Abstract
ROS1 gene rearrangements are reported in 1–2% of lung adenocarcinomas (ACA) and are associated with response to the multitargeted tyrosine kinase inhibitor, crizotinib. ROS1 rearrangements can be detected using fluorescence in situ hybridization (FISH) however immunohistochemistry (IHC) for ROS1 protein is a promising alternate screening modality. In this study we examine the correlation between ROS1 IHC and FISH and describe the clinicopathologic characteristics of ROS1-rearranged lung tumors. ROS1 IHC was performed using clone D4D6 (Cell Signaling Technology, Danvers, MA) on whole tissue sections. In a validation cohort, IHC was compared to ROS1 break-apart FISH in 53 cases of lung ACA enriched for an absence of known genetic alterations and never-smoking status. In a screening cohort, we performed ROS1 IHC on 167 consecutive cases of lung ACA from a routine molecular diagnostics practice and confirmed positive results by FISH. In the validation cohort, 6 cases (11%) were both FISH and IHC positive. One FISH-negative case was strongly ROS1 IHC positive. All IHC negative cases were FISH negative. In the screening cohort, 2 of 167 (1.2%) had strong, diffuse ROS1 protein expression; a rearrangement was confirmed by FISH in both. ROS1-translocated tumors were wild type for EGFR, KRAS, and ALK and commonly had solid growth with mucinous/cribriform features and psammomatous calcification. ROS1 protein expression in tumor cells is 100% sensitive and 92% specific for ROS1 rearrangements by FISH. ROS1 IHC is an effective screening tool for this rare but clinically important subset of lung ACA.
Keywords: ROS1, lung adenocarcinoma, immunohistochemistry
INTRODUCTION
Lung adenocarcinomas (ACA) contain a number of defined oncogenic alterations, some of which predict response to targeted therapies. EGFR kinase domain mutations occur in 10–20% of lung ACA and predict response to EGFR tyrosine kinase inhibitors (TKIs). (1,2) Rearrangements involving the anaplastic lymphoma kinase (ALK) gene occur in about 5% of lung ACA and predict response to the multitargeted kinase inhibitor crizotinib. (3) KRAS mutations occur in approximately 25% of lung ACA, (4) typically in a mutually exclusive manner with EGFR and ALK alterations, and are associated with a lack of response to EGFR TKIs.(5)
Rearrangements involving the ROS1 oncogene were first described in glioblastoma multiforme(6) and later discovered in non small cell lung carcinomas (NSCLC) using a phosphoproteomic screen. (7) Recent studies have described ROS1 rearrangements in approximately 1–2% of NSCLC using fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) screens. (8–10) ROS1 fusion partners include CD74, SLC34A2, and SDC4, all of which lead to oncogenic transformation in cell culture and/or in vivo. (8–10) Importantly, in vitro studies and early clinical reports have demonstrated that ROS1-translocated lung ACA can be inhibited by crizotinib. (10,11) Therefore, the detection of ROS1 translocations identifies a subset of patients with lung ACA who may benefit from targeted therapy.
The routine detection of ROS1-rearranged ACAs in clinical practice has been limited by the lack of clinically validated, commercially-available reagents. The lack of diagnostic reagents has also prevented the collection of cases for epidemiological studies. To date, most testing has relied on laboratory-specific FISH assays that utilize a break-apart probe spanning the common break point region (including exons 32, 34, and 35) in the ROS1 gene. The presence of a ROS1 rearrangement leads to a split signal in the majority of cases, or the loss of 5’ probe signal in the case of the FIG1-ROS1 translocation. (8–10) Initial studies suggested that ROS1 rearrangements are more common in young never-smokers of Asian ethnicity, (10) however the data on these clinicopathologic correlations remains limited. Given the relative rarity of these alterations, large numbers of patient specimens will need to be screened to identify and better characterize ROS1-translocated lung tumors.
For many laboratories, FISH is an expensive and cumbersome assay to support, requiring specialized microscopy equipment and technical expertise. In contrast, IHC is performed in many pathology laboratories and can be readily interpreted by surgical pathologists in the course of clinical diagnostic practice. A ROS1 IHC antibody has recently been described that appears to specifically detect ROS1-fusion proteins in NSCLC. (9) However, confirmation of the sensitivity and specificity of this antibody in clinically and genetically characterized cohorts is required before it can be adopted for routine clinical use.
In this study, we validate the performance of the ROS1 (D4D6) IHC antibody as compared to ROS1 break-apart FISH in lung tumors from 53 patients clinically selected for testing based on smoking history and mutation status. We then utilize ROS1 IHC to screen a cohort of 167 consecutive lung adenocarcinomas without regard to clinical history or mutation status. Using the cases identified in these cohorts, we describe the clinicopathologic features associated with ROS1 translocation and fusion protein overexpression.
METHODS
CASE SELECTION
The validation cohort was comprised of 53 cases that were clinically selected for ROS1 FISH and IHC testing based on a diagnosis of lung ACA, eligibility for chemotherapy, and availability of materials for further testing. This cohort was enriched for tumors from never- or light-smokers that were wild type for EGFR, KRAS, ALK, BRAF, ERBB2, and PIK3CA (Table 1). (12) Specimens tested in this cohort were obtained from 2000 to 2012 and included 27 open lung biopsies, 12 lymph node or soft tissue biopsies, 3 fine needle aspirations (FNA), 9 core biopsies, and 2 brain metastatectomies. 167 cases included in the IHC screening cohort were comprised of lung ACA diagnosed between 2006–2012 and were captured following clinical molecular testing including EGFR and KRAS mutation analysis, BRAF, ERBB2, and PIK3CA mutation analysis (in some cases), and ALK FISH and/or ALK IHC. Specimen types included 65 open lung biopsies, 45 lymph node or soft tissue biopsies, 20 FNAs, 31 core biopsies and 6 brain metastatectomies. Clinical histories were obtained from the electronic medical record following approval by the Dana Farber Cancer Institute Institutional Review Boards. All patients provided written informed consent for participation in correlative science studies.
