Abstract
Background
Attenuated Listeria monocytogenes vaccine strains have been administered intravenously (Le et al. [1], Maciag et al. [2]) and orally (Angelakopoulos et al. [3], Johnson et al. [4]) to humans. Here, one was given transcutaneously with cholera toxin adjuvant.
Methods
Eight healthy volunteers were studied (5 active, 3 placebo). Safety was assessed by physical exam and labs. Systemic immunological responses were measured by ELISA and IFN-gamma ELISpot.
Results
4/5 active volunteers had cellular responses to listerial antigens. 5/5 active volunteers showed humoral responses to cholera toxin.
Conclusions
An attenuated L. monocytogenes vector was safely administered transcutaneously. Topical administration appeared at least as immunogenic as previously studied oral delivery.
Keywords: Listeria monocytogenes, Transcutaneous, Vaccination, ELISpot, attenuated vector, Clinical trial
1. Introduction
The skin contains a complete complement of the body’s immune cells. An adult may have as many as 20 billion T cells of various subtypes in the skin, exceeding the number in circulation (reviewed in [5]). Dermal dendritic cells capture antigens and deliver them to the skin-draining lymph nodes and activate naïve or central memory T cells stimulating both local and systemic immune responses [5].
Intradermal injections can be vaccine-sparing and effective [6]. Intradermal delivery can be done using traditional needles, microneedles, air pressure insufflation, iontophoresis, scarification, or by simple absorption. The microbial enterotoxins cholera toxin and Escherichia coli heat labile toxin and mutants thereof have been shown to be mucosal and cutaneous immunogens and potent adjuvants. Transcutaneous administration of LT has enhanced immune responses to a co-administered bacterial protein [7], and an injected influenza vaccine [8].
Live attenuated bacterial vectors based upon enteric pathogens like Listeria and Salmonella species are relatively easily engineered to express foreign antigens. Unfortunately, they have not proved highly successful in human studies in stimulating immune responses to engineered heterologous antigens. Live bacterial vectors administered orally are plagued by several (at least theoretical) concerns: inadequate attenuation, reversion, recombination, shedding, and the potential for transmission. We previously evaluated a highly attenuated Listeria monocytogenes organism expressing an influenza A nucleoprotein antigen by the oral route in single escalating oral doses in volunteers, and found this organism safe, transiently shed, but poorly immunogenic and probably over-attenuated for oral delivery [4].
Despite this apparent failure, we hypothesized that this would be an ideal, highly attenuated organism to test transcutaneous application of a live attenuated enteric vector. In mice L. monocytogenes stimulates potent CD4 and CD8T cell responses and effectively delivers both viral and tumor antigens (reviewed in [9]). Besides vertical transmission in utero, L. monocytogenes is not transmitted person-to-person. Unlike oral delivery, cutaneous delivery (though not presentation or antigen processing) can be rapidly stopped by topical disinfection, and minimizes concerns related to shedding. We included a native commercially available cholera toxin adjuvant, in an attempt to maximize immune responses. The project was proposed as a novel physiological study of a bacterial vector delivered transcutaneously, and not as development of a new influenza vaccine.
We show that cellular immune responses to complex listerial antigens can be engendered by the transcutaneous route. Though not compared “head-to-head” these results may be superior to those engendered by delivery of a single large dose orally.
2. Materials and methods
2.1. Bacterial strain
L. monocytogenes strain BMB72 expressing a secreted influenza A nucleoprotein antigen was derived from L. monocytogenes strain 10403S as described [4].
2.2. Human subjects
The study was reviewed and approved by the Partners IRB, the Harvard Institutional Biosafety Committee, the FDA (IND # 13937) and registered at CT.gov (NCT01311817). Healthy adults who were 18–55 years old and provided written informed consent underwent a complete medical screening by history, physical exam and laboratory evaluation as described [4]. Subjects were not screened for previous infection with L. monocytogenes or influenza and were paid per IRB norms. There is no clinically validated serological test for prior or active infection with L. monocytogenes.
2.3. Inoculum and administration
Research laboratory GMP methodologies and Standard Operating Procedures acceptable to the US FDA were used to grow and characterize bacterial inocula for clinical use. Bacteria from a master cell bank were grown aerobically with rotary shaking in 2 L glass flasks in trypticase soy broth (DIFCO, Sparks, MD) to optical density 1.0600 nm, harvested by centrifugation, and re-suspended in normal saline/20% USP glycerol (15:1 concentration by volume). Bacteria were not washed. Individual cryovials were filled by manual pipetting, frozen at −80 °C, and assessed at manufacture and every 3–6 months thereafter, for purity and stability, using microbial limits testing and CFU/ml determinations respectively over time. There were pre-specified acceptance criteria for use in humans.
