Abstract
The dopamine transporter (DAT) controls the spatial and temporal dynamics of dopamine (DA) neurotransmission by driving reuptake of extracellular transmitter into presynaptic neurons. Many diseases such as depression, bipolar disorder, Parkinson’s disease, and attention deficit hyperactivity disorder are associated with abnormal DA levels, implicating DAT as a factor in their etiology. Medications used to treat these disorders and many addictive drugs target DAT and enhance dopaminergic signaling by suppressing transmitter reuptake. We now understand that transport and binding properties of DAT are regulated by complex and overlapping mechanisms that provide neurons the ability to modulate DA clearance in response to physiological demands. These processes are controlled by endogenous signaling pathways and affected by exogenous transporter ligands, demonstrating their importance for normal neurotransmission, drug abuse, and disease treatments. Increasing evidence supports the disruption of these mechanisms in DA disorders, implicating dysregulation of transport in disease etiologies and suggesting these processes as potential points for therapeutic manipulation of DA availability.
Keywords: cocaine, amphetamine, drug addiction, attention deficit hyperactivity disorder, bipolar disorder, Parkinson’s disease
Dopamine transporter function and regulation
The neurotransmitter dopamine (DA) controls many functions including movement, cognition, mood, and reward. The availability of DA in the brain is modulated by the dopamine transporter (DAT), a plasma membrane protein that actively translocates released transmitter from the extracellular space into the presynaptic neuron. DAT is a target for addictive drugs including cocaine, amphetamine (AMPH), and methamphetamine (METH), and for agents such as Adderall™, Ritalin™, and Wellbutrin™ prescribed for the treatment of attention deficit hyperactivity disorder (ADHD), depression, and other dopamine imbalance conditions. These drugs affect DAT in two ways, as some such as cocaine bind to the protein and inhibit transport, while others such as AMPH and METH are transported and stimulate reverse transport (efflux) of intracellular DA. Both processes induce DA overflow, governing the strength of DA signaling during drug abuse and therapeutic treatments.
DAT belongs to the SLC6 family of transporters that couple inward solute transport to downhill movement of Na+ and Cl− [1, 2]. Solute translocation occurs by an alternating access mechanism in which the protein cycles between outwardly and inwardly facing conformations that bind and release substrate on opposite sides of the membrane. The kinetics of these conformational changes and the precise structures attained establish transporter velocity and functionality, as the inward conformation normally releases DA to the cell interior, efflux requires binding and outward translocation of intracellular DA, and cocaine binds to the outward facing conformation [3, 4]. DAT also moves ions non-stoichiometrically via channel-like mechanisms, generating currents sufficient to impact membrane potential [5, 6]. As substrate translocation occurs at the plasma membrane, overall transport capacity of DAT is also a function of its surface density.
It is now well established that these transporter activities are regulated by mechanisms that allow neurons to modulate DA clearance rates in response to short- and long-term physiological demands [7, 8]. Many of these processes are interactive or overlapping, and affected by substrates and inhibitors. Regulation is achieved by post-translational modifications and binding partner interactions, with additional layers of modulation imparted by cholesterol and membrane raft association. Increasing evidence indicates that many of these processes are dysregulated in dopamine imbalance disorders, suggesting them as factors in disease etiology and as potential therapeutic targets. Here we discuss some of the most well-characterized of these mechanisms, focusing on events related to acute signaling pathways and transporter post-translational modifications, and conclude with analysis of processes altered in dopaminergic diseases.
Cytoplasmic domain regulatory elements
DAT consists of 12 transmembrane (TM) spanning helices with TMs 1, 3, 6, and 8 forming the substrate permeation pathway. Unwound sections of TM1 and TM6 form the core of the active site and separate the helices into functional segments (Figures 1 and 2A). Extracellular and intracellular gates above and below the active site dictate inwardly and outwardly facing conformations and control the direction of DA movement [9]. Large N- and C-terminal tails extend into the cytoplasm and contain sites for post-translational modifications, binding partner interactions, and regulatory motifs (Figure 1). Great insights on the core structure have been obtained from homology modeling to the bacterial leucine transporter LeuT [1, 10], but far less is known about the cytoplasmic domains, which are not conserved in LeuT and whose structures have not been solved. The N-terminus contains intracellular gate residue Arg60 which stabilizes the outwardly facing form and has been implicated in control of transport by influencing the conformation of TM1a, which is thought to undergo major structural rearrangements during the outward-to-inward transition [11-13]. Less is known about the C-terminal domain or its potential impacts on TM12.
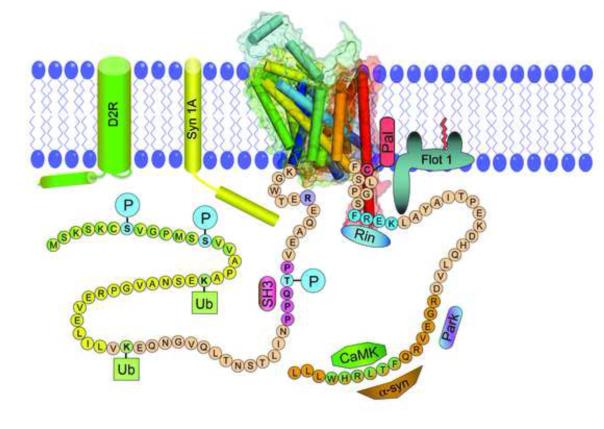
Figure 1. Regulatory elements of rat dopamine transporter N- and C-terminal domains.