Table 1.
Clinical characteristics of patients tested in ROS1 IHC validation and screening cohorts.
| Validation cohort N=53 |
Screening cohort N=167 |
|
|---|---|---|
| Mean age (range) | 61 (31–88) | 65 (29–94) |
| Gender | ||
| Male | 27 (51) | 73 (44) |
| Female | 26 (49) | 94 (56) |
| Smoking status | ||
| Never | 31 (58) | 38 (23) |
| Former | 20 (38) | 82 (49) |
| Mean pack years (range) | 13.25 (1–69) | 30 (1–100) |
| Current | 2 (4) | 45 (27) |
| Mean pack years (range) | 24.5 (5–44) | 60.6 (20–150) |
| Race | ||
| Asian | 6 (11) | 10 (6) |
| Black | 4 (8) | 3 (2) |
| Caucasian | 41 (77) | 145 (87) |
| Hispanic | 2 (4) | 3 (2) |
| Unknown | 0 | 6 (4) |
| Stage at initial diagnosis | ||
| IA | 8 (15) | 20 (12) |
| IB | 6 (11) | 13 (8) |
| IIA | 6 (11) | 8 (5) |
| IIB | 0 (0) | 8 (5) |
| IIIA | 11 (21) | 35 (21) |
| IIIB | 4 (8) | 3 (2) |
| IV | 18 (34) | 80 (48) |
| Tumor histology | ||
| Adenocarcinoma | 53 (100) | 167 (100) |
| Mutation status | ||
| “Pan-WT” | 50 (94) | 93 (56) |
| EGFR | 2 (4) | 19 (11) |
| KRAS | 0 | 40 (25) |
| ALK | 1 (2) | 9 (5) |
| BRAF | 0 | 3 (2) |
| ERBB2 | 0 | 0 (0) |
| PIK3CA | 0 | 3 (2) |
| ROS1- altered | 7 (13) | 2 (1.2) |
PATHOLOGY REVIEW
Hematoxylin and eosin-stained slides were reviewed by a surgical pathologist with thoracic expertise (LMS) and were graded and subtyped according to WHO and IASLC recommendations.(13,14)
FISH
Four µm-thick formalin-fixed paraffin-embedded (FFPE) tissue sections were evaluated for ROS1 translocation by FISH using probes flanking the ROS1 gene (RP11-59K17 and RP1-92C8 (BACPAC Resource, Oakland, California)). Briefly, tissue sections were mounted on positively charged slides and air-dried. Targeted tumor areas were circled with a diamond pen following review of the corresponding hematoxylin and eosin (H&E) slide by a pathologist (LMS). Slides were deparaffinized, dehydrated, incubated in Tris-EDTA solution (100mM Tris-HCL,50mM EDTA, pH 7.0) at 100°C for 45 minutes. Slides were washed in 1× PBS and then incubated in Pepsin solution Digest-All 3 (Life Technologies, Grand Island, NY) at 37°C for 20 minutes. Slides were washed and dried at 40–50°C on a slide warmer for 2–5 minutes. The tissue was then fixed in 10% buffered formalin at room temperature (RT) for 1 minute, washed and dehydrated at RT in 70%, 85%, 100% ethanol for 2 minutes each. ROS1 probes were added to the slide, and denatured simultaneously with the target DNA at 95oC for 3 minutes. Hybridization was carried out at 37°C for 48hours. Post-hybridization wash was performed in 0.5X SSC at 72°C for 5 minutes, followed by 3 washes in 0.025% Tween 20-PBS at RT, 2 minutes each. Slides were counterstained with DAPI and stored in the dark at −20°C before microscopic examination.
Results were analyzed with an OLYMPUS BX51 fluorescent microscope (Olympus, Center Valley, PA). A minimum of 50 nuclei from two separate areas of tumor were scored. Representative images were captured using CytoVision Imaging System (Leica Microsystems, Buffalo Grove, IL) and/or the BioView Duet System (Image Solutions, Ltd., Preston, UK).
Samples were classified as positive for ROS1 rearrangement when ≥15% of nuclei showed split signals or single red signals (3' ROS1).
Immunohistochemistry
Immunohistochemistry for ROS1 was performed on 4 µm-thick FFPE tissue sections using clone D4D6 (Cell Signaling Technology (CST), Danvers, MA). Slides were baked, deparaffinized in xylene, passed through graded alcohols, and then antigen retrieved with 1mM EDTA, pH8.0 (Invitrogen, Carlsbad, CA) in a steam pressure cooker (Decloaking Chamber; BioCare Medical, Walnut Creek, CA) at 125°C for 30 seconds. All further steps were carried out at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (Dako USA, Carpentaria, CA) for 5 minutes to quench endogenous peroxidase activity, and then washed in 50mM Tris-Cl, pH7.4. Slides were blocked using normal goat serum (Dako, 250ul normal goat serum added to 5ml 50mM Tris-Cl), and subsequently incubated with rabbit anti-ROS1 monoclonal antibody (clone D4D6, 1:1000; CST) diluted in Signalstain antibody diluent (#8112, CST) for 1 hour. Slides were then washed in 50mM Tris-Cl, pH7.4 and treated with Signalstain boost IHC detection reagent (HRP, rabbit, #8114, CST) for 30 minutes. After further washing, immunoperoxidase staining was developed using a 3,3’diaminobenzidine (DAB) chromogen (Dako) for 5 min. Slides were counterstained with hematoxylin, dehydrated in graded alcohol and xylene, mounted and coverslipped.
