Abstract
The outcome of T-cell-mediated responses, immunity or tolerance, critically depends on the balance of cytopathic versus regulatory T (Treg) cells. In the creation of stable tolerance to MHC incompatible allografts, reducing the unusually large mass of donorreactive cytopathic T effector (Teff) cells via apoptosis is often required. Cyclosporine (CsA) blocks activation-induced cell death (AICD) of Teff cells, and is detrimental to tolerance induction by costimulation blockade, whereas Rapamycin (RPM) preserves AICD, and augments the potential of costimulation blockade to create tolerance. While differences between CsA and RPM in influencing apoptosis of activated graft-destructive Teff cells are apparent, their effects on graft-protective Treg cells remain enigmatic. Moreover, it is unclear whether tolerizing regimens foster conversion of naïve peripheral T cells into alloantigen-specific Treg cells for graft protection. Here we show, using reporter mice for Treg marker Foxp3, that RPM promotes de novo conversion of alloantigen-specific Treg cells, whereas CsA completely inhibits this process. Upon transfer, in vivo converted Treg cells potently suppress the rejection of donor but not third party skin grafts. Thus, the differential effects of RPM and CsA on Teff and Treg cells favor the use of RPM in shifting the balance of aggressive to protective type alloimmunity.
Keywords: Antigen specific, cyclosporine, de novo generation, Foxp3, Rapamycin, regulatory T cells, tolerance
Introduction
In the past two decades, a large array of immunosuppressive agents has expanded the armamentarium used by transplant physicians and surgeons to prevent acute allograft rejection, evidenced by the greatly improved rates of short-term graft survival. The focus of transplantation medicine is now more shifted towards tackling issues associated with side effects of long-term immunosuppression and chronic rejection. The goal is to achieve transplantation tolerance that is specific and indefinite acceptance of transplanted graft without ongoing immunosuppression. Animal studies indicate that the hallmark of transplantation tolerance is the generation of donor-specific regulatory T (Treg) cells that are capable of suppressing cytopathic T effector (Teff) cells. Among different subsets of T cells with regulatory properties, naturally occurring CD4+CD25+ Treg cells are the most often and thoroughly studied.
It has recently been reported that the forkhead/winged-helix transcription factor Foxp3 is the ‘master switch’ for the development and function of CD4+CD25+ Treg cells, and serves as their lineage-specific marker (1–3). These thymus-derived cells are essential in maintaining self-tolerance (4), and are also indispensable for induction of peripheral tolerance in animal models of transplantation (5). Nonetheless, it is a matter of debate whether upon antigen stimulation, formerly naïve T cells in the extrathymic compartment can adopt a Treg phenotype and exert antigen-specific regulatory function (6–8). In transplantation setting, it has not been addressed at molecular details whether peripheral generation of Treg cells occurs and whether such adaptive or induced Treg (iTreg) cells contribute to donor-specific tolerance. Hence we aim to examine the existence and role of de novo-generated Treg cells by potential tolerance-inducing protocols. However, the intracellular localization of Foxp3 limits its usefulness in isolating and transferring live cells for functional studies. To overcome this difficulty, we created a knock-in mouse (Foxp3GFP) with a bicistronic EGFP reporter introduced into the endogenous Foxp3 locus (9). Compared with the CD4+GFP− T cells, CD4+GFP+ T cells are anergic and immunosuppressive in vitro, similar to the wild type CD4+CD25+ counterparts. The GFP+ cells also robustly express Treg-associated markers, including Foxp3, CTLA-4, CD25 and GITR (Supplementary Figure 1). The coordinated but independent expression of wild type Foxp3 and GFP proteins allows us to faithfully track Foxp3-expressing cells with the green fluorescence marker and study the factors that modulate Foxp3 expression (9). With this system, we compared the effects of the two commonly used immunosuppressive drugs, CsA and RPM, on de novo generation of alloantigen-specific Treg cells.
Materials and Methods
Mice
The Foxp3GFP knock-in mice in C57BL/6 background (CD45.2+) were generated as reported (9). They were further crossed once with the CD45.1 + homozygous mice in C57BL/6 background (Jackson Laboratories, Bar Harbor, ME) to obtain both the CD45 congenic alleles. The other mouse strains used in this study were all purchased from the Jackson Laboratories. All animal experiments were performed in compliance with the approval of the Harvard Medical Area Standing Committee on Animals (Boston, MA).
In vitro Foxp3 induction
FACS-sorted CD4+GFP− cells (2 × 105) from naïve Foxp3GFP mice were stimulated in 48-well culture plates coated with anti-CD3 (3 μg/mL) and anti-CD28 (5 μg/mL) (Pharmingen). In some wells, TGF-β1 (eBioscience, 1 ng/mL), anti-TGF-β1 (R & D Systems, 20 μg/mL), and different doses of RPM or CsA (both from LC Laboratories) were added. After 3 days, cells were analyzed on FACS for GFP expression or processed for realtime PCR quantification of Foxp3 message, using RNeasy Mini Kit (Qiagen), cDNA reverse transcription kit and Taqman primer-probe set (both from Applied Biosystems). A comparative threshold cycle (CT) method was used to determine Foxp3 gene relative expression analyzed against the endogenous gene of murine GAPDH. For each sample, the Foxp3 CT value was normalized with the formula ΔCT= CT Foxp3 − CT GAPDH. The mean ΔCT was determined, and relative Foxp3 expression was calculated with the formula 2−ΔCT.