A 3D model of rat DAT based on the A. aeolicus LeuT transporter was generated using PyMol (Schrödinger, LLC), with TM helices shown as barrels and light shading indicating semitransparent Connolly surfaces. The structure was positioned in a membrane bilayer with schematic depictions of N- and C-terminal tails extending into the cytoplasm. Posttranslational modifications shown are Ser7, Ser13, and Thr53 phosphorylation (blue, P), Lys19 and Lys35 ubiquitylation (light green, Ub), and Cys580 palmitoylation (red, Pal). Motifs and sequences indicated are intracellular gate residue Arg60 (R, purple), putative Src homology domain epitope (mauve, SH3), PKC endocytosis motif (blue, FREK), and domains for interactions with Syntaxin 1A (Syn1A, yellow), D2 DA receptor (D2R, green) Ras-like GTPase Rin 1 (Rin, blue), Calcium-Calmodulin-Dependent Protein Kinase (CaMK, green), and α-synuclein (α-Syn, orange) and Parkin (Park, dark blue-lavender). Flotillin 1 (Flot 1, olive green) is shown with palmitic acid modification (red line) but without a known DAT interaction site.
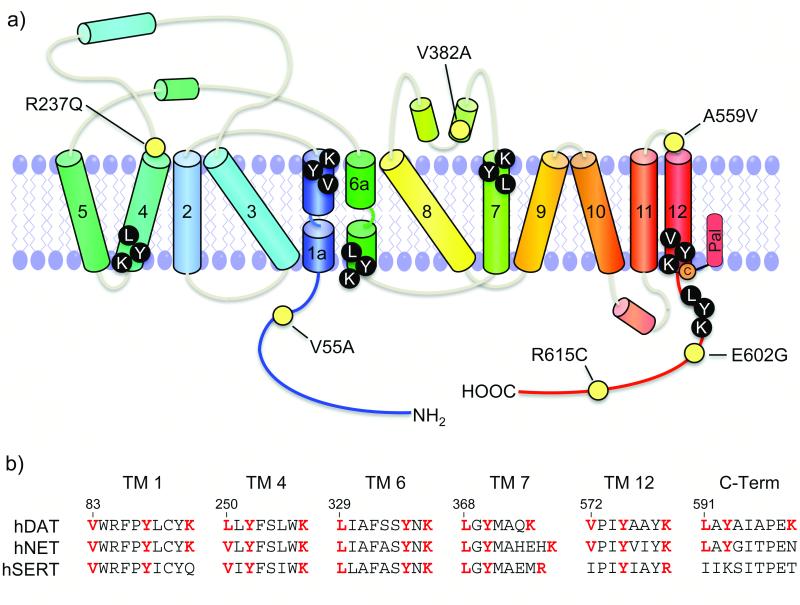
Figure 2. Selected coding variants and potential CRAC motifs in DAT.
(a) Coding variants known to alter DAT function (numbered yellow circles) and helical topological 2D architecture of DAT depicting essential cholesterol interacting residues in putative CRAC motifs (black circles with white letters). (b) Sequence alignment of human DAT, NET, and SERT showing homology within putative CRAC motifs. Residues that are key components of the motifs are shown in red; the numbers above the sequence correspond to hDAT.
The N-terminus undergoes extensive modification by phosphorylation and ubiquitylation. Phosphorylation is catalyzed by different classes of kinases on two distinct regions of the domain. The most well-studied site is a cluster of serines at positions 2, 4, 7, 12, and 13 that undergoes increased phosphorylation by protein kinase C (PKC) activation and by in vitro and in vivo exposure to AMPH and METH [14, 15]. AMPH/METH-induced phosphorylation is PKC-dependent, with kinase activation potentially resulting from drug-induced increases in cytosolic Ca2+ or reactive oxygen species [16]. Within this cluster multiple serines are modified, but to date the only verified phosphorylation site is Ser7 [17]. The presence of these sites at the distal end of a long and potentially flexible domain suggests the possibility for regulation of binding partner interactions, although such effects have not yet been demonstrated. The second phosphorylation site is at membrane proximal residue Thr53 [18, 19]. This residue is followed by proline, making it specific for proline-directed kinases such as Extracellular Signal Regulated Kinase (ERK). Phosphorylation of proline-directed sites substantially alters protein structure by regulating cis-trans isomerization of the phosphoacceptor-prolyl peptide bond [20], and the location of this site suggests its potential to regulate transporter functions via impacts on TM1a or Arg60. The sequence flanking Thr53 (P-P-X-X-P) may also constitute an SH3 domain ligand for protein scaffolding [21]. Between the two phosphorylation domains is a region that undergoes ubiquitylation on Lysines 19 and 35 (and on hDAT Lys27), catalyzed by the ubiquitin E3 ligases Nedd4-2 and Parkin [22-24]. Modification by Nedd4-2 is likely monubiquitylation and is increased by PKC activation as a mechanism for stimulated endocytosis [22, 25].