Native, sterile, preservative-free biologically active cholera toxin (List Biological Laboratories, Inc.) was used. Based upon spread plate cultures, the thawed inoculum contained 2.0 × 1010 CFU/dose and also contained approximately 2× that number of dead organisms. Cholera toxin (50 µg) was added to the bacterial inoculum (Lot ELH072910), and a 1 mL volume pipetted onto the absorbent pad of a Tegaderm patch (3 M Company) (Fig. 1). Preclinical cutaneous toxicology studies were performed using CT and the bacterial strain in animals by both the oral and cutaneous routes.
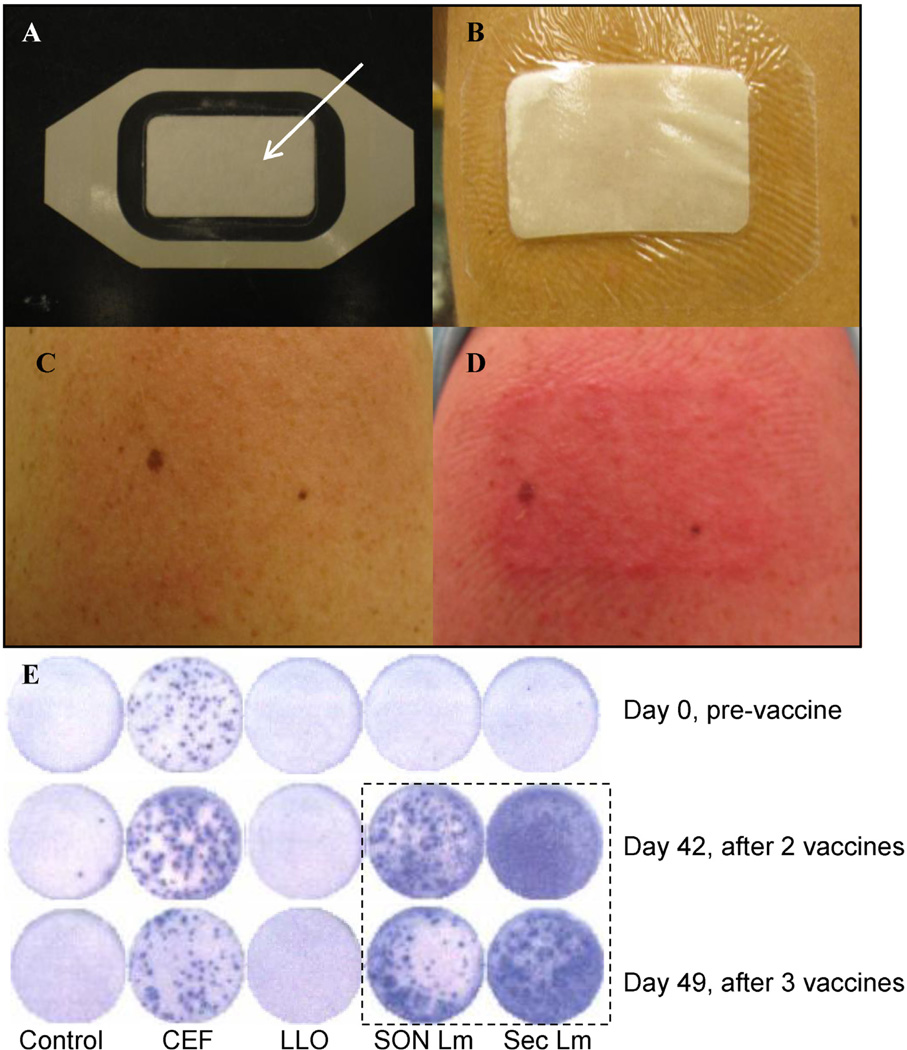
Fig. 1.