In vivo Treg conversion
FACS-sorted CD4+GFP− cells (1 × 107, CD4+GFP+ <0.1%) from naïve Foxp3GFP mice (CD45.1+CD45.2+) were i.v. injected into nonirradiated BDF1 hosts. After cell transfer, BDF1 mice were treated on days 0, 1, 2 with HBSS or RPM (3 mg/kg, i.p.) or CsA (20 mg/kg, s.c.), alone or together with anti-CD154 (MR1, BioExpress, 0.25 mg, i.p.). On day 4, lymph node and spleen cells from BDF1 hosts were analyzed on FACS by gating on CD45.1 +CD4+ cells. In some experiments, de novo-generated CD4+GFP+ Treg cells were FACS-sorted for in vitro and in vivo function assays.
CFSE labeling, Foxp3 and Annexin V staining
Lymph node and spleen cells from naïve C57BL/6 mice were depleted of CD4+CD25+ cells by anti-CD25 beads (Miltenyi) and labeled with CFSE (5 μM, Invitrogen) at room temperature for 6 min. Cells (4–6 × 107) were then i.v. injected into BDF1 mice. The doses and times for treating recipients with RPM, CsA and/or anti-CD154 were the same as above. After 4 days, spleen and lymph node cells from the treated animals were intracellularly stained with anti-Foxp3 (eBioscience), and gated on the H-2D(d)−CD4+ fraction. Annexin V staining was carried out with a kit from BD Pharmingen.
In vitro suppression assay
Spleen cells from DBA/1 or DBA/2 mice were depleted of T cells by anti-CD4/CD8 beads (Miltenyi), treated with Mitomycin C (Sigma) at 50 μg/mL for 30 min, and used as stimulators (8 × 104) in round-bottomed 96-well plates. Naïve CD4+GFP− cells from Foxp3GFP mice were FACS-sorted and used as responders (8 × 104) in MLR. Varying ratios of FACS-sorted, de novo-induced CD4+GFP+ Treg (iTreg) cells from BDF1 hosts treated with RPM plus anti-CD154 were added to the MLR culture for 4 days. Cells were pulsed with [3H]methylthymidine (0.5μCi/well; NEN) for the last 8 h before harvesting, and incorporated radioactivity of triplicate wells was counted.
Skin transplantation
FACS-sorted CD4+GFP− T cells (2 × 105, from naïve knock-in mice) were transferred by tail vein injection into C57BL/6 RAG-1-deficient mice. One day later, mice were transplanted with allogeneic tail skin grafts from DBA/2 donor. One group of animals was treated with RPM (3 mg/kg, i.p.) for three consecutive days, then every other day for total 14 days. The second group was treated with CsA (20 mg/kg, s.c.) every day for 14 days. The third group was left untreated. In some recipients of the three groups, spleen and lymph node cells were examined for GFP expression by FACS on day 18 and day 30.
To test the alloantigen-specific suppressive activity of iTreg cells, allogeneic tail skin grafts from DBA/1 and DBA/2 mice were simultaneously transplanted onto C57BL/6 RAG-1-deficient mice on the opposite sides of the flank. CD4+GFP− Teff cells (2 × 105, from naïve knock-in mice) with or without de novo-induced iTreg cells (3 × 104, from BDF1 hosts treated with RPM plus anti-CD154) were transferred by tail vein injection. Each graft was examined daily beginning at day 7 post-adoptive transfer and was considered rejected when the graft is completely necrotic. Difference of graft survival times was assessed by Kaplan-Meier survival analysis with StatView software. P < 0.01 is considered statistically significant.
Results
RPM, but not CsA, induces Foxp3 expression in activated CD4+ Foxp3− T cells in vitro
First, we examined in vitro whether RPM or CsA can affect de novo expression of Foxp3 by peripheral T cells. Naïve CD4+GFP− T cells were sorted by FACS (99% purity), and stimulated with plate-bound anti-CD3 and anti-CD28. Addition of TGF-β1 resulted in the conversion of 30–40% of naïve CD4+GFP− T cells into the Foxp3+ Treg phenotype, as previously reported (9). RPM also promotes conversion, albeit less vigorously, with about 10% of CD4+Foxp3− cells stimulated with plate-bound anti-CD3 and anti-CD28 converting into the Foxp3+ phenotype after 3 days of culture (Figure 1A). Induction of Foxp3 by RPM is dose-dependant, though transient peaking at day 3 (Figure 1B).
Figure 1. RPM but not CsA induces Foxp3 expression in CD4+Foxp3− T cellsin vitro.