On the C-terminus DAT is modified by S-palmitoylation, the addition of a saturated fatty acyl moiety via a thioester bond. This occurs on Cys580 near the membrane-cytoplasm interface of TM12 and at one or more currently unknown residues [26]. Just downstream of this site is a motif at residues 587-590 (FREK) that binds the small ras-like GTPase Rin1 and dictates PKC-stimulated endocytosis [27, 28]. Other DAT regulatory partners include Syntaxin 1A (Syn1A), which binds N-terminal residues 1-33 [29, 30], D2 DA receptors, which bind residues 1-15 [31], Calcium-Calmodulin Dependent Protein Kinase (CaMK) which binds C-terminal residues 612-617 [32], α-synuclein (α-Syn) which binds residues 606-620 [33], Parkin, which binds to the C-terminus [34], and the membrane raft protein Flotillin 1 (Flot1) whose binding site is not known [35].
Regulation by kinases, post-translational modifications, and binding partners
DAT is regulated by multiple signaling systems, with PKC and ERK being two of the most well-characterized [8]. Many neurotransmitter receptor systems including DA, opioids, and glutamate that impact DA uptake, dopaminergic signaling, and drug abuse characteristics could feed into these pathways [7, 36]. Although in most cases specific mechanistic links between these receptors and DAT remain to be established, functional interactions have been identified between DAT and D2 DA receptors and G-protein βγ subunits [31, 37]. DAT regulation mediated by signaling systems is complex, with some functions regulated by more than one pathway and some pathways regulating more than one function, indicating the presence of convergent and divergent mechanisms. Several recent reviews have discussed aspects of these issues in greater detail [2, 7, 38, 39].
Protein Kinase C
Activation of PKC rapidly down-regulates DA transport capacity through effects on DAT endocytosis, transport Vmax, and efflux. Acute endocytotic regulation of DAT controls the number of transporter surface copies available to translocate DA [40], whereas extended activation of PKC funnels DAT into lysosomal degradation for long-term regulation of transporter levels [25, 41, 42]. Most studies on acute endocytosis support clathrin-dependent processes [41-43], and targeting to early, late, and recycling endosomes [25, 44]. Multiple mechanisms have been linked to PKC-stimulated DAT endocytosis, including N-terminal ubiquitylation [22], Rin1 binding to the C-terminal endocytosis motif [27], and possibly Flot1 interactions [35, 42]. It is not known if these processes function coordinately or independently, or drive DAT into the same or different subcellular compartments. PKC-stimulated endocytosis of DAT is not prevented by removal of distal N-terminal serines [45], indicating that an accessory protein rather than the transporter is the kinase target in this process. One possibility for this is Flot1, which loses ability to induce DAT endocytosis with S315A mutation [35]. PKC also reduces the rate of DAT plasma membrane recycling [46], further enhancing the level of transporter internalization.
While endocytosis has been largely considered to represent the primary mechanism underlying transport down-regulation, several studies have now shown that DAT retains substantial levels of PKC-induced down-regulation when endocytosis is inhibited or cannot be detected [26, 47, 48], indicating the presence of a kinetic mechanism for regulation of surface transporter activity. This may be driven by Ser7 phosphorylation, as S7A DAT shows no PKC-dependent down-regulation when internalization is blocked [49]. S7A DAT possesses altered conformational equilibrium that could underlie its loss of kinetic regulation, and this is accompanied by reduced cocaine analog affinity, suggesting a role for phosphorylation in modulating cocaine effects [17]. Activation of PKC by phorbol esters or Gq-coupled glutamate receptors also stimulates DA efflux in slices of rat striatum or substantia nigra, [50, 51], and may, like AMPH-induced efflux, be driven by DAT phosphorylation. PKC thus regulates DA reuptake capacity by multiple processes, an endocytotic mechanism driven by phosphorylation of DAT accessory proteins, a kinetic down-regulation mechanism mediated by Ser7 phosphorylation, and enhancement of efflux. In vivo these processes would be expected to increase extracellular dopamine and thus would be suitable targets for treatment of dopamine imbalance conditions.
Extracellular Signal Regulated Kinase
In contrast to PKC, which dampens DA reuptake, ERK increases DA transport capacity, demonstrating the ability of DAT to undergo bidirectional regulation and indicating that establishment of overall uptake set points involves integration of signals from these pathways. The role of ERK in regulation of DAT is most clearly seen in actions of DA and kappa-opioid receptors, which upregulate DA transport and DAT surface levels by ERK-dependent processes [52-55]. ERK inhibitors also suppress constitutive DAT surface levels and uptake activity [8, 36], strongly indicating that ERK tonically exerts a positive effect on DA transport capacity that can be enhanced via receptor activation. One possibility for these effects involves DAT Thr53, as T53A mutation reduces DA transport Vmax [18], consistent with the potential for ERK-mediated phosphorylation of this site to increase transport activity. In addition, while D2 receptors activate ERK, they also directly interact with DAT to promote surface expression [31, 54], and this interaction may be disrupted in schizophrenia [56]. PKCβ may also be involved in the mechanism of D2 receptor regulation of DAT by ERK [57], highlighting the complexity of these processes.
