Abstract
Feedlot calves (n = 3784) were systematically randomized and allocated in a 2 × 2 factorial study to receive metaphylactic oxytetracycline (OTC) on arrival or no antimicrobial, as well as florfenicol once subcutaneously or twice intramuscularly (48 h apart) if diagnosed with bovine respiratory disease (BRD). Calves of different treatment groups were comingled and followed from placement to re-implantation (~100 days). Animals receiving OTC had a reduced risk of BRD, an increased risk of arthritis, and no significant differences in average daily gain, BRD relapse, overall mortality, or BRD mortality. There were no significant differences between treatment protocols. Deep nasal swabs (n = 233) taken at arrival (n = 122), treatment (n = 77), and swabs from lungs and joints at postmortem (n = 34) were cultured for Mycoplasma bovis from 61 animals ill or dying of chronic pneumonia and arthritis and from 61 healthy calves. There was significant variation in diversity among isolates (n = 51) between study years and different cattle. Metaphylaxis or antimicrobial treatment did not affect the diversity of isolates. Except for tilmicosin, isolates were largely susceptible to tested antimicrobials.
Résumé
Effet du traitement antimicrobien et des stratégies préventives sur le complexe respiratoire bovin ainsi que la relation génétique et l’antibiorésistance des isolats deMycoplasma bovisdans un parc d’engraissement de l’Ouest canadien. Les veaux d’un parc d’engraissement (n = 3784) ont été systématiquement randomisés et répartis dans une étude factorielle 2 × 2 pour recevoir de l’oxytétracycline métaphylactique (OTC) à l’arrivée ou pas d’antimicrobien, ainsi qu’une injection sous-cutanée ou deux injections intramusculaires (à intervalle de 48 h) de florfénicol s’ils étaient diagnostiqués avec le complexe respiratoire bovin (CRB). Les veaux de différents groupes de traitement ont été regroupés pêle-mêle et suivis du placement à la réimplantation (~100 jours). Les animaux recevant l’OTC avaient un risque réduit de CRB, un risque accru d’arthrite et ne présentaient pas de différences significatives pour le gain de poids quotidien moyen, la rechute du CRB, la mortalité globale ou la mortalité associée au CRB. Il n’y avait aucune différence significative entre les protocoles de traitement. Des écouvillonnages nasaux profonds (n = 233) prélevés à l’arrivée (n = 122), au traitement (n = 77) et des écouvillonnages des poumons et des articulations post mortem (n = 34) ont été cultivés pour Mycoplasma bovis à partir de 61 animaux malades ou mourants de pneumonie chronique et d’arthrite et de 61 veaux en santé. Il n’y avait aucune variation significative dans la diversité des isolats (n = 51) entre les années d’étude et les différents bovins. La métaphylaxie ou le traitement antimicrobien n’a pas affecté la diversité des isolats. Sauf pour la tilmicosine, les isolats étaient largement sensibles aux antimicrobiens testés.
(Traduit par Isabelle Vallières)
Introduction
Chronic pneumonia and polyarthritis syndrome (CPPS) is a common problem in feedlot cattle characterized by chronic and unresponsive pneumonia or lameness, often culminating in death or euthanasia (1–4). Since the early 1990’s in western Canada, high-risk stocker calves often receive metaphylactic long-acting antimicrobials on arrival at the feedlot for the control of bovine respiratory disease (BRD). Prior to long-acting antimicrobial formulations, calves with BRD were removed from their pen and held in a separate sick pen for the duration of their daily treatment regimen. Today, calves are treated with a long-acting antimicrobial and returned to their pens. Despite metaphylaxis and current treatment protocols, more calves appear to be developing CPPS. This study investigated if metaphylactic antimicrobials and the therapeutic antimicrobial regimen affected the outcome of BRD cases or the development of CPPS.