Tegaderm patch vaccination, cutaneous reaction and ELISpot wells for Volunteer #7. Panels A and B show the Tegaderm patch with pad (arrow) before and after application. Panels C and D show skin before and 24 h after the second vaccination, the worst rash observed. The lower panel E shows ELISpot wells over time for various conditions, left to right: control (medium alone), commercial CMV, EBV and influenza peptide pool (CEF) listeriolysin peptide pool (LLO), sonicated WT L. monocytogenes (Son Lm) and L. monocytogenes spent medium antigen (Sec Lm). The hatched boxed shows wells with a marked increase over time to listerial antigens compared with baseline (immediately above), in the absence of any apparent change in controls and listeriolysin O wells over time.
The deltoid regions were first exfoliated with dry ECG prep pads (Marquette Medical Systems, Jupiter, FL), then with electrode skin prep pads containing alcohol and silica (Dynarex Company) to remove excess stratum corneum. Vaccine patches were then applied and left in place for up to 24 h (Fig. 1). Each volunteer received two patches: either one active and one placebo (saline), or two placebos. Two patches were used, to assess reaction to the patch alone and the volunteer’s assignment of active vs. placebo patch. Subjects were randomly assigned to active vs. placebo and the study physician and subjects were blinded to assignments. After removal, patch sites were sequentially cleaned with alcohol wipes, a surgical scrub sponge with 3% chloroxylenol (Beckton, Dickinson and Company), and Calstat antiseptic hand rub (Steris).
2.4. Clinical assessments
Subjects were seen as outpatients. Vaccinations were given on days 0, 21, and 35. Volunteers were examined 24 and 48 h after removal of patches. Safety labs (CBC, liver function tests) were done 48 h after each vaccine removal and at a final visit. Temperature was monitored at each visit, and by subjects at home. PBMC were isolated from heparinized blood on days 0, 35, and 42. Serum was collected weekly starting on day 21 (Supplementary Fig. 1, study schema; online).
2.5. Interferon-γ ELISpot studies
IFN-γ ELISpot studies were performed as described [3,4] using freshly isolated PBMC on Immobilon P (MAIPS4510; Millipore) plates containing various peptide pools and complex antigens. Bulk, freshly isolated mononuclear cells isolated by Ficoll gradient centrifugation and counted using a slide-based Nexcelcom automated cell counter were used without further characterization. Control wells included PHA and CEF, a commercial standard peptide pool including 32 CMV, EBV and influenza virus peptides 8–12 amino acids in length (AnaSpec). Test peptides included 3 influenza nucleoprotein peptide pools and a single LLO peptide pool used in our prior oral administration study [4], the latter a gift of Cerus corporation. Complex antigens included whole sonicated L. monocytogenes, and a soluble antigen from spent L. monocytogenes culture medium. Spots were counted by an automated reader (Immunospot 3; CTL). ELISpot results are presented as mean values of duplicate wells per condition as SFC/106 PBMC. A positive response was defined as more than 2-fold greater than baseline spots for that antigen and over 100 SFC/106 PBMC as in our earlier study [4]. Groups were compared using Fisher’s exact test (two tailed).
2.6. Seroconversion/ELISA
Serum samples were studied by ELISA to quantify immunoglobulin G [10] directed against the complex listerial antigens, recombinant his-tagged LLO, cholera toxin, and recombinant influenza A nucleoprotein over time. Antigens (10 µg/mL) were used to coat Maxisorp 96-well plates (Nalge Nunc International). Assays were performed as described [4] with goat-anti-human IgG affinity-purified peroxidase-labeled antibody and read on a Vmax kinetic microplate reader (Molecular Devices). Endpoint titers are reported as the highest dilution at which a serum sample read at ≥0.15 OD450 nm, an empirically chosen cutoff value. Fourfold or greater increases in endpoint titer were considered a positive result. Groups were compared using Fisher’s exact test (two tailed).
3. Results
3.1. Subjects
A total of 22 people were screened by phone, 12 underwent complete screening, and 8 completed the study (7 men, 1 woman; all Caucasian).