Palmitoylation
Palmitoylation is a dynamic and reversible lipid modification that regulates proteins in a manner analogous to phosphorylation-dephosphorylation. Acute pharmacological inhibition of DAT palmitoylation strongly reduces transport Vmax and enhances PKC-induced down-regulation without affecting total or surface transporter levels, whereas longer-term inhibition or C580A mutation leads to enhanced DAT degradation [26]. These results indicate that palmitoylation functions to promote transport capacity and oppose both short- and long-term transporter down-regulation. These actions are opposite those induced by PKC, and DAT phosphorylation and palmitoylation mutants display reciprocal levels of these modifications [49], indicating that phosphorylation and palmitoylation may define transporter populations possessing opposing levels of uptake activity or metabolic stability. Because overall DAT levels would help establish DA tone, it is possible that loss of appropriate DAT palmitoylation and degradation could lead to hypo- or hyper-dopaminergic conditions. The close proximity of Cys580 to the PKC endocytosis motif also suggests the potential for connections between these mechanisms, although this has not been investigated.
Syntaxin 1A
Syn1A is a plasma membrane protein originally characterized to interact with synaptic vesicle fusion proteins during exocytotic transmitter release, and is now known to regulate other proteins including neurotransmitter transporters. Syn1A binds to the distal N-terminus of DAT, and conditions that reduce its levels or prevent its interaction with the transporter lead to multiple effects including increased uptake and channel activity and decreased efflux and transporter phosphorylation [29, 30, 58]. These findings are consistent with Syn1A functioning to suppress transport and promote efflux by affecting N-terminal phosphorylation and/or TM1 conformation. Our current understanding of the physiological significance of these events is low, but it has been speculated that the ability of Syn1A to induce vesicular neurotransmitter release but inhibit transmitter reuptake may represent a mechanism for spatial or temporal coordination of these processes [59, 60].
Cholesterol and membrane rafts
Membrane rafts are cholesterol- and sphingolipid-rich microdomains that are specialized for particular cellular functions such as receptor signaling via segregation of specific protein populations [61]. DATs are distributed relatively equally between membrane raft and nonraft domains [47, 62] with raft localization reducing transporter lateral membrane mobility [63]. DAT targeting to rafts is positively correlated with membrane cholesterol content [62, 63], and requires the palmitoylated membrane raft organizing protein Flot1 [35, 42]. It is not yet known if palmitoylation of DAT plays a role in its raft distribution or interactions with Flot1. Raft partitioning has significant consequences for regulation of DAT, as rafts represent the primary sites of its PKC-stimulated phosphorylation [47], interactions with Syn1A [30], Rin1 [28], and Flot1 [35], and may represent sites for PKC-stimulated endocytosis [35, 42]. Mis-targeting of DAT could thus affect processes such as efflux or down-regulation that are dependent on these mechanisms.
Cholesterol itself plays a crucial role in optimal DAT function. Depletion of cholesterol leads to reductions in DA transport activity, AMPH-stimulated efflux, and PKC-stimulated down-regulation, as well as alterations in transporter conformational equilibrium [47, 62-64], suggesting the potential for links between DAT dysfunction and disorders of cholesterol. Because these processes are linked to transporter phosphorylation and binding partner interactions, their cholesterol dependence may result from membrane raft localization. However, some of these effects do not correlate with raft partitioning levels, suggesting the presence of raft-independent mechanisms [47, 64]. Cholesterol interacts with many proteins through Cholesterol Recognition Amino Acid Consensus (CRAC) motifs, which consist of the sequence L/V–X(1-5)–Y–X(1-5)–K/R and bind sterol via hydrophobic, aromatic, and H-bonding interactions [65]. Although it is not known if they are functional, DAT contains six CRAC motifs in regions of known importance including TM1a, TM6b, TM12 near the palmitoylation site, and the C-terminus near the endocytosis motif (Figure 2A). These sequences are highly conserved in the homologous norepinephrine and serotonin transporters, which are also cholesterol dependent and localized to membrane rafts (Figure 2B). DAT interaction with cholesterol via these motifs could thus conceivably contribute to its raft partitioning or influence functions irrespective of raft localization.
DAT dysregulation in disease
Dysregulation of DAT resulting in aberrant DA clearance has long been postulated to result in dopaminergic dysfunction [66]. While non-coding single nucleotide polymorphisms and variable number tandem repeats in the DAT gene have been associated with many DA disorders [67] more recent findings from animal and model systems of DA diseases have identified DAT coding polymorphisms and mutational/functional alterations in DAT regulatory partners. It is also well supported that abused drugs exogenously impact many crucial regulatory transporter properties.