Parenteral oxytetracycline (OTC) is licensed in Canada for treatment of BRD and commonly used as well for metaphylaxis (5). Numerous trials have compared the efficacy of OTC and other antimicrobials but no recent trials have evaluated the effects of OTC against a negative control (6–10). Given the rise in chronic pneumonia, current information is needed that considers the potential positive and negative effects of metaphylactic antimicrobials on animal health (1). Florfenicol is a synthetic broad spectrum antimicrobial registered in Canada to treat BRD. Single-dose subcutaneous [40 mg/kg body weight (BW)] and two-dose intramuscular (20 mg/kg BW) injections have been effective treatments for BRD (6,11–15). Given that animals receiving a single dose return to their home pen immediately, 1- and 2-dose protocols could affect treatment success through pharmacokinetics, detection of relapses, or disease transmission.
Cases of CPPS are attributed to Mycoplasma bovis. Several Canadian reports have identified M. bovis in tissue from over two-thirds of feedlot animals dying of BRD and/or animals that had been chronically ill prior to death (16–18). Improved knowledge of M. bovis ecology is needed to understand the apparent increase in CPPS. Management, the emergence of virulent clones, presence of bovine viral diarrhea virus (BVDV) or antimicrobial resistant strains could all be factors. To date, no studies have described the genetic relatedness or antimicrobial susceptibility of M. bovis from western Canada.
This report describes 3 simultaneous studies on a single population. A clinical trial evaluated the individual and combined effects of OTC on arrival and 2 different management protocols for treatment of BRD on the incidence, relapse, and mortality rates from BRD in cattle during the first 100 days on feed (DOF). A case control study compared the isolation rates of M. bovis from nasal swabs collected at processing into the feedlot between calves that were subsequently treated or died versus those that remained healthy. Finally, a cross-sectional study of M. bovis isolates collected from calves at treatment or postmortem during the first 130 days on feed (DOF) were compared for genetic similarity and antimicrobial susceptibility. These questions were addressed concurrently to explore potential connections between antimicrobial use, CPPS, and M. bovis.
Materials and methods
Experimental exposures
A target of 3750 trial animals was selected to meet the primary objective of evaluating the effects of metaphylactic OTC exposure on BRD morbidity in the first 100 DOF. Using the primary hypothesis that OTC would reduce the BRD treatment rate from 25% to 20%, a sample size of 1250 non-exposed animals and 2500 exposed animals was targeted to provide a 90% power and a 95% confidence. This incident rate would have resulted in 812 animals being treated for BRD.
There were 4 exposure groups based on a 2 × 2 factorial design. Systematic randomization was used to assign 2 animals to receive metaphylaxis (META) for every 1 receiving no metaphylaxis (NO META) at processing.
Animals assigned to META received OTC, (Liquamycin LA-200; Pfizer Animal Health, Montreal, Quebec), 10 mg/kg BW, SQ in the neck, while those assigned to NO META provided a negative control. If diagnosed with BRD, calves with an even identification number were allocated to a HOME treatment and received a single 40 mg/kg BW, SQ dose of florfenicol (Nuflor®; Merck Animal Health, Kirkland, Quebec) in the neck and were immediately returned to their pen. Those with an odd identification number were allocated to the HOLD strategy and received two 20 mg/kg BW IM doses of florfenicol 48 h apart. Between treatments HOLD animals remained in the hospital pen and were only returned to their pen after a second exposure.
Study population
This study was conducted at a commercial feedlot in Saskatchewan in 2007 and 2008. The feedlot was open-air with side-by-side dirt-floor pens separated by a central feed alley. The one-time capacity was 15 000 and pens held approximately 250 animals. There was 1 hospital facility and 1 enclosed processing facility each with a hydraulic chute, an individual animal scale, a chute-side computer for recording animal health data, and separation alleys to facilitate the return of animals to designated pens. There were 3 recovery and chronic pens, 3 receiving pens, and several shipping pens.
Clients of the feedlot voluntarily participated by enrolling half of the cattle they placed in the feedlot between October 26 and November 19, 2007 and between October 22 and December 10, 2008. The enrolled animals did not differ in any systematic way from the other animals in the feedlot. The crossbred beef steer and heifer calves were purchased from auction markets throughout western Canada and transported by truck to the feedlot.