3.2. Clinical responses
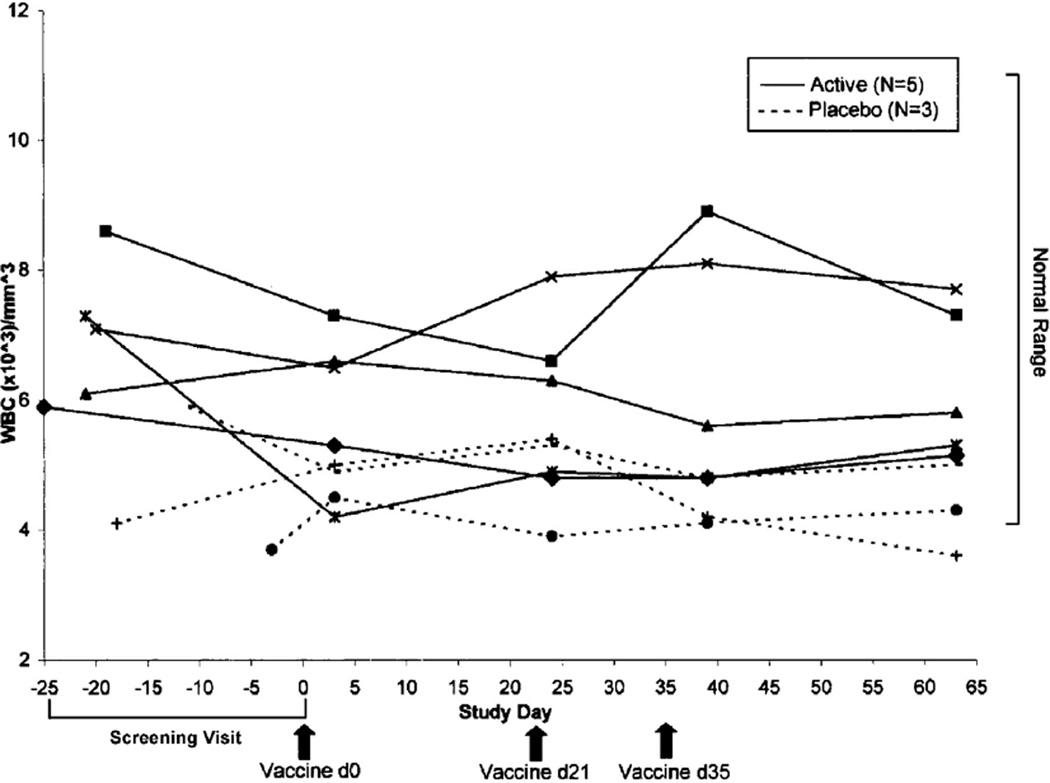
No volunteer had fever, serious or unexpected problems or clinically significant abnormal laboratory findings. No subject at any time point studied had liver function tests outside the normal range (ALT, AST, alkaline phosphatase and Total and Direct Bilirubin). Total WBC over time are shown in Fig. 2 and show minor excursions outside the normal reference range on the lower end which is common in young men; differentials were performed and were clinically unremarkable (data not shown). Rashes were graded as mild, moderate, or severe using pre-defined grading criteria. All volunteers receiving an active patch were able to correctly identify the active and placebo patches based upon pruritis and erythema. Volunteers #5 and #7, both active, developed localized moderate erythematous papular rashes following the second vaccination (Fig. 1 shows volunteer #7, with the worst rash). The protocol allowed 2.5% hydrocortisone cream for hypersensitivity reaction to cholera toxin, but only volunteer #7 used it. Volunteer #7 also requested delay of the third vaccination by one week because of the rash.
Fig. 2.
Total peripheral blood leukocyte (WBC) counts over time. No consistent trends were noted in total WBC or differential (not shown) over time. Volunteer 8 (placebo; closed circle) had known slightly low normal WBC at baseline and was included by formal exception request to the ethics committee.
One volunteer (#4, placebo) spontaneously reported nausea and brief severe diarrhea without fever approximately 2 weeks after removal of the first vaccination. He noted that his wife had similar symptoms; they attributed this to consuming “old” deli meats. He reported no fever or any other symptoms subsequently. There were no mishaps or intoxications related to cholera toxin application.
3.3. Humoral immune responses
Baseline end-point dilution IgG titers against recombinant LLO, complex listerial antigens (as used in ELISpot studies), whole bacterial cells (wild type and vaccine organisms) and recombinant nucleoprotein ranged from 1:160 to 1:10.240. All sera from an individual volunteer were evaluated concurrently on the same ELISA plate, and no individual had a four-fold increase in titer (data not shown). Modifications of ELISA conditions designed to decrease background titers (e.g. modified secondary antibody dilutions or blocking solutions) did not alter these findings. However, 5/5 active versus 0/3 placebo subjects had a four-fold or greater increase in endpoint titer cholera toxin (P = 0.018). Subjects had variable baseline titers (1:40 to 1: 5120) that increased an average of 36-fold in the active group but were essentially unchanged in those receiving 2 placebo patches (average fold increase 1.3).