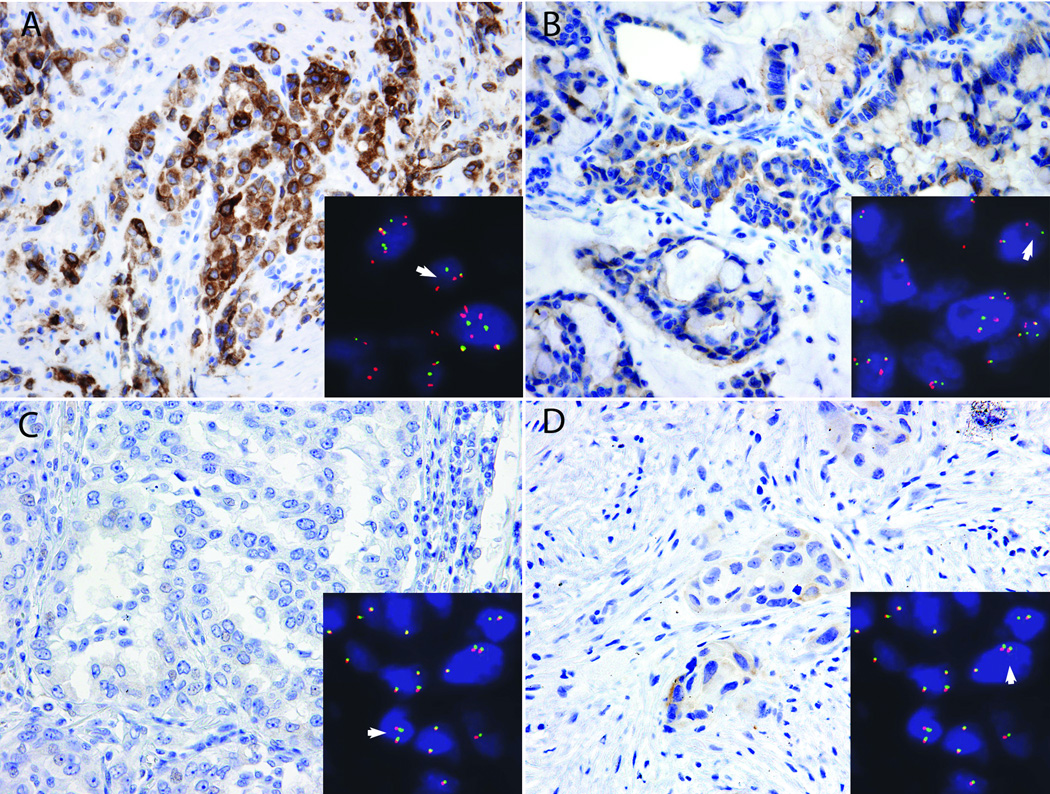
The stained slides were reviewed by a pathologist (LMS) blinded to FISH results. Staining was graded semiquantitatively as follows: 0 for absent expression or nuclear expression only, 1+ for cytoplasmic faint, barely perceptible staining not exceeding background in any percentage of cells, 2+ for cytoplasmic staining exceeding background in 0 to 50% of tumor cells, and 3+ for cytoplasmic staining exceeding background in >50% of tumor cells (Figure 1). Positive cases demonstrated a granular to diffuse cytoplasmic expression pattern, frequently of varying intensity within the tumor cell population.
Figure 1.
Representative images of ROS1 IHC and corresponding ROS1 FISH results. A) Strong, diffuse (3+) staining in a case with ROS1 FISH rearrangement (split red and green signals, arrowhead; inset). B) Weak to moderate but diffuse (3+) staining in a case with ROS1 FISH rearrangement (arrowhead, inset). C) Absent ROS1 IHC in a case with no rearrangement by FISH (fused red and green signals only, arrowhead; inset). D) Weak staining in scattered cells (2+) in a case without a rearrangement by FISH (arrowhead; inset).
Mutation analysis
For mutation analysis, DNA was extracted from dissected 4µm FFPE tissue sections containing more than 50% tumor cells. For EGFR mutation analysis, allele specific PCR for exon 21 Leu858Arg mutation and a PCR-based sizing assay for the exon 19 deletion mutation were performed (primer sequences available upon request). KRAS codons 12 and 13 were analyzed using pyrosequencing (primer sequences available upon request). ALK FISH was performed using commercially available, FDA-approved ALK FISH dual color breakapart probes (Abbott Molecular) as previously described. (15) ALK IHC was performed using clone 5A4 (Novocastra, UK) as previously described. (15) Tumors lacking an alteration using these targeted assays underwent Sanger sequencing of the EGFR kinase domain (exons 18 through 21), KRAS exons 2 and 3 and/or BRAF exons 11 and 15, ERBB2 exon 20, and PIK3CA exons 9 and 20, as previously described.(12)
Statistics
Time to death was calculated by subtracting the date of original diagnosis from date of death. Survival comparisons were performed using the log-rank test for equality. Fisher's exact test was used to compare dichotomous variables and p values were corrected for multiple comparisons using a Bonferroni correction. Statistical calculations were performed using Stata 12.1 (StataCorp LP, College Station, TX).
RESULTS
Validation cohort
53 cases of lung ACA were evaluated for ROS1 alterations using ROS1 IHC and breakapart FISH; the clinical and molecular features of this cohort are detailed in Table 1.
ROS1 FISH
In a clinically-selected cohort enriched for patients with limited smoking history and mutation-negative ACA (Table 1), 6 of 53 (11%) tumors were ROS1 rearranged by FISH (representative images in Figure 1). The frequency of split signals in the FISH-positive cases averaged 72%, and ranged from 46–89%. 44 cases were scored as FISH negative. The FISH negative cases contained an average of 5.7% split signals (range 0–12%). Three cases failed FISH.