The feedlot’s standard protocol was applied to all animals at arrival. Each animal was identified with a unique ear-tag, implanted with a trenbolone acetate and estradiol benzoate growth implant (Component EH/T or Component ES/T; Elanco Animal Health, Guelph, Ontario), and treated for internal and external parasites with a topical ivermectin product (0.5%; Vetomectin; Ivermectin Pour-On; Durvet, Blue Springs, Missouri, USA), 1.0 mL/10 kg BW. Animals were immunized with a multivalent clostridial bacterin–toxoid (Covexin 8; Intervet Schering-Plough Animal Health, Montreal, Quebec), an infectious bovine rhinotracheitis virus, parainfluenza-3 virus, BVD virus, and bovine respiratory syncytial virus combination vaccine (Starvac 4; Novartis Animal Health, Mississauga, Ontario), and a M. haemolytica and Pasteurella multocida bacterin–toxoid (Somnustar PH; Novartis Animal Health). At an average of 100 DOF for each pen, cattle were re-implanted and vaccinated with the same multivalent viral vaccine as given initially (Starvac 4; Novartis Animal Health). The weight of each animal was recorded at arrival and re-implantation.
At initial processing, study animals had a deep nasal swab collected for M. bovis culture; an ear notch collected for bovine viral diarrhea virus (BVDV) immunohistochemistry, and received OTC if allocated to META. Once processing was complete, animals were immediately moved into designated feedlot pens and managed according to standard practice. The feedlot’s standard health-checking procedure was used to identify ill animals. Pens were checked once or twice daily by animal health personnel who were blinded to the experimental status of each animal. Animals with evidence of sickness based on subjective criteria (general appearance and attitude, gauntness, reluctance to move) were moved to 1 of the hospital facilities for examination. The case definition of BRD was an elevated rectal temperature (≥ 40.0°C) and an absence of abnormal clinical signs directly attributable to organ systems other than the respiratory system. Animals meeting the case definition for BRD had a deep nasopharyngeal swab collected at the time of diagnosis and received the allocated florfenicol treatment.
All BRD relapses were treated in the same way. Animals were diagnosed as a BRD relapse if ill with an absence of abnormal clinical signs directly attributable to organ systems other than the respiratory tract, and at least 96 h had elapsed since the most recent course of BRD therapy. Animals received a single dose of enrofloxacin (Baytril 100; Bayer Animal Health, Mississauga, Ontario), 10 mg/kg BW, SQ, in the neck on the first relapse and trimethoprim-sulfadoxine (Trivetrin; Merck Animal Health, Kirkland, Quebec), 16 mg/kg BW, IM, in the neck, q24h for 3 d on the second relapse. All BRD relapses were returned to their original pen at the completion of the treatment. Animals diagnosed with a third BRD relapse did not receive antimicrobials. They were housed in a chronic pen with animals unsuitable to be returned to their designated feedlot pens after treatment, based on subjective appraisal of attitude and appearance. All other diseases were treated according to a standard feedlot protocol provided by consulting veterinarians. Feedlot veterinarians completed a postmortem examination on all dead animals, assigned a cause of death, and collected swabs from the joints and lungs.
Laboratory methods
Ear notches for BVDV immunohistochemistry were frozen at −20°C until testing was performed at Prairie Diagnostic Services, Saskatoon, Saskatchewan. Ear notches were fixed in 10% buffered formalin solution for a minimum of 24 to 48 h, embedded in paraffin within 1 wk, serially sectioned, mounted on poly-L-lysine–coated slides, and stained for BVDV using monoclonal antibody 15C5. Diaminobenzidine served as the chromogen. Positive controls stained concurrently with each batch were tissues from cattle that had died from mucosal disease. Each section was examined for the presence or absence of BVDV staining.