Psychostimulant Drugs
Chronic use of cocaine and AMPH leads to long-term impacts on DAT levels and activities by mechanisms that are not understood [68, 69]. Substantial evidence now supports the alteration of multiple DAT regulatory processes by abused drugs and other transporter ligands [7], indicating that these compounds not only act pharmacologically but also induce physiological changes that could lead to long-term transporter adaptations during addiction or therapeutic treatment regimens. In experiments done in vitro to distinguish direct impacts of drug pretreatments from those due to changes in neurochemistry, most studies have found little to no effect of uptake blockers on DAT regulation [70] while AMPH and METH induce pronounced alterations on DAT trafficking, Vmax, and efflux.
The effects of AMPH and METH on DAT surface levels and activity are complex and poorly understood. During the first few minutes of AMPH exposure, DAT undergoes plasma membrane recruitment and transport increases that presumably function homeostatically to clear the increased extracellular DA induced by the drug [71, 72]. Longer AMPH and METH treatments, however, induce transporter down-regulation [15, 73] and endocytosis [72, 74] that may serve to limit intracellular exposure to these neurotoxic compounds. Evidence indicates the presence of both kinetic and endocytotic down-regulation mechanisms, as AMPH-induced transport down-regulation has been found to precede or occur in the absence of endocytosis [73, 75]. Some [15, 73] but not all [25, 76] studies support the involvement of PKC in these processes, and a recent study suggests that AMPH and PKC drive DAT into distinct endocytotic compartments [25]. Thus the extent of overlap of these drug-induced regulatory processes with those activated by PKC remains unclear. Importantly, it has been demonstrated that acute down-regulation of DAT by AMPH and METH occurs in vivo, as low dose regimens of drugs delivered intraperitoneally or by self-administration induce rapid reductions in striatal DA transport activity that reverse by 24-48h and are not accompanied by DAT protein losses [73, 75, 77, 78]. Some evidence also supports brain-region specificity in these processes, as AMPH-induced down-regulation of DAT in synaptosomes from nucleus accumbens occurs more slowly and to a lesser extent than that observed in striatum [73], and in vivo METH treatments that down-regulate DAT in nucleus accumbens are without effect in hypothalamus [79]. This regionality indicates the presence of neuron-specific mechanisms that may provide the potential to target specific DA pathways in disease treatments.
AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72]. Deletion of the DAT distal N-terminal serines or CaMK binding domain impairs AMPH-stimulated efflux [32, 83], leading to the current paradigm that efflux requires CaMK-mediated phosphorylation of the transporter N-terminus. It is not known, however, if this occurs via direct CaMK phosphorylation of DAT, which has not yet been demonstrated, or if the CaMK dependency of these functions is mediated indirectly via cross-talk with PKC. Both CaMK and PKC phosphorylate the N-terminus of DAT on Ser13 in vitro (Figure 1), suggesting this as a potential locus for stimulation of efflux by these kinases, although in vivo phosphorylation of Ser13 has not yet been demonstrated [17]. In addition, although most efforts to link DAT phosphorylation and efflux mechanisms have focused on distal serines, T53A DAT shows total loss of AMPH-stimulated 1-methyl-4-phenylpyridinium efflux [18], suggesting a role for Thr53 and other N-terminal regions in this process. Hypotheses that have been forwarded to explain how the N-terminus and its phosphorylation could stimulate efflux include control of TM1a/transporter conformational equilibrium, regulation of DAT oligomerization, and enhancement of Na+ affinity/DA binding at the inward transporter face [84-86], but it is clear that we are still far from understanding the molecular events driving this process.
ADHD and bipolar disorder
Rare coding polymorphisms of DAT that result in amino acid substitutions at residues Val55, Arg237, Val382, Ala559, Glu602, and Arg615 (Figure 2A) have been identified from patients diagnosed with ADHD and bipolar disorder as well as from non-diagnosed individuals [87, 88]. In vitro analyses have identified regulatory changes in four of these variants. Val substitutions of Ala559, which is found at the extracellular end of TM12, have been identified from both ADHD and bipolar patients [88]. A559V DAT displays elevated tonic efflux that could result in the high extracellular DA levels thought to be associated with these disorders. The elevated efflux is CaMK-dependent and mediated by D2 DA receptors, consistent with involvement of endogenous signaling and phosphorylation mechanisms. In addition, the enhanced level of efflux is inhibited rather than stimulated by AMPH, providing a possible explanation for the paradoxical actions of AMPH-based treatments in ADHD [86, 89]. A second mutation identified from an ADHD patient occurs at Arg615, which is present within the C-terminal tail CaMK binding site. Cys substitution of this residue causes numerous regulatory alterations including increases in transporter phosphorylation and decreases in Flot1 binding, membrane raft partitioning, and PKC- and AMPH-induced endocytosis [90]. These properties have been interpreted as the transporter existing in a state of chronic down-regulation that would result in elevated extracellular DA. The other two polymorphisms generate Ala substitutions at Val382, present in extracellular loop 4, which stabilizes the extracellular gate, and Val55 located between Thr53 and Arg60 in the N-terminal tail. V382A DAT displays decreased DA affinity, increased cocaine affinity, and altered PKC-dependent regulation [91, 92], while V55A displays increased DA Km and decreased cocaine affinity [48, 92]. The position of these residues suggests the possibility that these transport and binding changes could result from impacts on transporter conformational equilibrium. The identification of DAT mutations in individuals not diagnosed with DA disorders supports the hypothesis that these complex diseases may result from the convergence of multiple mutations or dysregulated events that synergize to affect DAT functionality, such that a single mutation alone may be insufficient to induce dysfunction [88].