ROS Immunohistochemistry
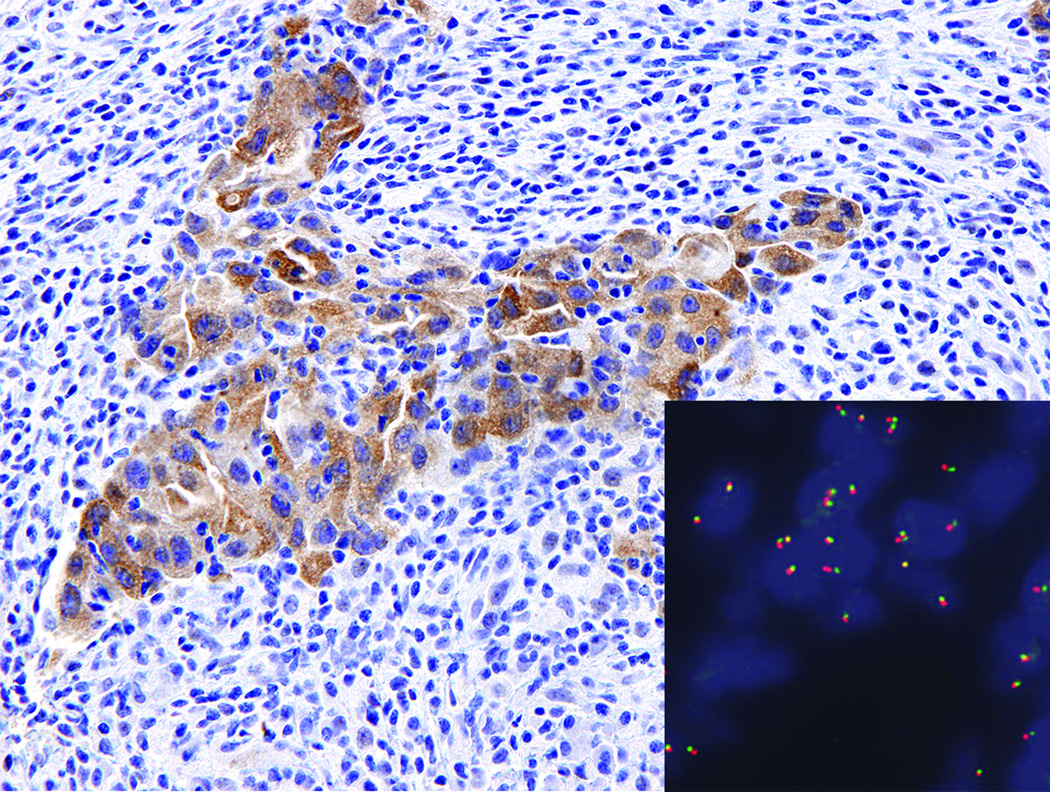
13 of the 53 cases tested showed any degree of ROS1 expression in tumor cells. Of these, six (11%) showed 3+ (>50% of cells staining with intensity ranging from weak to strong) (Figure 1, panels A and B), one (1.9%) showed 2+ (<50% of cells with weak to moderate staining), and six (11%) showed 1+ (focal, barely perceptible) cytoplasmic expression. Six of the seven cases with 2–3+ staining contained a ROS1 rearrangement by FISH. One case with 3+ ROS1 staining was negative for a rearrangement by FISH (Figure 2). The intensity of ROS1 protein expression did not correlate with percentage of split signals; in fact, the one case with 2+ ROS1 protein expression had the highest percentage of FISH positive tumor nuclei (89%) (other data not shown). All cases with absent to 1+ ROS1 staining were FISH negative (Figure 1, panel C). Two cases that failed FISH were ROS IHC negative. One case that failed ROS1 FISH was also inadequate for IHC.
Figure 2.
Moderate to strong, diffuse (3+) staining in a lymph node metastasis with no detectable rearrangement by FISH (fused signals only; inset).
Screening Cohort
167 cases of lung ACA with unknown ROS1 rearrangement status were screened using ROS IHC; the clinical and molecular features of this cohort are detailed in Table 1. 93 cases were considered wild type (WT). Of the cases included in this WT category, one case was inconclusive for all mutational analyses, three cases lacked sufficient tumor tissue for ALK analysis, and in an additional 8 cases KRAS mutation testing was either not performed or was inconclusive.
ROS Immunohistochemistry
Of 167 cases tested, two had insufficient tumor remaining to score the IHC. 17 cases total had any degree of ROS1 protein expression, including 12 with 1+, 3 with 2+, and 2 with 3+ staining.
ROS1 Fish
In light of the fact that none of the 1+ cases from the validation cohort were ROS1 rearranged by FISH, only cases with 2+ staining or greater from the screening cohort were further tested by FISH. One case with 2+ staining (Figure 1D) harbored a KRAS codon 12 mutation and the remaining 2+ ROS IHC positive cases were considered “pan-WT”. These three cases contained a mean of 7% FISH positive nuclei (range 4–9%) and thus were all considered to be negative for a ROS1 rearrangement. Only the two 3+ IHC positive cases were confirmed as ROS1 rearranged by FISH; one contained 72% positive nuclei and the other contained 24% positive nuclei. Thus, ROS1 rearrangements were detected in this screening cohort at a rate of 2 of 165 (1.2%) evaluable cases overall or 2 of 93 (2.2%) otherwise pan-WT cases.