A double-guarded uterine swab (item #636102, Reproduction Resources, Walworth, Wisconsin, USA) was passed through the nostril into the deep nasopharynx to collect a sterile swab for M. bovis culture and placed immediately in Ames transport medium and stored short-term at −20°C at the feedlot. The swabs were transported for 4 h in coolers with ice packs for long-term storage at −80°C at the University of Saskatchewan.
Upon completion of the clinical trial, nasal swabs were chosen retrospectively for culture and analysis of the isolates. All animals dying between 20 and 130 DOF and a random selection of calves treated for BRD and/or arthritis that survived the feeding period were selected for culture. For each dead and/or treated calf, the next animal processed at arrival that remained healthy during the first 130 DOF was also cultured to compare isolation rates for swabs collected at arrival. Calves that died or were treated had additional nasal swabs collected at treatment and/or joint and lung swabs collected at postmortem submitted for culture. A total of 120 nasal, lung, or joint swabs were cultured each year.
Swabs were submitted for isolation of M. bovis (Animal Health Laboratory, University of Guelph, Guelph, Ontario). Nasal swabs were streaked onto modified Hayflick’s medium containing 15% horse serum. Inoculated plates were incubated at 35+/−3°C with 5% CO2 for 3 to 10 d. Colonies were identified to the species level using an indirect fluorescent antibody test (19). Isolates were subjected to genomic analysis and antimicrobial susceptibility testing.
Mycoplasma were collected from 5-mL cultures by centrifugation at 13 000 × g for 10 min, and the genomic DNA was extracted from the cell pellet using the DNeasy tissue kit (QIAGEN, Mississauga, Ontario) following the manufacturer’s protocol for Gram-positive bacteria. The procedures used for Amplified Fragment Length Polymorphism (AFLP) analysis have been described (20).
Isolates were tested for antimicrobial susceptibility using custom Sensititre plates (Nova Century Scientific, Burlington, Ontario). Ten antimicrobials were tested over a range of dilutions (Table 1). Ampicillin and ceftiofur were not expected to show activity against Mycoplasma spp. and were included as negative controls. A standard dilution of culture was added to each well so that 2 × 103 to 2 × 105 color-changing units per well were delivered in a final 200-μL volume. Ten-fold dilutions of the challenge culture were also plated into wells without antimicrobial to confirm that the challenge was within the acceptable range of color-changing units per well. The sealed plates were incubated at 37° C in a 5% CO2 atmosphere for 48 h. The lowest concentration of antimicrobial suppressing growth, as expressed by blue to red shift, was recorded as the minimum inhibitory concentration (MIC) for that antimicrobial.
Table 1.
Minimum inhibitory concentrations (MICs) in μg/mL for 51 Mycoplasma bovis isolated from feedlot calves on arrival, at treatment for bovine respiratory disease, or death
| Antimicrobial | Dilution range | MIC range | MIC50 |
|---|---|---|---|
| Ampicillin | 0.25–32 | — | 32 |
| Ceftiofur | 0.125–64 | — | 64 |
| Chlortetracycline | 0.03–32 | 2–4 | 2 |
| Enrofloxacin | 0.03–4 | — | 0.12 |
| Erythromycin | 0.06–32 | — | 32 |
| Florfenicol | 0.06–32 | 1–8 | 2 |
| Oxytetracycline | 0.03–32 | 2–4 | 4 |
| Spectinomycin | 0.12–256 | 1–4 | 2 |
| Tilmicosin | 0.5–128 | — | 128 |
| Tulathromycin | 1–32 | 1–8 | 2 |
MIC50 — minimum concentration required to inhibit growth of 50% of the organisms. The MIC90 (minimum concentration required to inhibit growth of 90% of the organisms) was the same as the MIC50 for all antibiotics except tulathromycin for which it was 8 μg/mL.
Data management and statistical analysis
Data were maintained in a spreadsheet (Microsoft Excel) and descriptive statistics were performed using commercially available software (Microsoft Excel 2010; Microsoft Access 2010; Microsoft Corporation, Redmond, Washington, USA) and Stata 10.1 (StataCorp, College Station, Texas, USA).
