic carcinogenesis [62]. However, when animals were exposed to environmental tobacco smoke and the regulation of genes expressed by pancreatic ductal cells, such as cystic fibrosis transmembrane conductance regulator (CFTR), carbonic anhydratase, and ductal mucins was monitored, expression levels of these genes did not seem to be related to cigarette smoke exposure alone [21]. Alterations in the gene expression of CFTR and carbonic anhydratase analyzed in animals treated with 160 mg/m3 TSP cigarette smoke were not detected when compared to sham-treated animals. However, when pancreatic acinar cell lesions were detected and expression levels of these animals were compared to animals treated with the same dose of cigarette smoke that did not develop pancreatic morphologic lesions, CFTR and carbonic anhydratase expression was increased upon the development of acinar cell lesions [21]. These data imply the possible importance of pancreatic inflammation in pancreatic cancer carcinogenesis, because already small and focal pancreatic inflammatory foci induced these alterations.
Summary and future directives
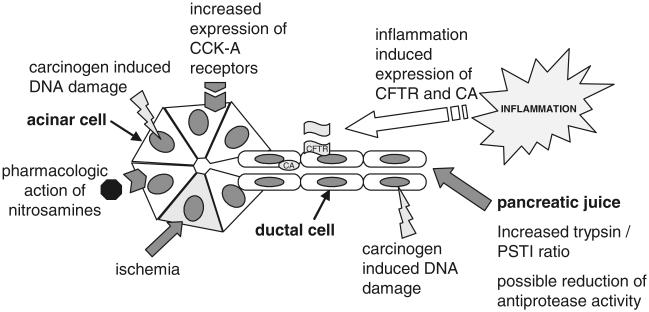
Recent data indicate a direct link between cigarette smoke inhalation and pancreatic damage in rats, which is defined by a chronic inflammatory state of the pancreas. These data resemble a possible experimental link between chronic pancreatitis and cigarette smoking but only gives indirect explanations for cigarette smoke-induced pancreatic cancer development. However, the occurrence of experimental cigarette smoke-induced pancreatic damage in rats is a model in which further studies clarifying the mechanism standing behind these observations can be performed. Current data give rise to the hypothesis that cigarette smoke-induced pancreatic damage is a multifactorial event based on cigarette smoke-induced interference with pancreatic regulation, interference of cigarette smoke components with anti-proteases, pancreatic inflammatory events, and carcinogenic or epigenetic events induced by carcinogens contained in cigarette smoke (Fig. 1). To resolve the mechanisms responsible for cigarette smoke-induced pancreatic damage, future studies will clarify the significance of each component shown to have adverse effects on the pancreas.
Fig. 1.
Experimental data on cigarette smoke-induced pancreatic damage shows several mechanisms by which detrimental effects are induced. These mechanisms include interference with pancreatic regulations by nicotine or nitrosamines, inflammatory events, carcinogen-induced DNA damage, interference of cigarette smoke constituents with anti-proteases, and possible ischemic events
Acknowledgments
We thank Mrs. Maria Schierenberg for editorial assistance.