Staining Pitfalls
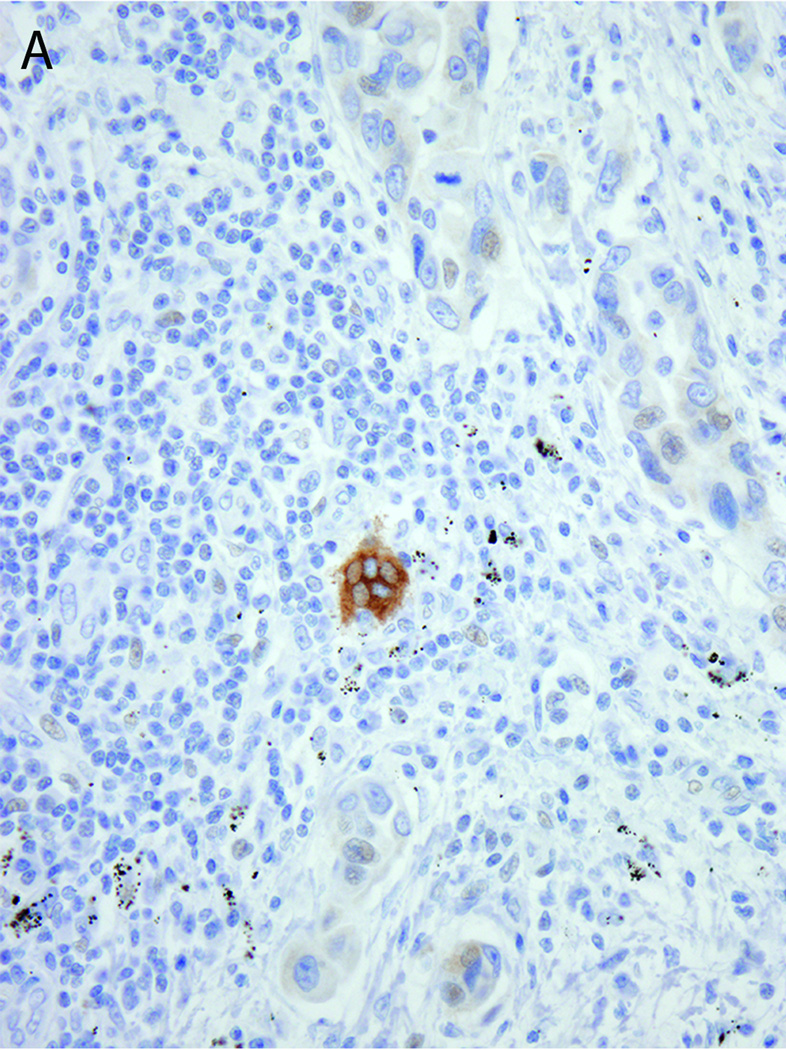
ROS1 protein expression was also detected in osteoclast-type giant cells (Figure 3A), when present, and in reactive epithelial proliferations, including in areas of type II pneumocyte hyperplasia and bronchiolar metaplasia. This expression was occasionally seen in nonneoplastic alveolar epithelium at the tumor periphery or in subpleural pneumocytes. In most cases, the expression in this epithelium was 1–2+ in intensity. However, one case from a patient with an ALK-rearranged tumor and concurrent pulmonary mycobacterial infection showed strong (3+) ROS1 protein expression in hyperplastic type II pneumocytes surrounding areas of organizing pneumonia (Figure 3B). ROS1 protein expression was also detected at a very low level (1+) in rare tumor cells. ROS1 FISH was performed on this specimen and was negative.
Figure 3.
ROS1 protein overexpression in A) an osteoclast-like giant cell adjacent to ROS1-negative tumor cells and B) reactive pneumocytes rimming an area of organizing pneumonia in a patient with concurrent ALK-rearranged lung adenocarcinoma and mycobacterial infection. ROS1 FISH was negative in both cases.
Sensitivity and Specificity of ROS1 Immunohistochemistry
Of the 218 total cases tested in the validation and screening cohorts, 56 underwent paired IHC and FISH. If only 3+ (diffusely) expressing tumors are considered positive, ROS1 IHC is 87.5% sensitive and 98% specific for the presence of a ROS1 translocation by FISH. If both 2 and 3+ ROS1 protein expression are considered positive, ROS1 IHC is 100% sensitive and 92% specific for the presence of a ROS1 translocation by FISH. (Table 2)
Table 2.
Sensitivity and specificity of ROS1 immunohistochemistry for ROS1 translocation by FISH calculated based on 3+ protein expression cutoff (upper panel) and 2+ protein expression cutoff (lower panel).
| FISH negative |
FISH positive |
Total | ||
|---|---|---|---|---|
| ROS1 IHC positive (3+ only) | 1 | 7 | 8 | Sensitivity = 88% Specificity = 98% |
| ROS1 IHC negative (0–2+) | 47 | 1 | 48 | |
| Total | 48 | 8 | 56 | |
| ROS1 IHC positive (2–3+) | 4 | 8 | 12 | Sensitivity = 100% Specificity = 92% |
| ROS1 IHC negative (0–1+) | 44 | 0 | 44 | |
| Total | 48 | 8 | 56 |
Clinicopathologic Features of ROS1-altered Lung Tumors
The clinical features of ROS1-altered tumors are detailed in Table 3. Including both the validation and screening cohorts, ROS1-rearranged and/or 3+ protein-expressing tumors were detected in 5 women and 4 men. When compared with the relatively unselected population of patients represented in the screening cohort, patients with ROS1-altered lung ACA showed a trend toward disease presentation at a younger age (average 60 years vs. 65 years, Student’s t test, p = 0.17), were significantly more likely to be never-smokers (89% vs. 23% in the screening cohort, Fisher’s exact test p <0.0001), and were more commonly non-Caucasian (67% vs 87% Caucasian in the screening cohort, Fisher’s exact test p=0.05). In the tested cohorts, ROS1 rearrangements occur in a mutually exclusive manner with all other tested genetic alterations.
Table 3.
Clinical characteristics of patients with ROS1- altered lung adenocarcinomas.
| ROS1-altered tumors N=9 |
|
|---|---|
| Mean age (range) | 60 (38–75) |
| Gender | |
| Male | 4 (44) |
| Female | 5 (56) |
| Smoking status | |
| Never | 8 (89) |
| Former | 1 (11) |
| Current | 0 |
| Race | |
| Asian | 2 (22) |
| Black | 1 (11) |
| Caucasian | 6 (67) |
| Stage at initial diagnosis | |
| IA | 2 (22) |
| IIIA | 4 (44) |
| IV | 3 (33) |
In this small and largely retrospectively tested cohort, the presence of ROS1 rearrangement and/or protein expression did not alter therapy for most patients. All but one patient received adjuvant chemotherapy with or without radiation as per standard protocols for treatment of lung adenocarcinoma. One patient received erlotinib therapy, with subsequent progression. A single patient received crizotinib at the time of disease relapse; the patient showed response to this therapy, however only three months of followup was available at the time of this writing. Because many of the patients in the screening cohort were diagnosed shortly before the time of this study, this group of patients was heavily censored, prohibiting meaningful outcomes analysis. The median overall survival (OS) in the validation cohort was shorter for patients with ROS1 altered tumors than those without (40 months vs 59 months); however, survival analysis revealed no significant difference in OS based on ROS1 status (log rank test p=0.95), although this analysis is likely under-powered due to small numbers.