Antimicrobial exposures
For each health event occurring between entry and re-implantation (~100 DOF) the date, presumptive diagnosis/cause of death, antimicrobial(s), dose(s) administered, and samples collected were recorded in the chute-side computer system. The incident rates for initial BRD, arthritis, mortality due to all causes, and mortality due to BRD were tabulated for all animals and stratified by META. The incidence rates for BRD relapse and BRD mortality were tabulated for all animals diagnosed with BRD and stratified by META and HOME.
Statistical analysis to compare average daily gain and the risk of each animal health outcome between treatment groups was evaluated using generalized linear mixed models to account for clustering within pen. The intra-class correlation coefficient was calculated for pen and reported for each of the health outcome models (21). Year was retained in all models as a fixed effect regardless of significance and was evaluated for its potential interaction with other significant terms. Data were analyzed at the individual animal level. Health outcomes were evaluated using a multilevel logistic regression model (xtmelogit command) with a random intercept for pen, while average daily gain was evaluated using a xtmixed command. The effects of metaphylaxis and treatment were considered as main effects and as an interaction term, except when comparing initial treatment rates, in which only metaphylaxis was considered as a main effect. The health outcomes of animals with and without a BVDV positive animal in the pen were compared at the individual animal level, with metaphylaxis and treatment considered as independent predictors, and potential confounders or interaction terms. Confounders were retained in the model if they altered the coefficient of another predictor by > 25%. For all analyses, associations were considered statistically significant at P < 0.05. The Hosmer-Lemeshow goodness-of-fit criterion was used to evaluate the basic logistic regression models, prior to calculation of the random-effect parameter estimates. Pen-level standardized residuals were tested for normality using a quantile plot and homoscedasticity was confirmed visually by graphing the standardized residuals against the predicted outcomes. Influential pens were also identified using Cook’s distance.
Mycoplasma bovis
Isolation success was compared between years, sick (dead or treated) and healthy, and the intervention point when swabs were collected using a multilevel logistic regression with a random intercept for pen. The files generated using the GeneMapper software were input into the BioNumerics v5.1 software (Applied Maths, Austin, Texas, USA), and the fragments were compared using the Band Matching function with optimal settings as verified by visual inspection. Uncertain bands were excluded in the analysis by using the software’s Uncertain Bands function. The similarities among the fingerprints of each sample were calculated using the Binary Dice coefficient. Dendrograms were constructed by using Unweighted Pair Group Method with Arithmetic Mean (UPGMA). The cluster grouping was based on a 95% similarity of the fingerprints. The AFLP fingerprints of representative groups were visually examined to ensure the software algorithm was performing correctly and matched with the visual pattern determination. The range and average dice coefficients of isolates from an animal, pen, year, treatment group and site of swab collection (nasal, lungs, joint) were summarized. A multiple membership model was used to determine what factors influenced the pair-wise Dice coefficients using Markov Chain Monte Carlo (MCMC) estimation in MLWin (Version 2.1, Centre for Multilevel Modelling, University of Bristol) to account for the relationship between isolates and pens of calves. With all self-comparison of isolates removed, the dataset consisted of 1275 pair-wise comparisons from 51 isolates. For each pair-wise comparison of isolates the following dummy variables were created: were the isolates collected from the same calf (yes or no), pen (yes or no), year (yes or no) or site (nasal, lungs, joint or different sites), and were both isolates collected from calves given metaphylaxis (yes or no) or antibiotic treatment (yes or no). Analysis was carried out using manual backwards elimination at a 95% level of significance. Initial estimates were generated by restricted maximum likelihood, followed by an initial burn-in of 1000 iterations and a run-length of 10 000 iterations. Diagnostics (MCMC) were completed for each parameter (assessing the trajectory of each parameter in trace plots, posterior distribution, autocorrelation, and accuracy diagnostics) (21).
Antimicrobial susceptibility data were summarized by the central tendency and distribution of the MIC values. In the absence of accepted resistance breakpoints for M. bovis, susceptibility was inferred based on the distribution of MIC values, the Clinical Laboratory Standards Institute interpretive criteria for other respiratory bacteria, and previous publications (22,23).