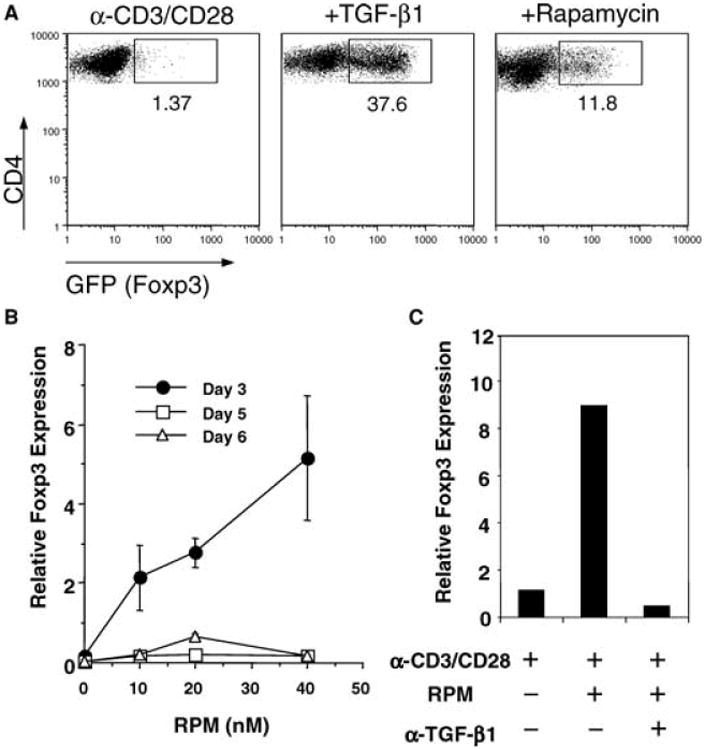
(A) FACS-sorted CD4+Foxp3−(GFP−) cells from naïve Foxp3GFP knock-in mice were stimulated with plate-bound anti-CD3 and anti-CD28 alone or in the presence of TGF-β1 (1 ng/mL) or RPM (20 nM) for 3 days. Foxp3 induction was monitored by the GFP signal by FACS. The percentages of the Foxp3+(GFP+) cells within the total CD4+ T-cell population are indicated. (B) Kinetics of Foxp3 induction by different doses of RPM. Error bars represent two measurements of Foxp3 message (relative to that of GAPDH) by real-time PCR in cells stimulated in vitro as above. (C) Anti-TGF-β1 blocked the induction of Foxp3 message by RPM. The neutralizing antibody (20 μg/mL) was added at the beginning of the 3-day culture. (D) Differential effects of RPM and CsA on TGF-β induction of Foxp3+(GFP+) cells. FACS-sorted CD4+Foxp3−(GFP−) cells were stimulated as in (A) in the presence of TGF-β1 (1 ng/mL) with increasing doses of RPM or CsA. After 3 days, total cells were gated for Annexin V staining. The percentages of GFP+ cells in Annexin V negative population were plotted against the drug doses in (E). Data represent three independent experiments.
Previous work has demonstrated that RPM induces TGF-β1 expression (10). We found that anti-TGF-β1 antibody completely blocked RPM-induced Foxp3 expression (Figure 1C), suggesting a potential link between RPM effect and TGF-β1 signaling. Although CsA is also known to induce TGF-β1 (11), it not only failed to induce Foxp3 (not shown), but also blocked Foxp3 induction by TGF-β1 (Figure 1D) or RPM (not shown). Interestingly, TGF-β-converted GFP+(Foxp3+) Treg cells are mainly Annexin V negative, indicating that they are more resistant to apoptosis than GFP−(Foxp3−) Teff cells. Contrary to RPM being permissive to TGF-β, CsA blocked TGF-β-induced Foxp3 expression in cells that were still Annexin V negative (Figure 1D,E), suggesting its inhibitory effect is not simply due to compromising cell viability. Another calcineurin inhibitor FK506 (Tacrolimus) exerted the same effects as CsA (not shown). Thus, both TGF-β1 and calcineurin-dependent signals are required for de novo induction of Foxp3.
RPM, but not CsA, induces in vivo conversion of naïve T cells into Treg cells
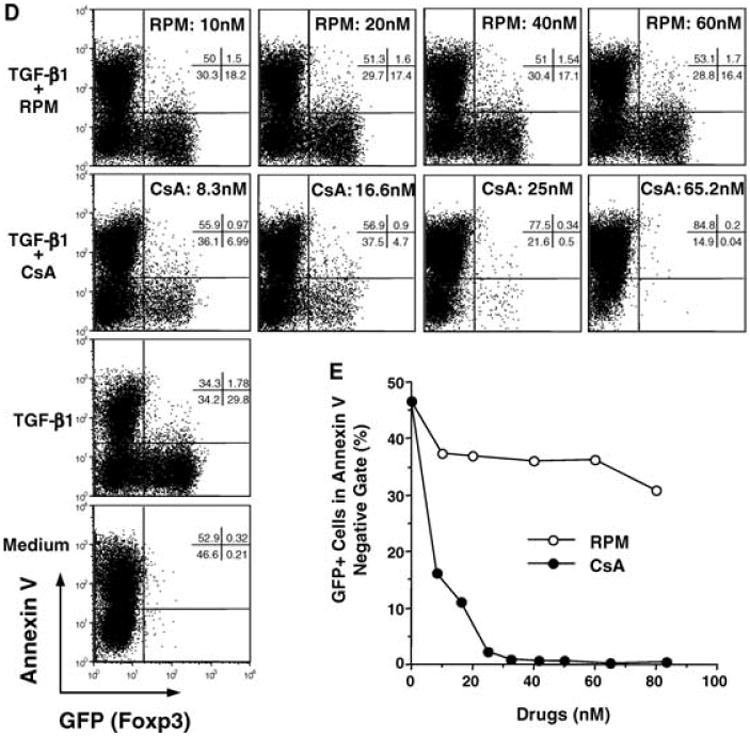
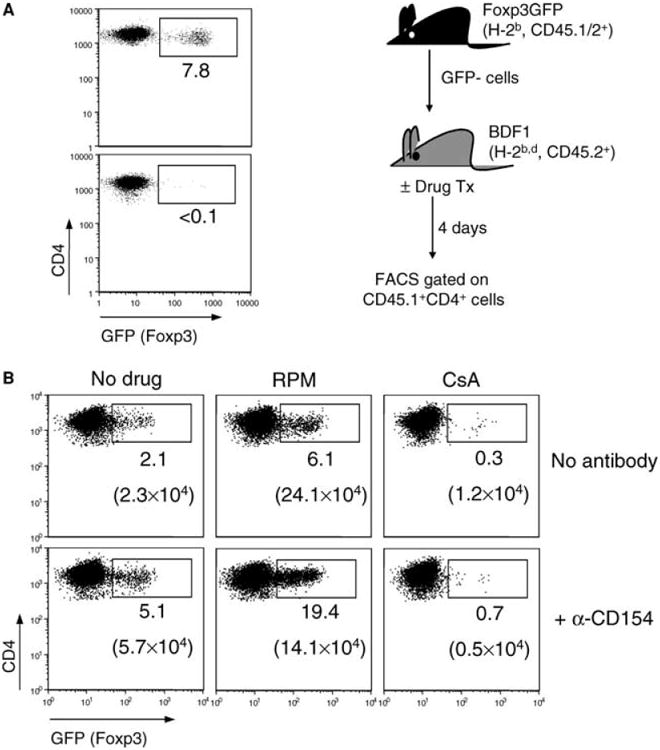
Next, we compared the effects of RPM and CsA upon in vivo conversion of naïve CD4+GFP− T cells into Treg cells. To optimally stimulate naïve T cells with alloantigen, FACS-sorted CD4+GFP− cells (GFP+ cells <0.1%) from the knock-in mice (C57BL/6 background, H-2b, CD45.1/2+) were adoptively transferred into naïve nonirradiated BDF1 mice (F1 of C57BL/6 and DBA/2, H-2b,d, CD45.2+) (Figure 2A). In this GVHD-like model, transferred alloreactive CD4+ T cells respond to host H-2d alloantigen, proliferate and complete 7–8 cell divisions within 3 days as demonstrated by CFSE dye-dilution assay (not shown). After transfer of CD4+GFP− C57BL/6 T cells, BDF1 hosts were treated on days 0, 1, 2 with HBSS or RPM (3 mg/kg, i.p.) or CsA (20 mg/kg, s.c.), alone or with MR1 anti-CD154 mAb (0.25 mg, i.p.).