Angelman syndrome
Angelman syndrome is a neurological disorder characterized by tremor and Parkinson’s Disease (PD)-like symptoms, suggesting a link to DA [93]. It results from a defective maternally inherited allele of an E3 ubiquitin ligase that causes reduction of CaMK activity through increased inhibitory autophosphorylation [94]. In a mouse model of Angelman syndrome, DAT showed suppressed AMPH-induced efflux that could result in decreased synaptic DA levels consistent with a PD-like phenotype [82]. These effects occurred without alterations of DAT levels or changes in CaMK binding to DAT, suggesting that loss of CaMK enzymatic activity drives the response, consistent with in vitro mechanistic studies of efflux.
Parkinson’s disease
PD is characterized by the gradual loss of dopaminergic neurons in the substantia nigra. Because DAT is a dopaminergic specific gateway for neurotoxic compounds including DA, 6-OHDA and 1-methyl-4-phenylpyridinium, dysregulated transport has been postulated as a mechanism that could contribute to disease development [66]. Although most forms of PD are idiopathic, two familial forms are caused by mutations or overexpression of α-Syn and Parkin, both of which affect DAT regulatory processes.
α-Syn is a small membrane-interacting scaffolding protein whose function and role in inducing PD are poorly understood. α-Syn binds to the distal C-terminus of DAT, where it could conceivably compete with CaMK and affect related regulatory properties, and recent work indicates that α-Syn impacts DAT-mediated ion currents that may alter DA neuron function [95]. Some studies support α-Syn acceleration of DA-induced apoptosis [33], supportive of a role in induction of neuronal death through enhanced transport of neurotoxic compounds. However, many conflicting results regarding the effects of α-Syn on DAT levels, trafficking, and activity have been found [96, 97], leaving us without a clear mechanistic understanding of the significance of this interaction.
The E3 ubiquitin ligase Parkin exerts a positive role in normal DAT function by regulating transporter ubiquitylation and degradation. Mutations of Parkin that cause PD have been linked to loss of DAT quality control via failure of the mutant proteins to ubiquitylate misfolded transporters [24], which accumulate and induce dominant-negative effects on DA uptake that could be associated with DA toxicity. Parkin also interacts with the C-terminus of DAT and in vitro has been found to disrupt DAT–β-Syn interactions and suppress α-Syn–induced DA neurotoxicity [34]. In addition, although the underlying mechanisms are not known, several inactivating point mutations in DAT have been identified in patients suffering from Infantile Parkinsonism-Dystonia [98].
Concluding remarks
The findings described here indicate that DAT functionality exists within a regulatory continuum that could potentially be exploited therapeutically to modulate extracellular DA levels in disease states. This could be accomplished by activating or inhibiting enzymes that catalyze DAT post-translational modifications or by targeted modulation of its binding partner interactions to drive increased or decreased DA reuptake. Improved understanding of these modifications and binding partner interactions as well as elucidation of N- and C-terminal domain structures will be crucial towards further understanding these regulatory events and how they may serve as therapeutic targets or contribute to diseases. These studies have also shown that uptake dysregulation can occur with no obvious alterations in transporter levels, demonstrating that some deficits may not be revealed by measurement of DAT protein or uptake/binding activities alone and that identification of transporter functional adaptations in various experimental or disease conditions may require analyses specifically designed to probe regulatory responses.
Highlights.
DAT is the major mechanism responsible for clearance of extracellular dopamine.
DAT is regulated by post-translational modifications and binding partner interactions.
Dysregulation of DAT is found in many dopaminergic diseases.
DAT regulatory mechanisms may provide therapeutic targets in DA diseases.