This study was conducted under the guidelines of the Canadian Council of Animal Care as approval by the University of Saskatchewan Council for Animal Care and Supply (protocol #20060097).
Results
Antimicrobial
Between October 26 and November 19, 2007 and October 22 and December 10, 2008, 3790 study calves arrived at the feedlot. Four calves were excluded from the study in 2007 due to treatment on arrival with tilmicosin (Micotil®; Elanco Animal Health Canada). The survival outcome of 2 calves was lost to follow-up; both animals entered the trial in 2007 with 1 assigned to META-HOLD and the other to META-HOME. These 6 animals were excluded from analysis. The 3784 study calves were allocated to the 4 treatments in equal proportions in 2007 and 2008 (Table 2). Calves were assigned to 7 pens in 2007 and 8 pens in 2008 with an average of 253 calves (range: 236 to 269) per pen and an approximately equal allocation of treatment and metaphylaxis groups among pens.
Table 2.
Treatment allocation for a clinical trial in a southern Saskatchewan feedlot in 2007 and 2008. Animals received oxytetracycline metaphylaxis on arrival at the feedlot (META) or no antimicrobials on arrival and, at first BRD treatment, either two 20 mg/kg body weight (BW) IM doses of florfenicol administered 48 h apart (HOLD) or a single 40 mg/kg BW SQ dose of florfenicol (HOME)
| META | NO META | ||||
|---|---|---|---|---|---|
|
|
|
||||
| HOLD | HOME | HOLD | HOME | Total | |
| Both years | 1256 | 1254 | 641 | 633 | 3784 |
| 2007 | 581 | 578 | 295 | 295 | 1749 |
| 2008 | 675 | 676 | 346 | 338 | 2035 |
Animals weighed an average of 248 kg [95% confidence interval (CI): 247.4 to 248.8] on arrival with no significant differences in weights between treatment groups (difference = 1.3 kg, 95% CI: −0.8 to 3.3; P = 0.4). Calves arriving in 2008 were an average of 2.3 kg lighter (95% CI: 0.9 to 3.8; P = 0.002) than those in 2007. The time from arrival to re-implantation averaged 133 DOF (95% CI: 132 to 134) in 2007 and 87 DOF (95% CI: 86 to 88) in 2008. Complete ancillary production data were not available for 59% of calves in 2007 so the analysis of average daily gain was limited to calves entering in 2008. Of these 2035 animals, 2002 (98.4%) survived and the data describing weight change and DOF were recorded for 1984 (99% of survivors). Animals had an average daily gain of 0.97 kg/day (95% CI: 0.961 to 0.985) and there was no significant difference between META groups (META: 0.97 kg/d, 95% CI: 0.88 to 1.03; NO-META: 0.98 kg/d, 95% CI: 0.91 to 1.05; P = 0.42). Average daily gain was 0.22 kg lower (95% CI: 0.17 to 0.27; P < 0.001) in animals treated for BRD but among those treated the treatment protocol was not a significant predictor or a confounder.
In total, 388 (10.5%) animals were treated 1 or more times during the study period. Of these, 268 (7.1%) were treated for BRD (Table 3). Animals allocated to META had reduced odds of being diagnosed with BRD, but had significantly increased odds of receiving their first treatment for arthritis (Table 4). Calves in 2007 had increased odds of being initially treated for arthritis than calves in 2008, but there was no interaction between the use of metaphylaxis and year; 14 calves were diagnosed with a BRD relapse. In the 268 calves at risk, relapse was not significantly associated with META or HOLD (Table 4).
Table 3.