References
- 1.Weiss W, Benarde MA. The temporal relation between cigarette smoking and pancreatic cancer. Am J Public Health. 1983;73:1403–1404. doi: 10.2105/ajph.73.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi F, Lucchini F, Negri E, La VC. Pancreatic cancer mortality in Europe: the leveling of an epidemic. Pancreas. 2003;27:139–142. doi: 10.1097/00006676-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Pisani P, Lopez AD, Masuyer E. At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. Int J Cancer. 1994;59:494–504. doi: 10.1002/ijc.2910590411. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez E, La VC, Porta M, Negri E, Lucchini F, Levi F. Trends in pancreatic cancer mortality in Europe, 1955-1989. Int J Cancer. 1994;57:786–792. doi: 10.1002/ijc.2910570605. [DOI] [PubMed] [Google Scholar]
- 5.Mulder I, Hoogenveen RT, van Genugten ML, Lankisch PG, Lowenfels AB, de Hollander AE, Bueno-de-Mesquita HB. Smoking cessation would substantially reduce the future incidence of pancreatic cancer in the European Union. Eur J Gastroenterol Hepatol. 2002;14:1343–1353. doi: 10.1097/00042737-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Prokopczyk B, Hoffmann D, Bologna M, Cunningham AJ, Trushin N, Akerkar S, Boyiri T, Amin S, Desai D, Colosimo S, Pittman B, Leder G, Ramadani M, Henne-Bruns D, Beger HG, El-Bayoumy K. Identification of tobacco-derived compounds in human pancreatic juice. Chem Res Toxicol. 2002;15:677–685. doi: 10.1021/tx0101088. [DOI] [PubMed] [Google Scholar]
- 7.IARC . Vol. 83. WHO International Agency for Research on Cancer; Lyon, France: 2004. Tobacco smoke and involuntary smoking. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 8.Rao MS. Animal models of exocrine pancreatic carcinogenesis. Cancer Metastasis Rev. 1987;6:665–676. doi: 10.1007/BF00047473. [DOI] [PubMed] [Google Scholar]
- 9.Wei D, Xiong HQ, Abbruzzese JL, Xie K. Experimental animal models of pancreatic carcinogenesis and metastasis. Int J Gastrointest Cancer. 2003;33:43–60. doi: 10.1385/IJGC:33:1:43. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury P, MacLeod S, Udupa KB, Rayford PL. Pathophysiological effects of nicotine on the pancreas: an update. Exp Biol Med (Maywood) 2002;227:445–454. doi: 10.1177/153537020222700708. [DOI] [PubMed] [Google Scholar]
- 11.Schuller HM. Neurotransmitter receptor-mediated signaling pathways as modulators of carcinogenesis. Prog Exp Tumor Res. 2007;39:45–63. doi: 10.1159/000100045. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann D, Rivenson A, Abbi R, Wynder EL. A study of tobacco carcinogenesis: effect of the fat content of the diet on the carcinogenic activity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Cancer Res. 1993;53:2758–2761. [PubMed] [Google Scholar]
- 13.Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48:6912–6917. [PubMed] [Google Scholar]
- 14.Standop J, Schneider MB, Ulrich A, Pour PM. Experimental animal models in pancreatic carcinogenesis: lessons for human pancreatic cancer. Dig Dis. 2001;19:24–31. doi: 10.1159/000050650. [DOI] [PubMed] [Google Scholar]
- 15.Bockman DE, Guo J, Buchler P, Muller MW, Bergmann F, Friess H. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest. 2003;83:853–859. doi: 10.1097/01.lab.0000074918.31303.5a. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury P, Doi R, Tangoku A, Rayford PL. Structural and functional changes of rat exocrine pancreas exposed to nicotine. Int J Pancreatol. 1995;18:257–264. doi: 10.1007/BF02784950. [DOI] [PubMed] [Google Scholar]