3.4. IFN-γ ELISpot results
ELISpot data were analyzed as pre-immune vs. peak value, and peaks typically occurred on days 35 or 42 (vaccines received on days 0, 21, 35). All subjects had marked positive control responses to the lectin (PHA), and to the CEF control pool at baseline which were generally stable over time (Table 1). As expected, all subjects had detectable baseline spots to at least one of three influenza nucleoprotein peptide test pools. No subject demonstrated an increase over time directed against influenza peptides (data not shown). All 8 subjects had baseline responses to both complex listerial antigens, with a mean value of 105 SFC/106 PBMC (range 2 to 282). IFN-γ secreting cells responsive to one or both of the complex L. monocytogenes antigens increased after vaccination, and were significantly increased in 4/5 active subjects versus 1/3 placebo (Table 1; P = 0.464). Volunteer 7, with the marked rash, had the most prominent IFN-γ ELISpot response, as shown pictorially in Fig. 1 and numerically in Table 1 (~50-fold increases). Placebo subject #4 had a marked sustained response to complex listerial antigens at days 35 and 42, following diarrhea in the interval between study days 14 and 21. No significant increases were detected in response to any of the 3 influenza nucleoprotein pools or LLO peptides in any volunteer and responses to the control CEF pool were generally unchanged over time (Table 1).
Table 1.
Interferon-gamma ELISpot responses to control and listerial antigens. Peripheral blood mononuclear cells isolated on days 0, 35, and 42/49 were cultured with 5 µg/mL antigen. Volunteers 4, 6, and 8 received a control immunization (saline). Counts represent mean values of IFN-secreting spots per million cells in 2 wells using CTL Immunospot ELISPOT Reader with Immunospot 5 counting software. Antigens from left to right: Saline (negative control); PHA (phytohaemagglutinin, positive control) and CEF (cytomegalovirus, Epstein–Barr virus, influenza virus peptides, positive control); LLO (listeriolysin-O peptides); SonWT (sonicated wild-type L. monocytogenes); Sec Lm (secreted listeria antigen).
| Assignment | Volunteer | Saline | CEF | LLO | Son Lm | Sec Lm |
|---|---|---|---|---|---|---|
| Placebo | #4 day 0 | 5 | 200 | 3 | 8 | 3 |
| day 42 | 13 | 215 | 5 | 295* | 470* | |
| #6 day 0 | 0 | 0 | 0 | 83 | 33 | |
| day 42 | 10 | 33 | 58 | 43 | 60 | |
| #8 day 0 | 5 | 15 | 3 | 58 | 390 | |
| day 42 | 10 | 20 | 5 | 68 | 198 | |
| Active | #1 day 0 | 20 | 140 | 10 | 283 | 148 |
| day 42 | 3 | 108 | 8 | 473 | 128 | |
| #2 day 0 | 13 | 530 | 35 | 175 | 83 | |
| day 42 | 15 | 933 | 35 | 200 | 218* | |
| #3 day 0 | 13 | 90 | 10 | 100 | 18 | |
| day 42 | 8 | 30 | 13 | 955* | 828* | |
| #5 day 0 | 8 | 1285 | 5 | 128 | 110 | |
| day 42 | 3 | 1013 | 50 | 433* | 260* | |
| #7 day 0 | 8 | 628 | 5 | 13 | 38 | |
| *day 49 | 13 | 645 | 5 | 778* | 1548* |
The asterisks denote a significant increase as defined in methods.
4. Discussion
Volunteers tolerated the 3 transcutaneous applications well. Cutaneous reactions were generally mildly pruritic and erythematous and seemed similar to prior reports of hypersensitivity related to cutaneous application of LT [7]. There is a syndrome of primary cutaneous listeriosis, a pustular rash that may lead to disseminated listeriosis, typically in farmers or veterinarians after exposure to infected abortuses [11]. We did not observe skin findings suggestive of cutaneous listeriosis and no subject had fever or systemic symptoms.