Number (and proportion) of animals experiencing adverse health outcomes in a southern Saskatchewan feedlot during the fall of 2007 and 2008. Cattle were allocated to receive oxytetracycline metaphylaxis on arrival to the feedlot (META) or no antimicrobials on arrival and by first BRD treatment of two 20 mg/kg body weight (BW) IM doses of florfenicol administered 48 h apart (HOLD) or a single 40 mg/kg BW SQ dose of florfenicol on first diagnosis (HOME)
| META | NO META | ||||
|---|---|---|---|---|---|
|
|
|
||||
| HOLD | HOME | HOLD | HOME | Overall | |
| BRD treatment | 79 (6.3) | 81 (6.5) | 47 (7.3) | 61 (9.6) | 268 (7.1) |
| Arthritis treatment | 23 (1.8) | 17 (1.4) | 6 (0.9) | 4 (0.6) | 50 (1.3) |
| BRD relapsea | 4 (5.0) | 5 (6.2) | 3 (6.3) | 2 (3.4) | 14 (5.2) |
| Overall mortality | 16 (1.2) | 22 (1.8) | 14 (2.2) | 11 (1.7) | 63 (1.7) |
| BRD mortalityb | 11 (69) | 16 (73) | 10 (71) | 7 (64) | 44 (70) |
Proportion of animals treated for BRD requiring a second treatment.
Proportional mortality due to BRD.
Table 4.
Results of a mixed logistic regression analyses for adverse health outcomes in cattle at a southern Saskatchewan feedlot during the fall of 2007 and 2008. Cattle were allocated to receive oxytetracycline metaphylaxis on arrival to the feedlot (META) or no antimicrobials (NO-META) on arrival and by first BRD treatment of two 20 mg/kg body weight (BW) IM doses of florfenicol administered 48 hours apart (HOLD) or a single 40 mg/kg BW SQ dose of florfenicol on first diagnosis (HOME). The independent variables, odds ratio, 95% confidence interval (CI), P-value and intra-class correlation (ICC) are reported for each health outcome model
| Outcome | Independent variable | Odds ratio | 95% CI | P-value | ICCd |
|---|---|---|---|---|---|
| BRD treatment | META versus NO-META | 0.73 | 0.57–0.95 | 0.02 | 0.14 |
| Year (2007 versus 2008) | 0.91 | 0.49–1.7 | 0.76 | ||
| BVD PI (Yes versus No)c | 11 | 1.54–85 | 0.02 | ||
| BVD PI in pen (Yes versus No)c | 1.6 | 0.81–3.1 | 0.18 | ||
| Arthritis treatment | META versus NO-META | 2.1 | 1.02–4.1 | 0.04 | < 0.01 |
| Year (2007 versus 2008) | 1.9 | 1.1–3.4 | 0.03 | ||
| BVD PI (Yes versus No)c | — | — | — | ||
| BVD PI in pen (Yes versus No)c | 0.82 | 0.40–1.7 | 0.60 | ||
| BRD relapsea | META versus NO-META | 0.91 | 0.31–2.7 | 0.91 | 0.19 |
| HOME versus HOLD | 1.0 | 0.35–2.9 | 0.84 | ||
| Year (2007 versus 2008) | 0.89 | 0.23–3.5 | 0.97 | ||
| BVD PI (Yes versus No)c | 4.6 | 2.1–7.1 | < 0.01 | ||
| BVD PI in pen (Yes versus No)c | 2.6 | 0.59–11 | 0.20 | ||
| Overall Mortality | META versus NO-META | 0.77 | 0.46–1.3 | 0.31 | 0.13 |
| HOME versus HOLD | 1.1 | 0.67–1.8 | 0.69 | ||
| Year (2007 versus 2008) | 0.77 | 0.38–1.6 | 0.48 | ||
| BVD PI (Yes versus No)c | 4.1 | 2.1–6.2 | < 0.01 | ||
| BVD PI in pen (Yes versus No)c | 1.6 | 0.75–3.6 | 0.22 | ||
| BRD Mortalityb | META versus NO-META | 0.80 | 0.44–1.5 | 0.75 | 0.11 |
| HOME versus HOLD | 1.1 | 0.61–2.0 | 0.5 | ||
| Year (2007 versus 2008) | 0.72 | 0.35–1.5 | 0.37 | ||
| BVD PI (Yes versus No)c | 3.4 | 1.1–5.8 | 0.01 | ||
| BVD PI in pen (Yes versus No)c | 1.2 | 0.50–2.7 | 0.73 |
— model not able to converge and estimates available.