- 17.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der PT. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury P, Hosotani R, Rayford PL. Inhibition of CCK or carbachol-stimulated amylase release by nicotine. Life Sci. 1989;45:2163–2168. doi: 10.1016/0024-3205(89)90083-0. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury P, Rayford PL, Chang LW. Induction of pancreatic acinar pathology via inhalation of nicotine. Proc Soc Exp Biol Med. 1992;201:159–164. doi: 10.3181/00379727-201-43494b. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury P, Hosotani R, Chang L, Rayford PL. Metabolic and pathologic effects of nicotine on gastrointestinal tract and pancreas of rats. Pancreas. 1990;5:222–229. doi: 10.1097/00006676-199003000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Wittel UA, Singh AP, Henley BJ, Andrianifahanana M, Akhter MP, Cullen DM, Batra SK. Cigarette smoke-induced differential expression of the genes involved in exocrine function of the rat pancreas. Pancreas. 2006;33:364–370. doi: 10.1097/01.mpa.0000240601.80570.31. [DOI] [PubMed] [Google Scholar]
- 22.Wittel UA, Pandey KK, Andrianifahanana M, Johansson SL, Cullen DM, Akhter MP, Brand RE, Prokopczyk B, Batra SK. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101:148–159. doi: 10.1111/j.1572-0241.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 23.Shi MM, Chong IW, Long NC, Love JA, Godleski JJ, Paulauskis JD. Functional characterization of recombinant rat macrophage inflammatory protein-1 alpha and mRNA expression in pulmonary inflammation. Inflammation. 1998;22:29–43. doi: 10.1023/a:1022391623063. [DOI] [PubMed] [Google Scholar]
- 24.Rau B, Paszkowski A, Lillich S, Baumgart K, Moller P, Beger HG. Differential effects of caspase-1/interleukin-1beta-converting enzyme on acinar cell necrosis and apoptosis in severe acute experimental pancreatitis. Lab Invest. 2001;81:1001–1013. doi: 10.1038/labinvest.3780312. [DOI] [PubMed] [Google Scholar]
- 25.Rane SG, Lee JH, Lin HM. Transforming growth factor-beta pathway: role in pancreas development and pancreatic disease. Cytokine Growth Factor Rev. 2006;17:107–119. doi: 10.1016/j.cytogfr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Closa D, Motoo Y, Iovanna JL. Pancreatitis-associated protein: from a lectin to an anti-inflammatory cytokine. World J Gastroenterol. 2007;13:170–174. doi: 10.3748/wjg.v13.i2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiesser M, Bimmler D, Frick TW, Graf R. Conformational changes of pancreatitis-associated protein (PAP) activated by trypsin lead to insoluble protein aggregates. Pancreas. 2001;22:186–192. doi: 10.1097/00006676-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Carter KJ, Rutledge PL, Steer ML, Silen W. Secretagogue-induced changes in intracellular pH and amylase release in mouse pancreatic acini. Am J Physiol. 1987;253:G690–G696. doi: 10.1152/ajpgi.1987.253.5.G690. [DOI] [PubMed] [Google Scholar]
- 29.Rosendahl J, Bodeker H, Mossner J, Teich N. Hereditary chronic pancreatitis. Orphanet J Rare Dis. 2007;2:1. doi: 10.1186/1750-1172-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin-Toth M. Biochemical models of hereditary pancreatitis. Endocrinol Metab Clin North Am. 2006;35:303–312. doi: 10.1016/j.ecl.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teich N, Rosendahl J, Toth M, Mossner J, Sahin-Toth M. Mutations of human cationic trypsinogen (PRSS1) and chronic pancreatitis. Hum Mutat. 2006;27:721–730. doi: 10.1002/humu.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archer H, Jura N, Keller J, Jacobson M, Bar-Sagi D. A mouse model of hereditary pancreatitis generated by transgenic expression of R122H trypsinogen. Gastroenterology. 2006;131:1844–1855. doi: 10.1053/j.gastro.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 33.Simon P, Weiss FU, Sahin-Toth M, Parry M, Nayler O, Lenfers B, Schnekenburger J, Mayerle J, Domschke W, Lerch MM. Hereditary pancreatitis caused by a novel PRSS1 mutation (Arg-122 → Cys) that alters autoactivation and autodegradation of cationic trypsinogen. J Biol Chem. 2002;277:5404–5410. doi: 10.1074/jbc.M108073200. [DOI] [PubMed] [Google Scholar]