Four of 5 active subjects had apparent increases in cellular responses to complex listerial antigens after vaccination. We believe these are bona fide given the absence of changes to control antigens over time, as exemplified in Fig. 1. We acknowledge these complex bacterial antigens contain inflammatory agents and that the responses likely represent a combination of innate and adaptive responses, from CD4, CD8 and possibly other cell types. The positive response in one of three control subjects is strikingly related to a spontaneous report of self-limited gastroenteritis in the subject and his wife, after eating “suspect” delicatessen cold cuts, one of the foods most likely to transmit L. monocytogenes in the USA per annum and per serving [12]. It is possible that listerial gastroenteritis explains his response, though this represents conjecture. As he reported these symptoms late, we were not able to perform a stool culture for Listeria during symptoms, where it might have been positive. The relative lack of cellular immune responses to LLO peptides is not surprising. Although LLO peptide pools result in large increases in IFN-γ response in mice vaccinated parenterally with listerial vectors, it appears to engender less of a response in humans as noted in our prior oral study [4].
The study was originally designed to include a third group of subjects who would have randomly received the L. monocytogenes organism alone, without cholera toxin. Funding limitations necessitated scaling back to include only placebo and active groups, which precluded assessing the contribution of CT adjuvant.
As expected, we did not detect cellular or humoral immune responses to the vectored influenza antigen. In our prior study of this organism, we administered single oral doses of up to 5 × 1010 after neutralization of stomach acid, and subjects shed the organism in fecal samples for up to 5 days [4]. There we saw smaller increases in IFN-ELISpot numbers (assays performed with the same listerial antigens) in about 50% of subjects, and no responses to influenza antigens. No subject’s response to listerial antigens was as prominent as that seen here in Volunteer #7.
Transcutaneous vaccination has practical and perhaps also immunological advantages. Resident effector memory T cells in the skin may contribute to the production of a robust, long-lasting response [10]. Our data support further exploration of live bacterial vaccines and vectors by this route, as both primary vaccines, and as “boosters.”
Supplementary Material
Acknowledgments
We thank the volunteers who participated in this project.
Funding: This work was supported in part by National Institute for Allergy and Infectious Diseases at the National Institutes of Health [RO1AI51206] to ELH. The funder had no role in the design or execution of the study nor in the preparation or submission of the article.
Abbreviations
- CBC
complete blood count; CEF, CMV, EBV, and Influenza virus
- CFU
colony forming units
- GMP
good manufacturing processes
- IFN
interferon
- LLO
listeriolysin
- LT
E. coli heat labile toxin
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- SFC
spot-forming cells
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributors: All authors participated in data acquisition and analysis. EHE and ELH drafted and finalized the manuscript.
Conflicts of interest statement: EIP is a full-time employee of List Biologicals. EHE, PVJ, ELH have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2013.05.028.
References
- 1.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 3.Angelakopoulos H, Loock K, Sisul DM, Jensen ER, Miller JF, Hohmann EL. Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation. Infect Immun. 2002;70:3592–3601. doi: 10.1128/IAI.70.7.3592-3601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson PV, Blair BM, Zeller S, Kotton CN, Hohmann EL. Attenuated Listeria monocytogenes vaccine vectors expressing influenza A nucleoprotein: preclinical evaluation and oral inoculation of volunteers. Microbiol Immunol. 2011;55:304–317. doi: 10.1111/j.1348-0421.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark RA. Skin-resident Tcells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson KS, Janssen JM, Troy SB, Maldonado Y. Intradermal fractional dose inactivated polio vaccine: a review of the literature. Vaccine. 2012;30:121–125. doi: 10.1016/j.vaccine.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Guerena-Burgueno F, Hall ER, Taylor DN, Cassels FJ, Scott DA, Wolf MK, et al. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect Immun. 2002;70:1874–1880. doi: 10.1128/IAI.70.4.1874-1880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frech SA, Kenney RT, Spyr CA, Lazar H, Viret JF, Herzog C, et al. Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch. Vaccine. 2005;23:946. doi: 10.1016/j.vaccine.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Le DT, Dubensky TW, Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol. 2012;39:311–322. doi: 10.1053/j.seminoncol.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLauchlin J, Low JC. Primary cutaneous listeriosis in adults: an occupational disease of veterinarians and farmers. Vet Rec. 1994;135:615–617. [PubMed] [Google Scholar]
- 12.Whiting R, Carrington C, Hicks J, Dennis S, Buchanan R, Brandt M, et al. quantitative assessment of relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. FDA report. 2003 http://www.fda.gov/downloads/food/scienceresearch/researchareas/riskassessmentsafetyassessment/ucm197330.pdf (accessed 2012)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.