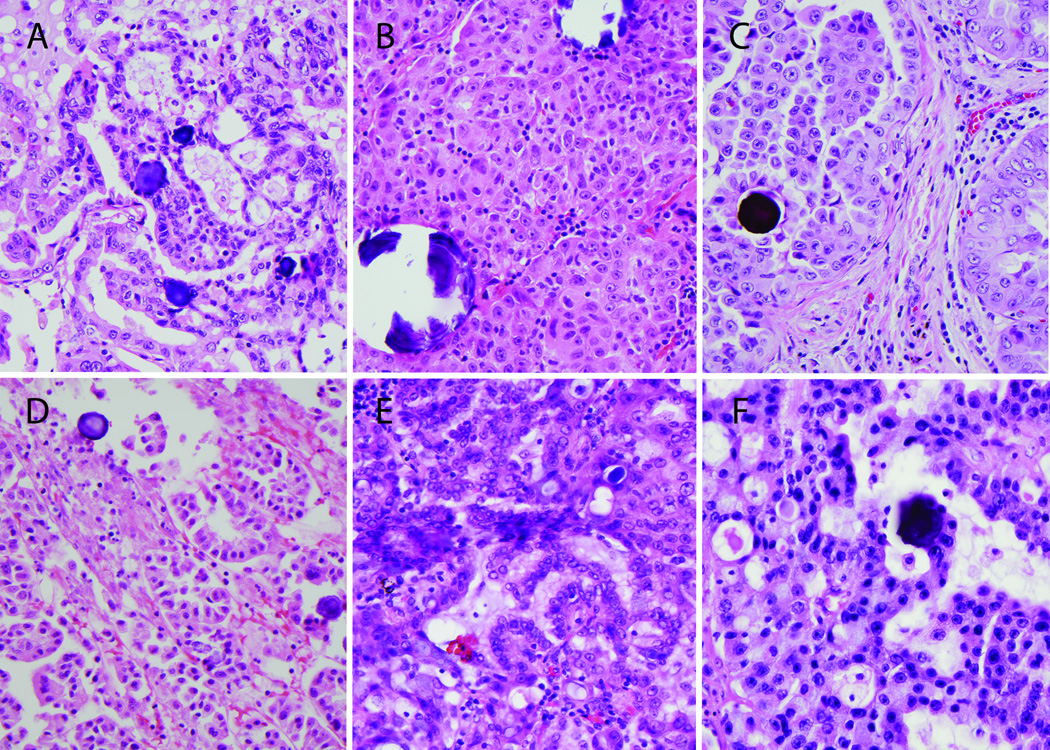
The pathologic features of the ROS1-altered lung tumors are detailed in Table 4 and illustrated in Figure 4. Common features in this subgroup included solid and papillary growth pattern, mucinous and/or signet ring cell features, and frequent psammomatous calcifications. These tumors typically had intermediate-sized polygonal cells with moderate amounts of eosinophilic cytoplasm, smooth nuclear contours with vesicular chromatin and distinct nucleoli.
Table 4.
Pathologic features of ROS1-altered tumors.
| Case | Specimen type | ROS1 IHC score |
ROS1 FISH status |
Dominant Histologic pattern |
Secondary pattern |
Cribriform features |
Mucinous and/or signet ring features |
Psammomatous calcification |
|---|---|---|---|---|---|---|---|---|
| 1 | Lymph node biopsy | 3+ | Negative | Solid | none | no | no | no |
| 2 | Pleural biopsy | 3+ | Rearranged | Solid | Papillary | yes | yes | no |
| 3 | Lymph node biopsy | 3+ | Rearranged | Solid | Papillary | no | no | yes |
| 4 | Brain metastatectomy | 3+ | Rearranged | Solid | Papillary | yes | yes | yes |
| 5 | Lung resection | 3+ | Rearranged | Solid | Papillary | yes | yes | yes |
| 6 | Soft tissue core biopsy | 2+ | Rearranged | Papillary | n/a | no | no | yes |
| 7 | Lung resection | 3+ | Rearranged | Solid | Papillary | yes | yes | yes |
| 8 | Lung resection | 3+ | Rearranged | Solid | Lepidic | yes | yes | yes |
| 9 | Brain metastatectomy | 3+ | rearranged | Solid | n/a | no | yes | no |
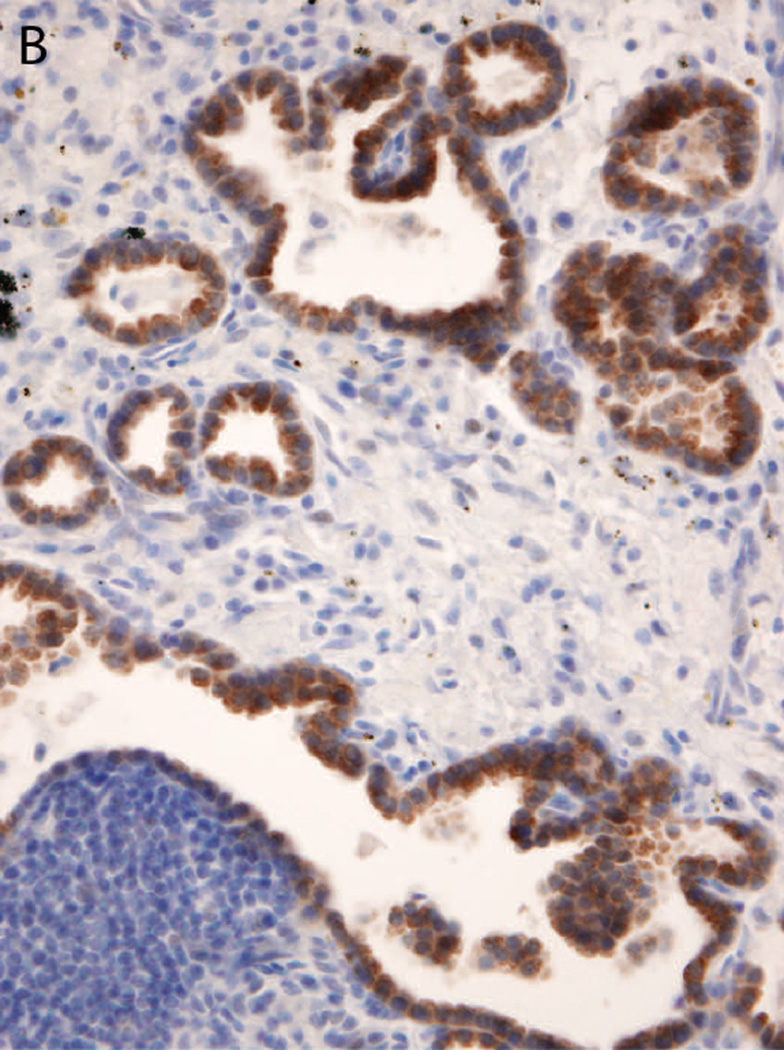
Figure 4.
Representative images of the histopathology of six different ROS1-translocated lung tumors: A primary lung tumor with cribriform features (A); pronounced solid growth pattern in a lymph node metastasis; (B) papillary growth pattern in a primary lung tumor resection (C) and soft tissue core biopsy (D); prominent mucinous features in a primary lung tumor (E); and cribriform features in a brain metastasis. Psammomatous calcifications are common (all panels).
The dominant histologic features of tumors with and without ROS1 alterations were compared. 26 cases with FNA or core biopsy samples were not included in this analysis because limited or poorly preserved tissue architecture prevented assessment of tumor growth pattern. ROS1-altered tumors were significantly more likely to have solid-predominant histology and mucinous features (Table 5). When compared by genotype, ROS1-altered tumors were most similar to ALK rearranged tumors, which also had a high frequency of solid-predominant growth and mucinous features (Table 5). Psammomatous calcifications were highly associated with ROS1 alterations (6 of 9 or 66% vs. 1 of 185 or 0.5%; p<0.001) as compared to all others; the single non-ROS1 altered tumor with psammomatous calcifications was pan WT.
Table 5.
Histopathology of ROS1-altered tumors as compared to tumors with other genotypes.
| Genotype | Total | Solid- predominant |
p value* | Mucin production |
p value* |
|---|---|---|---|---|---|