Figure 2. The influence of RPM and CsA upon alloantigen-driven extrathymic de novo generation of Foxp3+ cells.
(A) Naïve CD4+GFP− cells from Foxp3GFP mice (H-2b, CD45.1+CD45.2+) were enriched by FACS sorting. The pre- (upper) and post-sort (lower) FACS plots, and the scheme of adoptive cell transfer are shown. BDF1 hosts (H-2b,d, CD45.2+) were injected (i.v.) with 10 million of sorted C57BL/6 CD4+GFP− cells, and treated on days 0, 1, 2 with HBSS, RPM (3 mg/kg, i.p.), CsA (20 mg/kg, s.c.), alone or together with anti-CD154 (MR1, 0.25 mg, i.p.). (B) C57BL/6 CD4+ T cells residing in the spleens of BDF1 hosts were analyzed on day 4 via FACS gating on the CD45.1+ population. The percentages of induced Foxp3+(GFP+) cells within the gated C57BL/6 CD4+ T cells are indicated. Note that induced Treg cells (RPM and/or anti-CD154 treatment in B) express lower levels of GFP than natural Treg cells (upper panel in A). The absolute numbers of GFP+ cells recovered from the spleens of animals under different treatments were presented in parenthesis. See Supplementary Tables 1a and b for detailed calculations. Data are representative of the results obtained in 10 different experiments.
On day 4 post cell transfer, lymph node and spleen cells from BDF1 hosts were analyzed by flow cytometry via gating onto the CD45.1+CD4+ cells. Six percent of C57BL/6 cells residing in the spleens of the hosts treated with RPM became GFP+(Foxp3+), while 2% were positive in hosts receiving buffered saline. Provision of anti-CD154 mAb alone elicited a similar outcome. Combined RPM and anti-CD154 treatment was synergistic as nearly 20% of transferred C57BL/6 CD4+ T cells residing in the host spleens were Foxp3+ (Figure 2B), and the ratio reached 25% for C57BL/6 cells harvested from the host lymph nodes (not shown). In sharp contrast, CsA not only abrogated the positive effect of anti-CD154 upon conversion of Foxp3− into the Foxp3+ phenotype, but also blocked the low basal level conversion (about 2%) in control nontreated (HBSS) hosts. Clearly, RPM induces, whereas CsA blocks, the in vivo conversion of naïve CD4+Foxp3− T cells into Foxp3+ cells that arises with alloantigen stimulation.
The kinetics of in vivo conversion is fairly fast. In a separate experiment, we determined the percentages and absolute numbers of GFP+ cells among transferred CD4+ cells at different time points. The peak of conversion induced with RPM+anti-CD154 occurs by day 4 in spleen and by day 7 in lymph nodes (Supplementary Figure 2). Conversion declines afterwards, and on day 15, much fewer GFP+ cells can be recovered from spleen and lymph nodes (Supplementary Table 2). Two months after cell transfer, no GFP+ cells can be detected in CD45.1+CD4+ population (not shown). As suggested by the in vitro observation (Figure 1B), RPM-induced conversion may also be transient in vivo.