- 34.Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 35.Kuwata K, Hirota M, Shimizu H, Nakae M, Nishihara S, Takimoto A, Mitsushima K, Kikuchi N, Endo K, Inoue M, Ogawa M. Functional analysis of recombinant pancreatic secretory trypsin inhibitor protein with amino-acid substitution. J Gastroenterol. 2002;37:928–934. doi: 10.1007/s005350200156. [DOI] [PubMed] [Google Scholar]
- 36.Kiraly O, Boulling A, Witt H, Le MC, Chen JM, Rosendahl J, Battaggia C, Wartmann T, Sahin-Toth M, Ferec C. Signal peptide variants that impair secretion of pancreatic secretory trypsin inhibitor (SPINK1) cause autosomal dominant hereditary pancreatitis. Hum Mutat. 2007;28:469–476. doi: 10.1002/humu.20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulling A, Le MC, Trouve P, Raguenes O, Chen JM, Ferec C. Functional analysis of pancreatitis-associated missense mutations in the pancreatic secretory trypsin inhibitor (SPINK1) gene. Eur J Hum Genet. 2007;15:936–942. doi: 10.1038/sj.ejhg.5201873. [DOI] [PubMed] [Google Scholar]
- 38.Kiraly O, Wartmann T, Sahin-Toth M. Missense mutations in pancreatic secretory trypsin inhibitor (SPINK1) cause intracellular retention and degradation. Gut. 2007;56:1434–1439. doi: 10.1136/gut.2006.115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masamune A, Kume K, Takagi Y, Kikuta K, Satoh K, Satoh A, Shimosegawa T. N34S mutation in the SPINK1 gene is not associated with alternative splicing. Pancreas. 2007;34:423–428. doi: 10.1097/mpa.0b013e3180335fd0. [DOI] [PubMed] [Google Scholar]
- 40.Witt H, Sahin-Toth M, Landt O, Chen JM, Kahne T, Drenth JP, Kukor Z, Szepessy E, Halangk W, Dahm S, Rohde K, Schulz HU, Le MC, Akar N, Ammann RW, Truninger K, Bargetzi M, Bhatia E, Castellani C, Cavestro GM, Cerny M, stro-Bisol G, Spedini G, Eiberg H, Jansen JB, Koudova M, Rausova E, Macek M, Jr., Malats N, Real FX, Menzel HJ, Moral P, Galavotti R, Pignatti PF, Rickards O, Spicak J, Zarnescu NO, Bock W, Gress TM, Friess H, Ockenga J, Schmidt H, Pfutzer R, Lohr M, Simon P, Weiss FU, Lerch MM, Teich N, Keim V, Berg T, Wiedenmann B, Luck W, Groneberg DA, Becker M, Keil T, Kage A, Bernardova J, Braun M, Guldner C, Halangk J, Rosendahl J, Witt U, Treiber M, Nickel R, Ferec C. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet. 2006;38:668–673. doi: 10.1038/ng1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morosco GJ, Goeringer GC. Pancreatic elastase and serum alpha 1-antitrypsin levels in beagle dogs smoking high- and low-nicotine cigarettes: possible mechanism of pancreatic cancer in cigarette smokers. J Toxicol Environ Health. 1979;5:879–890. doi: 10.1080/15287397909529797. [DOI] [PubMed] [Google Scholar]
- 42.Janoff A, Carp H, Lee DK, Drew RT. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979;206:1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- 43.Evans MD, Church DF, Pryor WA. Aqueous cigarette tar extracts damage human alpha-1-proteinase inhibitor. Chem Biol Interact. 1991;79:151–164. doi: 10.1016/0009-2797(91)90079-m. [DOI] [PubMed] [Google Scholar]
- 44.Aubertin A. Vitamin C: how it may protect against cancer is unclear. J Natl Cancer Inst. 1991;83:396–397. doi: 10.1093/jnci/83.6.396. [DOI] [PubMed] [Google Scholar]
- 45.Pryor WA, Dooley MM, Church DF. The inactivation of alpha-1-proteinase inhibitor by gas-phase cigarette smoke: protection by antioxidants and reducing species. Chem Biol Interact. 1986;57:271–283. doi: 10.1016/0009-2797(86)90002-5. [DOI] [PubMed] [Google Scholar]
- 46.Stockley RA, Afford SC. The interaction of cigarette smoke solution with alpha 1-antitrypsin: effect on inhibitory capacity, electrophoretic mobility and immunological measurement. Clin Sci (Lond) 1983;64:223–230. doi: 10.1042/cs0640223. [DOI] [PubMed] [Google Scholar]
- 47.Janoff A, Dearing R. Alpha 1-proteinase inhibitor is more sensitive to inactivation by cigarette smoke than is leukocyte elastase. Am Rev Respir Dis. 1982;126:691–694. doi: 10.1164/arrd.1982.126.4.691. [DOI] [PubMed] [Google Scholar]