Acknowledgements
We thank Dr. Keith Henry for the molecular model of DAT used in Figure 1 and for advice and discussions. Support for our work comes from NIH grants DA13147 (RAV), DA27845 (RAV and LKH), DA31991 (JDF), P20 RR017699 from the COBRE program of the National Center for Research Resources (to the University of North Dakota), and P20 RR016741 from the IDeA Networks of Biomedical Research Excellence (INBRE) program of the National Center for Research Resources (to the University of North Dakota).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manepalli S, et al. Monoamine transporter structure, function, dynamics, and drug discovery: a computational perspective. The AAPS journal. 2012;14:820–831. doi: 10.1208/s12248-012-9391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pramod AB, et al. SLC6 transporters: Structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shan J, et al. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS One. 2011;6:e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuming T, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram SL, et al. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- 6.Carvelli L, et al. Dopamine transporters depolarize neurons by a channel mechanism. Proc Natl Acad Sci U S A. 2004;101:16046–16051. doi: 10.1073/pnas.0403299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann NY Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramamoorthy S, et al. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuming T, et al. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita A, et al. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 11.Guptaroy B, et al. Site-directed mutations near transmembrane domain 1 alter conformation and function of norepinephrine and dopamine transporters. Mol Pharmacol. 2011;79:520–532. doi: 10.1124/mol.110.069039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guptaroy B, et al. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Mol Pharmacol. 2009;75:514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sucic S, et al. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J Biol Chem. 2010;285:10924–10938. doi: 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster JD, et al. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- 15.Cervinski MA, et al. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- 16.Gnegy ME. The effect of phosphorylation on amphetamine-mediated outward transport. Eur J Pharmacol. 2003;479:83–91. doi: 10.1016/j.ejphar.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 17.Moritz AE, et al. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. The Journal of biological chemistry. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster JD, et al. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. The Journal of biological chemistry. 2012;287:29702–29712. doi: 10.1074/jbc.M112.367706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorentla BK, et al. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry. 2009;48:1067–1076. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu KP, et al. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 21.Saksela K, Permi P. SH3 domain ligand binding: What’s the consensus and where’s the specificity? FEBS letters. 2012;586:2609–2614. doi: 10.1016/j.febslet.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Vina-Vilaseca A, Sorkin A. Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes. J Biol Chem. 2010;285:7645–7656. doi: 10.1074/jbc.M109.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miranda M, et al. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol Biol Cell. 2007;18:313–323. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, et al. Parkin increases dopamine uptake by enhancing the cell surface expression of dopamine transporter. J Biol Chem. 2004;279:54380–54386. doi: 10.1074/jbc.M409282200. [DOI] [PubMed] [Google Scholar]
- 25.Hong WC, Amara SG. Differential targeting of the dopamine transporter to recycling or degradative pathways during amphetamine- or PKC-regulated endocytosis in dopamine neurons. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013 doi: 10.1096/fj.12-218727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster JD, Vaughan RA. Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation. J Biol Chem. 2011;286:5175–5186. doi: 10.1074/jbc.M110.187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudanova E, et al. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol Cell Neurosci. 2008;39:211–217. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navaroli DM, et al. The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13758–13770. doi: 10.1523/JNEUROSCI.2649-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvelli L, et al. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A. 2008;105:14192–14197. doi: 10.1073/pnas.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binda F, et al. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee FJ, et al. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fog JU, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Lee FJ, et al. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. Faseb J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 34.Moszczynska A, et al. Parkin disrupts the alpha-synuclein/dopamine transporter interaction: consequences toward dopamine-induced toxicity. J Mol Neurosci. 2007;32:217–227. doi: 10.1007/s12031-007-0037-0. [DOI] [PubMed] [Google Scholar]
- 35.Cremona ML, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nature neuroscience. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tejeda HA, et al. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cellular and molecular life sciences : CMLS. 2012;69:857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Olivares J, et al. Inhibition of Dopamine Transporter Activity by G Protein betagamma Subunits. PLoS One. 2013;8:e59788. doi: 10.1371/journal.pone.0059788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen R, et al. Dopamine transporter trafficking: rapid response on demand. Future Neurol. 2010;5:123. doi: 10.2217/fnl.09.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksen J, et al. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem. 2010;113:27–41. doi: 10.1111/j.1471-4159.2010.06599.x. [DOI] [PubMed] [Google Scholar]
- 40.Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 42.Sorkina T, et al. Flotillins regulate membrane mobility of the dopamine transporter but are not required for its protein kinase C dependent endocytosis. Traffic. 2013 doi: 10.1111/tra.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorkina T, et al. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 44.Rao A, et al. Differential subcellular distribution of endosomal compartments and the dopamine transporter in dopaminergic neurons. Molecular and cellular neurosciences. 2011;46:148–158. doi: 10.1016/j.mcn.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granas C, et al. N-terminal truncation of the dopamine transporter abolishes phorbol esterand substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- 46.Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster JD, et al. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazei-Robison MS, Blakely RD. Expression studies of naturally occurring human dopamine transporter variants identifies a novel state of transporter inactivation associated with Val382Ala. Neuropharmacology. 2005;49:737–749. doi: 10.1016/j.neuropharm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Moritz AE, et al. Abstract Viewer/Itinerary Planner. Society for Neuroscience Program; Washington, DC: 2011. Reciprocal phosphorylation and palmitoylation of the dopamine transporter regulate transport capacity. No. 344.16. [Google Scholar]