Differences between Teff cells and de novo-generated Treg cells in cell cycle progression and susceptibility to apoptosis
Although RPM and anti-CD154 synergize in inducing higher percentage of converted Treg cells, RPM itself paradoxically induces more Treg cells by absolute number in both spleen and lymph nodes (Figure 2B, Supplementary Table 1a and 1b). It might be that anti-CD154 differentially inhibits the proliferation of GFP− Teff cells, thus increasing the percentage of GFP+ Treg cells. To resolve this issue, we studied in vivo Treg conversion using a method that combines staining of Foxp3 intracellular proteins and CFSE labeling for cell division analysis. We adopted this strategy because both GFP (Foxp3) and CFSE dye emit green fluorescence, and cannot be simultaneously analyzed on the FL1 channel of FACScan. Lymph node and spleen cells from naïve C57BL/6 mice were depleted of CD4+CD25+ Treg cells by magnetic beads, labeled with CFSE and transferred into BDF1 hosts. RPM alone or RPM+anti-CD154 vigorously induced conversion of alloactivated CD4+ T cells into the Treg phenotype in the early cell divisions (e.g., 30–40% cells became Foxp3+ within divisions 1 and 2), whereas T cells undergoing more extensive proliferation (divisions 5–7) negligibly expressed Foxp3 (Figure 3A,B). Again, CsA completely blocked Foxp3 induction (Figure 3B). Interestingly, the percentages of Foxp3+ cells at divisions 1–4 were virtually the same between RPM and RPM+anti-CD154 treatments, suggesting anti-CD154 has little effect on the conversion process. Nonetheless, as compared to RPM treatment alone, treatment with RPM+anti-CD154 more significantly inhibited the proliferation of alloactivated Foxp3− Teff cells, but only slightly inhibited the apparent proliferation of Foxp3+ Treg cells (Figure 3C). In this experimental setting, however, the division peaks of Foxp3+ Treg cells do not necessarily represent their actual division rate, due to the fact that the initial CFSE label was on the Foxp3− Teff cells. Foxp3+ cells within a particular division could be the descendants of proliferating induced Treg cells and/or those converted “on spot” from proliferated Foxp3− Teff cells into Foxp3+ phenotype. Regardless of the interpretation, costimulation blockade with anti-CD154 shifts the balance of Treg vs. Teff by inhibiting more on the proliferation of Teff cells, albeit the absolute number of converted Treg cells is slightly reduced.
Figure 3. Differences between Teff cells and de novo generated Treg cells in cell cycle progression and susceptibility to apoptosis.
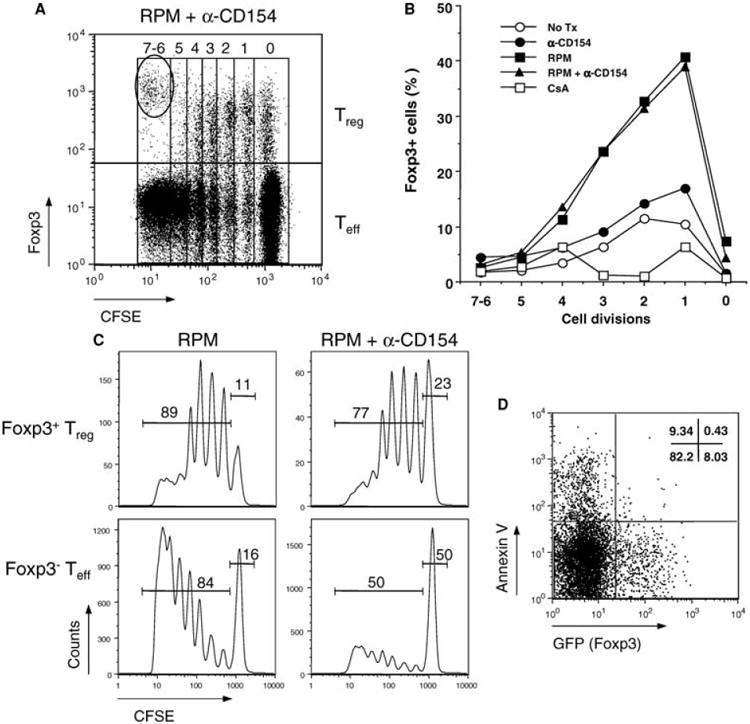
(A) BDF1 hosts injected with CFSE-labeled CD4+CD25− cells from naïve C57BL/6 mice were treated with RPM+anti-CD154 to induce de novo conversion of Treg cells as described in Methods. Lymph node cells from BDF1 hosts were stained with anti-Foxp3 and the gated H-2D(d)−CD4+ (C57BL/6) fraction was analyzed by FACS on day 4. The oval gate indicates the small contaminating natural Treg population within the starting CD4+CD25− pool, which has higher Foxp3 expression than de novo generated Treg cells. (B) Foxp3+ cells were induced in the early cell divisions. The percentages of C57BL/6 Foxp3+ cells induced 4 days after adoptive transfer into BDF1 hosts and subsequent drug treatment were plotted as a function of the number of cell divisions. (C) Anti-CD154, in conjunction with RPM, preferentially inhibits the proliferation of Foxp3− Teff cells. Day 4 CFSE dilution profiles of H-2D(d)−CD4+ Teff cells (Foxp3− gating in A) and Treg cells (Foxp3+ gating in A) with frequencies of divided and nondivided cells are shown. (D) The converted GFP+ Treg cells, but not GFP− Teff cells, are more resistant to apoptosis as demonstrated by Annexin V negative staining. Treg cells were de novo generated from GFP− naïve T cells of the knock-in mice upon adoptive transfer into BDF1 hosts and subsequent RPM+anti-CD154 treatment. Total H-2D(d)−CD4+ T cells were gated for Annexin V staining. Data are representative of three different experiments.