| ROS1 | 9 | 8 (89) | - | 6 (67) | - |
| ALK | 8 | 3 (38) | 0.5 | 3 (38) | 1 |
| EGFR | 19 | 3 (16) | <0.01 | 3 (16) | 0.1 |
| KRAS | 32 | 11 (34) | 0.06 | 7 (22) | 0.2 |
| Pan WT | 129 | 29 (22) | <0.001 | 22 (17) | 0.02 |
| Total | 185 | 46 (25) | <0.01 | 35 (19) | 0.03 |
P values are calculated using the Fisher's exact test and multiplied by 10 to correct for multiple comparisons (Bonferroni correction).
Discussion
We examined ROS1 expression in two cohorts of patients with lung adenocarcinoma. Patients tested in the first (validation) cohort were enriched for never-smoking status and wild type EGFR, KRAS, and ALK genotype. All tumors in this cohort underwent paired FISH and IHC to detect ROS1 translocation. ROS1 overexpression was detected in 7 of 53 (13%) tumors tested in this cohort, 6 of which also harbored ROS1 rearrangements by FISH. Patients in the second (screening) cohort represented 167 tumors sequentially tested in a clinical laboratory for EGFR, KRAS, and/or ALK alterations, as part of a standard oncologic workup. ROS1 overexpression was detected in 5 of 167 tumors and a ROS1 translocation was confirmed in 2 (1.2%).
Several important conclusions can be drawn from our study. First, ROS1 immunohistochemistry using the D4D6 antibody is highly sensitive and specific for detection of ROS1 translocations in lung adenocarcinoma. Because FISH-based testing is often cumbersome, expensive and logistically difficult for many pathology laboratories to support, immunohistochemistry-based tools are critical to widespread adoption of screening for rare but clinically important genetic alterations in tumors. Employed together, ALK and ROS IHC can quickly identify the 6–7% of lung adenocarcinoma patients that are likely clinically to respond to crizotinib therapy.
Second, our findings demonstrate that clinical and molecular selection can significantly enrich for the incidence of ROS1 translocations in a tested population. ROS1 overexpression was detected in 13% of tumors from a predominantly wild type cohort enriched for patients with limited to no smoking history. This compares to 1–2% of unselected lung adenocarcinomas, as seen in our screening cohort drawn from a clinical molecular diagnostics practice, and as previously reported in U.S. and Japanese studies.(8,10)
Third, we describe the clinical features of ROS1-rearranged tumors in a Western population. Similar to prior reports, (10) the majority (89%) of ROS1-rearranged tumors in our study occurred in never smokers, although statistical comparisons in this study are limited by the small number of ROS1-altered tumors and by the selection bias in the validation cohort. Rimkunas et al. reported concurrent EGFR mutations in two ROS1-rearranged tumors in a set of lung cancers from a Chinese population. (9) In our study, all ROS1 translocations occurred independent of the other tested oncogenic alterations, however the frequency and significance of dual alterations in different populations remains to be determined.