- 50.Cowell RM, et al. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol. 2000;389:59–65. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- 51.Opazo F, et al. PKC links Gq-coupled receptors to DAT-mediated dopamine release. Journal of neurochemistry. 2010;114:587–596. doi: 10.1111/j.1471-4159.2010.06788.x. [DOI] [PubMed] [Google Scholar]
- 52.Moron JA, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zapata A, et al. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282:35842–35854. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- 54.Bolan EA, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- 55.Thompson AC, et al. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee FJ, et al. Disruption of the dopamine transporter-dopamine D2 receptor interaction in schizophrenia. Synapse. 2009;63:710–712. doi: 10.1002/syn.20648. [DOI] [PubMed] [Google Scholar]
- 57.Chen R, et al. Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. Journal of neurochemistry. 2013;125:663–672. doi: 10.1111/jnc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cervinski MA, et al. Syntaxin 1A regulates dopamine transporter activity, phosphorylation and surface expression. Neuroscience. 2010;170:408–416. doi: 10.1016/j.neuroscience.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egana LA, et al. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cartier EA, et al. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. The Journal of biological chemistry. 2010;285:1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Hong WC, Amara SG. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J Biol Chem. 2010;285:32616–32626. doi: 10.1074/jbc.M110.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adkins EM, et al. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- 64.Jones KT, et al. Importance of cholesterol in dopamine transporter function. Journal of neurochemistry. 2012;123:700–715. doi: 10.1111/jnc.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Epand RM. Proteins and cholesterol-rich domains. Biochimica et biophysica acta. 2008;1778:1576–1582. doi: 10.1016/j.bbamem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 66.Miller GW, et al. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 67.Haddley K, et al. Molecular genetics of monoamine transporters: relevance to brain disorders. Neurochemical research. 2008;33:652–667. doi: 10.1007/s11064-007-9521-8. [DOI] [PubMed] [Google Scholar]
- 68.Mash DC, et al. Dopamine transport function is elevated in cocaine users. Journal of neurochemistry. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 69.Urban NB, Martinez D. Neurobiology of addiction: insight from neurochemical imaging. Psychiatr Clin North Am. 2012;35:521–541. doi: 10.1016/j.psc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Gorentla BK, Vaughan RA. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology. 2005;49:759–768. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Johnson LA, et al. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- 72.Chen R, et al. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. The Journal of pharmacology and experimental therapeutics. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem. 2009;108:1575–1584. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saunders C, et al. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.German CL, et al. Amphetamine and methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. Journal of neurochemistry. 2012;123:288–297. doi: 10.1111/j.1471-4159.2012.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boudanova E, et al. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology. 2008;54:605–612. doi: 10.1016/j.neuropharm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McFadden LM, et al. Methamphetamine self-administration acutely decreases monoaminergic transporter function. Synapse. 2012;66:240–245. doi: 10.1002/syn.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandoval V, et al. Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci. 2001;21:1413–1419. doi: 10.1523/JNEUROSCI.21-04-01413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu PW, et al. Differential regional effects of methamphetamine on dopamine transport. European journal of pharmacology. 2008;590:105–110. doi: 10.1016/j.ejphar.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goodwin JS, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2008 doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinkellner T, et al. Ca(2+)/calmodulin-dependent protein kinase IIalpha (alphaCaMKII) controls the activity of the dopamine transporter: implications for Angelman syndrome. The Journal of biological chemistry. 2012;287:29627–29635. doi: 10.1074/jbc.M112.367219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khoshbouei H, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sitte HH, Freissmuth M. The reverse operation of Na(+)/Cl(−)-coupled neurotransmitter transporters--why amphetamines take two to tango. J Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leviel V. Dopamine release mediated by the dopamine transporter, facts and consequences. Journal of neurochemistry. 2011;118:475–489. doi: 10.1111/j.1471-4159.2011.07335.x. [DOI] [PubMed] [Google Scholar]
- 86.Mazei-Robison MS, et al. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grunhage F, et al. Systematic screening for DNA sequence variation in the coding region of the human dopamine transporter gene (DAT1) Molecular psychiatry. 2000;5:275–282. doi: 10.1038/sj.mp.4000711. [DOI] [PubMed] [Google Scholar]
- 88.Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annual review of pharmacology and toxicology. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- 89.Bowton E, et al. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6048–6057. doi: 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakrikar D, et al. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin Z, Uhl GR. Human dopamine transporter gene variation: effects of protein coding variants V55A and V382A on expression and uptake activities. Pharmacogenomics J. 2003;3:159–168. doi: 10.1038/sj.tpj.6500169. [DOI] [PubMed] [Google Scholar]
- 93.Dichter GS, et al. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mabb AM, et al. Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends in neurosciences. 2011;34:293–303. doi: 10.1016/j.tins.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swant J, et al. alpha-Synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. The Journal of biological chemistry. 2011;286:43933–43943. doi: 10.1074/jbc.M111.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oaks AW, Sidhu A. Synuclein modulation of monoamine transporters. FEBS letters. 2011;585:1001–1006. doi: 10.1016/j.febslet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bellucci A, et al. From alpha-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain research. 2012;1476:183–202. doi: 10.1016/j.brainres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 98.Kurian MA, et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. The Journal of clinical investigation. 2009;119:1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]