Since activation-induced cell death (AICD) accompanies vigorous T-cell proliferation (12,13), we investigated whether Teff and newly converted Treg cells are differentially susceptible to apoptosis. Indeed, when recipients of allogeneic T cell transfers were treated with RPM+anti-CD154, CD4+ T cells with Annexin V positive staining were primarily noted within the GFP− compartment (Figure 3D). Therefore, RPM-based treatment fosters de novo conversion of naïve T cells into Foxp3+ Treg cells. Moreover, unlike Teff cells, the converted Treg cells are more resistant to apoptosis, a conclusion also supported by the in vitro finding (Figure 1D).
De novo-generated Treg cells are alloantigen-specific, and can protect donor-strain skin graft upon transfer
We then tested the antigen specificity of de novo-generated Treg cells in the mixed lymphocyte reaction (MLR). CD4+GFP− Teff cells from naïve knock-in mice were stimulated with Mitomycin C-treated DBA/1 (H-2q) or DBA/2 (H-2d) splenocytes. The de novo-generated Treg cells, sorted by FACS from RPM+anti-CD154 treated BDF1 mice, potently suppressed the proliferation of Teff cells against donor DBA/2 but not third-party DBA/1 allogeneic cells (Figure 4A), indicating they are alloantigen specific. To test the specificity of their graft protective function in vivo, we simultaneously grafted DBA/1 and DBA/2 skins onto recipient RAG-1-deficient mice on the opposite sides of the flank, and then transferred FACS-sorted naïve CD4+GFP− Teff cells, with or without GFP+ induced Treg (iTreg) cells sorted from RPM+anti-CD154 treated BDF1 hosts. Transfer of 3 × 104 iTreg cells powerfully suppressed the ability of 2 × 105 Teff cells to reject DBA/2, but not DBA/1, skin grafts (Figure 4B). Thus, such de novo-generated Foxp3+ cells are bona fide antigen-specific Treg cells that, like in vitro activated naturally occurring Treg cells (14, 15), can mediate donor graft protection, even at a low ratio of transferred 6 effectors to 1 regulatory T cell.
Figure 4. De novo generated Treg cells exert alloantigen-specific suppression and donor-selective graft protection.
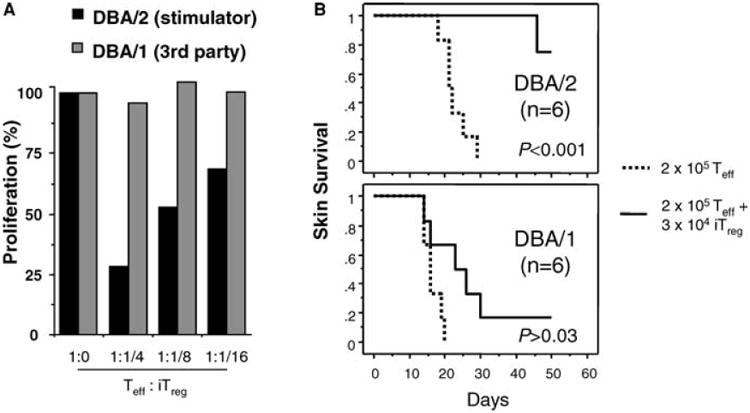
(A) In vitro MLR. Naïve CD4+GFP− Teff cells from the knock-in mice (H-2b) were stimulated with Mitomycin C-treated DBA/1 (H-2q) or DBA/2 (H-2d) splenocytes for 4 days. CD4+GFP+ induced Treg (iTreg) cells were FACS-sorted and added at varying ratios to Teff cells in the MLR. T-cell proliferation in these cultures, as measured by the mean values of incorporated thymidine of triplicate wells, is compared to that of MLR cultures with Teff alone (normalized as 100%). (B) Protection of donor but not third party skin grafts by iTreg cells. C57BL/6 RAG-1-deficient mice were simultaneously transplanted on the opposite sides of the flank with allogeneic tail skin grafts from DBA/1 and DBA/2 mice. CD4+GFP− Teff cells (2 × 105) alone (dotted line) or together with CD4+GFP+ iTreg cells (3 × 104) (solid line) were then transferred by tail vein injection. P<0.01 in Kaplan-Meier survival analysis is considered statistically significant.
RPM, but not CsA, induces long-term skin allograft survival with concomitant de novo generation of Foxp3+ cells
To correlate RPM-induced in vivo Treg cell generation with allograft tolerance in a transplant setting more physiological than the alloantigen-driven GVHD-like model (Figures 2 and 3), we adoptively transferred 2 × 105 CD4+GFP− naïve T cells into C57BL/6 RAG-1-deficient mice bearing transplanted DBA/2 skin grafts, and then treated the reconstituted recipients with RPM or CsA for 14 days. Untreated animals rejected DBA/2 skins promptly (MST = 25.7 days, n = 6). CsA treatment prolonged graft survival (MST = 40.7 days, n = 6) with statistical difference (p = 0.003), but all the grafts were eventually rejected. In contrast, RPM treatment induced indefinite graft survival (MST > 100 days, n = 7) (Figure 5A). FACS analysis on day 18 and day 30 post-treatment showed that RPM promoted a significant portion of transferred naïve T cells to become Foxp3+ Treg cells. These cells first appeared in the spleen, and later were enriched in the graft-draining lymph nodes of RPM-treated hosts. CsA, on the other hand, had a detrimental effect and even inhibited the basal level (1–3%) induction of Foxp3+ Treg cells by homeostatic proliferation (Figure 5B and W.G. unpublished observation). In a separate cell dosing experiment, 10-fold more (2 × 106) CD4+GFP− naïve T cells were adoptively transferred into RAG-1-deficient mice bearing DBA/2 skin grafts, and the recipient mice were treated with RPM or CsA by the same protocol. FACS analysis on day 18 post-treatment revealed a similar pattern of Treg cell induction as above (not shown). Moreover, the MST of DBA/2 skin grafts were 10.0 days (untreated, n = 3), 17.5 days (CsA-treated, n = 2) and 26.0 days (RPM-treated, n = 2) respectively. We terminated the experiment because the mice started to show signs of wasting disease. Nevertheless, these results collectively suggest that RPM-induced de novo generation of Foxp3+ Treg cells may contribute to its graft protecting activities.