- 48.Chowdhury P, Bone RC, Louria DB, Rayford PL. Effect of cigarette smoke on human serum trypsin inhibitory capacity and antitrypsin concentration. Am Rev Respir Dis. 1982;126:177–179. doi: 10.1164/arrd.1982.126.1.177. [DOI] [PubMed] [Google Scholar]
- 49.Ito H, Watanabe T, Shore SR, Aviado DM. Functional and biochemical effects on the lung following inhalation of cigarette smoke and its constituents. III. Serum antitrypsin and bronchomotor responses in rats. Toxicol Appl Pharmacol. 1976;35:403–412. doi: 10.1016/0041-008x(76)90063-6. [DOI] [PubMed] [Google Scholar]
- 50.Majumdar AP, Davis GA, Dubick MA, Geokas MC. Nicotine stimulation of protein secretion from isolated rat pancreatic acini. Am J Physiol. 1985;248:G158–G163. doi: 10.1152/ajpgi.1985.248.2.G158. [DOI] [PubMed] [Google Scholar]
- 51.Baskaran K, Laconi S, Reddy MK. Transformation of hamster pancreatic duct cells by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), in vitro. Carcinogenesis. 1994;15:2461–2466. doi: 10.1093/carcin/15.11.2461. [DOI] [PubMed] [Google Scholar]
- 52.Ho YS, Chen CH, Wang YJ, Pestell RG, Albanese C, Chen RJ, Chang MC, Jeng JH, Lin SY, Liang YC, Tseng H, Lee WS, Lin JK, Chu JS, Chen LC, Lee CH, Tso WL, Lai YC, Wu CH. Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFkappaB activation and cyclin D1 up-regulation. Toxicol Appl Pharmacol. 2005;205:133–148. doi: 10.1016/j.taap.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Schuller HM, Zhang L, Weddle DL, Castonguay A, Walker K, Miller MS. The cyclooxygenase inhibitor ibuprofen and the FLAP inhibitor MK886 inhibit pancreatic carcinogenesis induced in hamsters by transplacental exposure to ethanol and the tobacco carcinogen NNK. J Cancer Res Clin Oncol. 2002;128:525–532. doi: 10.1007/s00432-002-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuller HM. Life Sci. 2007;80:2274–2280. doi: 10.1016/j.lfs.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cancela JM, Van CF, Galione A, Tepikin AV, Petersen OH. Transformation of local Ca2+ spikes to global Ca2+ transients: the combinatorial roles of multiple Ca2+ releasing messengers. dEMBO J. 2002;21:909–919. doi: 10.1093/emboj/21.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bragado MJ, Garcia LJ, Montero A, San Roman JI, Calvo JJ, Lopez MA. Nicotinic cholinergic influences in pancreatic secretion induced by intraduodenal alkaline and acid solutions in the rabbit. Gen Pharmacol. 1993;24:687–692. doi: 10.1016/0306-3623(93)90232-m. [DOI] [PubMed] [Google Scholar]
- 57.Prokopczyk B, Leder G, Trushin N, Cunningham AJ, Akerkar S, Pittman B, Ramadani M, Straeter J, Beger HG, Henne-Bruns D, El-Bayoumy K. 4-Hydroxy-1-(3-pyridyl)-1-butanone, an indicator for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA damage, is not detected in human pancreatic tissue. Cancer Epidemiol Biomarkers Prev. 2005;14:540–541. doi: 10.1158/1055-9965.EPI-04-0626. [DOI] [PubMed] [Google Scholar]
- 58.Murthy SN, Dinoso VP, Jr., Clearfield HR, Chey WY. Simultaneous measurement of basal pancreatic, gastric acid secretion, plasma gastrin, and secretin during smoking. Gastroenterology. 1977;73:758–761. [PubMed] [Google Scholar]
- 59.Brown P. The influence of smoking on pancreatic function in man. Med J Aust. 1976;2:290–293. doi: 10.5694/j.1326-5377.1976.tb130184.x. [DOI] [PubMed] [Google Scholar]
- 60.Bynum TE, Solomon TE, Johnson LR, Jacobson ED. Inhibition of pancreatic secretion in man by cigarette smoking. Gut. 1972;13:361–365. doi: 10.1136/gut.13.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131:639–648. doi: 10.1007/s00432-005-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tai MH, Upham BL, Olson LK, Tsao MS, Reed DN, Jr, Trosko JE. Cigarette smoke components inhibited intercellular communication and differentiation in human pancreatic ductal epithelial cells. Int J Cancer. 2007;120:1855–1862. doi: 10.1002/ijc.22530. [DOI] [PubMed] [Google Scholar]