Lastly, we describe the distinctive pathologic features associated with ROS1 rearrangement, including solid and papillary-pattern growth with cribriform features, prominent mucin production, and frequent psammomatous calcifications. The pathology of ROS1-rearranged tumors overlaps with that of ALK-rearranged lung adenocarcinomas both in this study and as described in prior reports (16,17) including solid, mucinous and cribriform features. This is an interesting observation in light of the evolutionary relatedness of the ALK and ROS1 genes and similar clinical characteristics of patients with ALK and ROS1-translocated lung carcinomas.(18,19)
The D4D6 antibody can detect full length ROS1 protein, (9) and is reactive in some macrophages/giants cells and in non-neoplastic pneumocyte proliferations. In particular, care must be taken not to over-interpret the significance of ROS1 expression restricted to reactive pneumocytes at the tumor periphery that could be mistaken for lepidic growth of tumor. Although typically weak, in rare cases ROS1 protein expression can be strong in this reactive compartment.
Our study demonstrates that diffuse (3+) ROS1 protein expression is 89% sensitive and 98% specific for ROS1 translocations by FISH. Seven tumors with a documented ROS1 translocation had diffuse ROS1 expression of variable intensity, one case had multifocal weak to moderate expression in <50% of tumor cells. Overall, ~2% of tested cases had weak to moderate ROS1 expression in <50% of tumor cells. Faint (1+) staining in rare tumor cells was not uncommon, and was never associated with a ROS1 translocation. If 2+ and 3+ expression are considered positive, ROS1 IHC is 100% sensitive and 92% specific. Thus ROS1 IHC should serve as an excellent screening tool, with multifocal (2+) expression patterns triggering confirmatory FISH testing.
Pre-analytic variables, including duration of fixation, age of the tissue slide, and storage conditions, can have a significant impact on the antigenicity of some proteins. (20) It is unknown at this time if ROS1 protein stability is particularly sensitive to these variables, but as with other molecular and immunohistochemical tests, the tissue preparation should be optimized to maintain antigenicity and limit the risk of false negative results.
Interestingly, in one case the ROS1 protein was strongly expressed in tumor cells, however a translocation was not detected by FISH on multiple examinations. In this case of a metastasis to a lymph node, tumor cells were present as small nests in a background of abundant lymphocytes. It is unclear if this discrepant result reflects a falsely negative FISH result (for technical/interpretative reasons or due to a cryptic translocation), a falsely positive IHC result, or if the IHC result reflects an alternate mechanism of ROS1 overexpression.
ROS1 rearrangements occur infrequently in lung ACA, however given the frequency of lung cancer in the population, ROS1-rearranged tumors represent a significant number of cancer patients. In light of early reports of response to targeted inhibition using crizotinib, it is imperative that laboratories implement simple and cost-effective screening tools to identify patients with ROS1-rearranged lung cancer. For rapid screening of candidates for crizotinib therapy, ROS IHC can be readily incorporated into the diagnostic surgical pathology workup of lung adenocarcinoma, with results confirmed by FISH as needed.
Acknowledgments
Source of Funding
Cell Signaling Technology, Inc. provided ROS1 immunohistochemistry reagents under a research agreement with Brigham and Women’s Hospital and Dana Farber Cancer Institute. Dr. Sholl has received travel funding from Ventana Medical Systems, Inc. and receives consultancy fees from Genentech. Dr. Jänne is currently receiving a grant (#P50 CA090578) from the National Institutes of Health and receives consultancy fees from Pfizer, Boehringer Ingelheim, Roche, Sanofi, Astra Zeneca, and Chugai Pharmaceuticals and royalties from Lab Corp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
For the remaining authors none were declared.
References
- 1.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Angelo SP, Park B, Azzoli CG, et al. Reflex testing of resected stage I through III lung adenocarcinomas for EGFR and KRAS mutation: report on initial experience and clinical utility at a single center. J Thorac Cardiovasc Surg. 2011;141:476–480. doi: 10.1016/j.jtcvs.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birchmeier C, Sharma S, Wigler M. Expression and rearrangement of the ROS1 gene in human glioblastoma cells. Proc Natl Acad Sci U S A. 1987;84:9270–9274. doi: 10.1073/pnas.84.24.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 9.Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 10.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, et al. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol. 2012;7:1086–1090. doi: 10.1097/JTO.0b013e3182570919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardarella S, Ortiz TM, Joshi VA, et al. The Introduction of Systematic Genomic Testing for Patients with Non-Small-Cell Lung Cancer. J Thorac Oncol. 2012;7:1767–1774. doi: 10.1097/JTO.0b013e3182745bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis W, Brambilla E, Muller-Hermelink HK, et al., editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus, and Heart. Lyon: IARCPress; 2004. [Google Scholar]
- 14.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sholl LM, Weremowicz S, Gray SW, et al. Combined Use of ALK Immunohistochemistry and FISH for Optimal Detection of ALK- Rearranged Lung Adenocarcinomas. J.Thorac.Oncol. In press doi: 10.1097/JTO.0b013e31827db604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–5223. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011;35:1226–1234. doi: 10.1097/PAS.0b013e3182233e06. [DOI] [PubMed] [Google Scholar]
- 18.Ou SH, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the Treatment of ALK-Rearranged Non-Small Cell Lung Cancer: A Success Story to Usher in the Second Decade of Molecular Targeted Therapy in Oncology. Oncologist. 2012;17:1351–1375. doi: 10.1634/theoncologist.2012-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 20.DeLellis RA. Variability in achieving reproducible results in diagnostic immunohistochemistry laboratories. Hum Pathol. 1994;25:1109–1110. doi: 10.1016/0046-8177(94)90076-0. [DOI] [PubMed] [Google Scholar]