Figure 5. RPM induces long-term skin allograft survival with concomitant de novo generation of Foxp3+ cells.
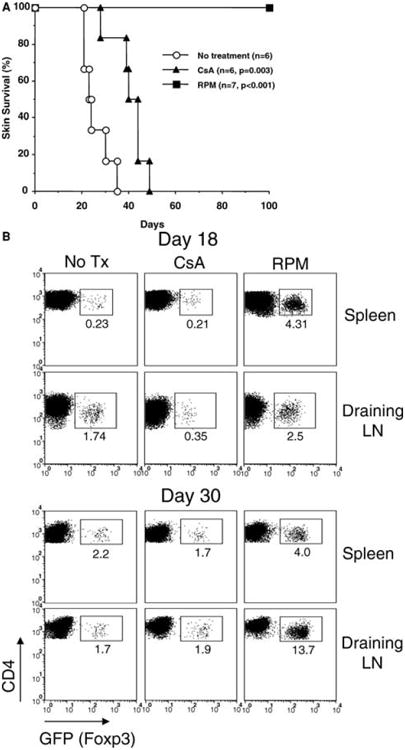
(A) RPM, but not CsA, induces long-term skin graft survival in an adoptive transfer model. FACS-sorted CD4+GFP− T cells (2 × 105, from naïve knock-in mice) were transferred into C57BL/6 RAG-1-deficient mice receiving allogeneic tail skin grafts from DBA/2 donor. Mice were either not treated, or treated with RPM (3 mg/kg, i.p., daily for the first 3 days and then every other day for 11 days) or CsA (20 mg/kg, s.c, daily for 14 days). (B) Cells from spleens and graft-draining lymph nodes were examined for GFP expression by FACS on day 18 and 30. The percentages of GFP+ cells among transferred CD4+ T cells were indicated. Data represent the mean values of two individual mice from each group.
Discussion
Cyclosporine (CsA) and Rapamycin (RPM) are widely used to effectively prevent transplant rejection. Both drugs are potent and reasonably well tolerated immunosuppressive agents, but their effects on graft-destructive Teff and graft-protective Treg cells are drastically different. In collaboration with the Turka laboratory, we have previously reported that RPM promotes, whereas CsA blocks, AICD of allore-active T cells (13,16). In several other models, RPM enhances while CsA abrogates the efficacy of costimulation blockade-based therapy to induce graft tolerance (17–20). These and work on CD8+ T cells (21) collectively indicate that AICD of alloreactive T cells in general is prerequisite for tolerance induction by costimulation blockade. Nonetheless, the effects of RPM and CsA on subsets of alloreactive T cells, namely Teff versus Treg cells, especially those de novo-generated Treg cells, were not tested in all the abovementioned studies. Here we demonstrated that RPM promotes and synergizes with anti-CD154, to convert peripheral alloreactive CD4+Foxp3− T cells into apoptosis-resistant Foxp3+ Treg cells that can mediate donor-specific skin graft protection upon transfer, whereas CsA completely inhibits this process. In a companion study using a pre-transplant conditioning regimen of donor-specific transfusion plus anti-CD154 mAb, the enhanced donor-directed Treg activity in the CD4+CD25+ pool could be further strengthened by addition of RPM but abolished by CsA cotreatment (Kang et al., submitted for publication). It has been reported that CsA treatment reduces Foxp3 expression in natural Treg cells (22), and fails to support the differentiation of the highly suppressive CD4+CD25+CD27+ subset upon alloantigen stimulation (23). On the contrary, RPM does not show adverse effects but sustains a high ratio of natural CD4+CD25+ Treg cells during IL-2-mediated expansion (24). In addition, naïve human T cells exhibit regulatory activities upon TCR stimulation in the presence of RPM (25), although direct evidence for Teff to Treg conversion was not fully established due to promiscuous expression of Foxp3 in human T cells after activation (26). The effects of CsA versus RPM on Treg conversion was not assessed in that study (25). In renal transplant recipients, calcineurin inhibitors, but not RPM, were found to reduce the frequencies of CD4+CD25+Foxp3+ Treg cells (27). The detrimental effect of calcineurin inhibitors on Treg cells could partly lie in their activity to block IL-2 production, which is required for Treg function and homeostasis (28), as replenishing CsA-treated hosts with exogenous IL-2/Fc (or IL-2) restores Treg activity in transplantation (Kang et al.) and GVHD models (29). Additionally, calcineurin inhibitors may more profoundly affect Treg cell programming by directly interfering with NFAT:Foxp3 interaction (30,31). Such an effect could be fatal for the newly converted Treg cells when Foxp3 levels are delicately low. In support of this notion, excessive amount of IL-2/Fc failed to revert CsA blockade on Treg conversion in vivo (not shown).
The extrathymic de novo generation of Treg cells is of considerable interest, as this process may underline producing allograft-specific suppressors important for transplant tolerance, as well as new Treg recruits in the vicinity of tumor or infection that deter specific immunity. By using a Foxp3-GFP fusion protein knock-in mouse, Fontenot et al. reported that induction of Foxp3 expression in CD4+GFP− T cells does not occur in vivo during pathogen-driven immune responses (7). Our study demonstrated for the first time that de novo generation of graft-protective Treg cells indeed occurs in vivo under tolerizing conditions. Such conversion of Teff into Treg may very likely depend on cytokine milieu, as proinflammatory cytokines highly secreted during alloactivation, pathogen infection or immunization with complete adjuvant would inhibit Foxp3 induction while favoring the development of pathogenic Th17 cells (9). RPM is a potent antiinflammatory agent (32), and as well an inducer of TGF-β directly and/or indirectly through causing apoptosis (10,33). Although RPM effect is TGF-β dependent (Figure 1C), TGF-β might not be the sole factor responsible for RPM-induced conversion. During in vitro MLR with allogeneic splenocytes, RPM and TGF-β showed a synergistic effect in inducing Treg cells. The effect by RPM cannot be supplemented by increasing TGF-β dose (Supplementary Figure 4). Suppression of cell cycle progression by RPM may also favor Treg conversion, as Teff cells undergoing extensive proliferation failed to induce Foxp3 ((8); Figure 3). This inverse correlation between proliferation and conversion is also supported by our findings that antigen presenting cells (APCs) possessing weak costimulatory activity promote better conversion of naïve T cells into the Treg phenotype than APCs with potent costimulatory properties, and co-stimulation blockade has an added beneficial effect (Zhong et al., submitted for publication).
In summary, RPM and CsA differentially affect both T-cell death and T-cell regulation. RPM induces de novo generation of biologically active Treg cells that mediate graft protection selectively for stimulating cells/tissues. Therefore, RPM but not CsA should be included in tolerance-inducing protocols, in order to recruit not only natural but also induced Foxp3+ cells into the overall Treg pool. How much natural and induced Treg cells contribute relatively to transplant tolerance warrants further study, especially with the use of genetically modified mouse lines that enable specific depletion of one or the other population at ease.
Supplementary Material
Supplementary Table 1a: Induction of GFP+ iTreg cells in spleen
Supplementary Table 2: Kinetics of GFP+ iTreg induction after RPM+α-CD154 treatment
Acknowledgments
We thank Pixu Liu and Zhigang Fan for technical assistance. This work is supported in part by grants from National Institutes of Health and Juvenile Diabetes Research Foundation (NIH NS 30843 to M.O. and V.K.K.; NIH A141521–08 and a grant from JDRF Center on Immune Tolerance in Type 1 Diabetes to T.B.S.; and JDRF 5–2006-19 to W.G.).
References
- 1.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25 + regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 2.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 5.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 6.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 10.Dodge IL, Demirci G, Strom TB, Li XC. Rapamycin induces transforming growth factor-beta production by lymphocytes. Transplantation. 2000;70:1104–1106. doi: 10.1097/00007890-200010150-00020. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Sehajpal PK, Khanna A, et al. Differential regulation of transforming growth factor beta and interleukin 2 genes in human T cells: demonstration by usage of novel competitor DNA constructs in the quantitative polymerase chain reaction. J Exp Med. 1991;174:1259–1262. doi: 10.1084/jem.174.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XC, Demirci G, Ferrari-Lacraz S, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 13.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 14.Trenado A, Sudres M, Tang Q, et al. Ex vivo-expanded CD4+ CD25 +immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J Immunol. 2006;176:1266–1273. doi: 10.4049/jimmunol.176.2.1266. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki S, Patel M, Harper A, et al. Effective expansion of alloantigen-specific Foxp3+ CD25+ CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc Natl Acad Sci USA. 2006;103:2758–2763. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Zheng XX, Li XC, Zand MS, Strom TB. Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation. 1998;66:1387–1388. doi: 10.1097/00007890-199811270-00021. [DOI] [PubMed] [Google Scholar]
- 18.Smiley ST, Csizmadia V, Gao W, Turka LA, Hancock WW. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement for NFkappaB: implications for tolerance induction. Transplantation. 2000;70:415–419. doi: 10.1097/00007890-200008150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Sho M, Sandner SE, Najafian N, et al. New insights into the interactions between T-cell costimulatory blockade and conventional immunosuppressive drugs. Ann Surg. 2002;236:667–675. doi: 10.1097/00000658-200211000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaha P, Bigenzahn S, Koporc Z, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. [DOI] [PubMed] [Google Scholar]
- 21.Dai Z, Arakelov A, Wagener M, Konieczny BT, Lakkis FG. The role of the common cytokine receptor gamma-chain in regulating IL-2-dependent, activation-induced CD8+ T cell death. J Immunol. 1999;163:3131–3137. [PubMed] [Google Scholar]
- 22.Baan CC, Van Der Mast BJ, Klepper M, et al. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation. 2005;80:110–117. doi: 10.1097/01.tp.0000164142.98167.4b. [DOI] [PubMed] [Google Scholar]
- 23.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–1023. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 24.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 25.Valmori D, Tosello V, Souleimanian NE, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 26.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segundo DS, Ruiz JC, Izquierdo M, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82:550–557. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 28.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25 +regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 Controls Regulatory T Cell Function through Cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Carlson RP, Hartman DA, Tomchek LA, et al. Rapamycin, a potential disease-modifying antiarthritic drug. J Pharmacol Exp Ther. 1993;266:1125–1138. [PubMed] [Google Scholar]
- 33.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1a: Induction of GFP+ iTreg cells in spleen
Supplementary Table 2: Kinetics of GFP+ iTreg induction after RPM+α-CD154 treatment