Animals treated for BRD requiring a second treatment.
Mortality due to BRD.
Variables (BVD PI and BVD PI in pen) were evaluated in 2 separate multivariable models where the beta-coefficients for the other variables remained constant.
An estimate of the correlation between calves in the same pen.
Overall mortality was 1.6% with 1.2% attributed to BRD (Table 3). The median DOF at death was 23 d (IQR 14 to 41). Among the 44 deaths from BRD, 13 (30%) had been treated for this condition prior to death.
Bovine viral diarrhea virus
Four calves were persistently infected (PI) with BVDV and found in separate pens (1 in 2007 and 3 in 2008). Two died during the study. The PI calves had an 11 times greater risk of being diagnosed with BRD (Table 4). No PI calves were treated for arthritis at their first treatment.
Mycoplasma bovis
From the 3684 study animals, there were 353 calves that were treated (for BRD or arthritis) or died before 130 DOF that were considered for M. bovis culture of their nasal, lung, or joint swabs. All 34 calves that died between 20 and 130 DOF were selected for comparison resulting in 104 swabs to be cultured. Twenty-seven calves were randomly selected from those treated for BRD or arthritis that survived until re-implantation giving an additional 68 samples for culture. In total 61 healthy and sick (dead or treated) calves were selected to achieve a distribution across years, pens, health outcomes, OTC exposure, and based on available swabs. The mean DOF for swabs collected at BRD or arthritis treatment and postmortem, were 17 (95% CI: 8 to 25), 47 (95% CI: 39 to 55), and 45 (95% CI: 41 to 49), respectively.
Culture was attempted on 233 swabs. In addition to the swab collected at arrival, an average of 1.8 (range: 1 to 5) additional swabs collected at treatment and death were submitted for culture. Mycoplasma bovis was isolated from 52 (22%) swabs, 2 (3.3%) of which were collected at arrival, 17 (23%) taken at treatment, and 33 (32%) taken at postmortem. The odds of isolating M. bovis were significantly higher in swabs taken at treatment and death compared with arrival (Table 5). The only association between DOF and isolation of M. bovis was for BRD treatment nasal swabs, where positive swabs were collected at a mean of 25 DOF (95% CI: 16 to 34) and negative swabs at a mean of 13 DOF (95% CI: 9 to 23; P = 0.031). Fifty-one isolates were tested by AFLP and 51 for antimicrobial susceptibility, but only 50 of the 52 total isolates underwent both analyses. One isolate did not re-grow for antimicrobial susceptibility testing and another was unable to be typed by AFLP.
Table 5.
Results of a logistic regression analysis to determine factors associated with the isolation of Mycoplasma bovis from nasal, joint, and lungs swabs collected at arrival, treatment, and postmortem of cattle at a southern Saskatchewan feedlot during the fall of 2007 and 2008. Cattle were allocated to receive oxytetracycline metaphylaxis on arrival to the feedlot (META) or no antimicrobials (NO-META) on arrival and by first BRD treatment of two 20 mg/kg body weight (BW) IM doses of florfenicol administered 48 h apart (HOLD) or a single 40 mg/kg BW SQ dose of florfenicol on first diagnosis (HOME). The independent variables, odds ratio, 95% confidence interval (CI), and P-values are reported
| Outcome | Independent variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|
| Isolation of Mycoplasma bovis | Treatment versus Arrival | 60 | 13–280 | < 0.01 |
| Death versus Arrival | 40 | 9–172 | < 0.01 | |
| 2007 versus 2008 | 1.2 | 0.82–1.6 | 0.38 | |
| META versus NO-META | 0.91 | 0.33–4.5 | 0.81 | |
| Treated for BRD (Yes or No) | 2.2 | 0.83–5.3 | 0.42 | |
| Treated for Arthritis (Yes or No) | 4.7 | 0.21–16 | 0.59 |
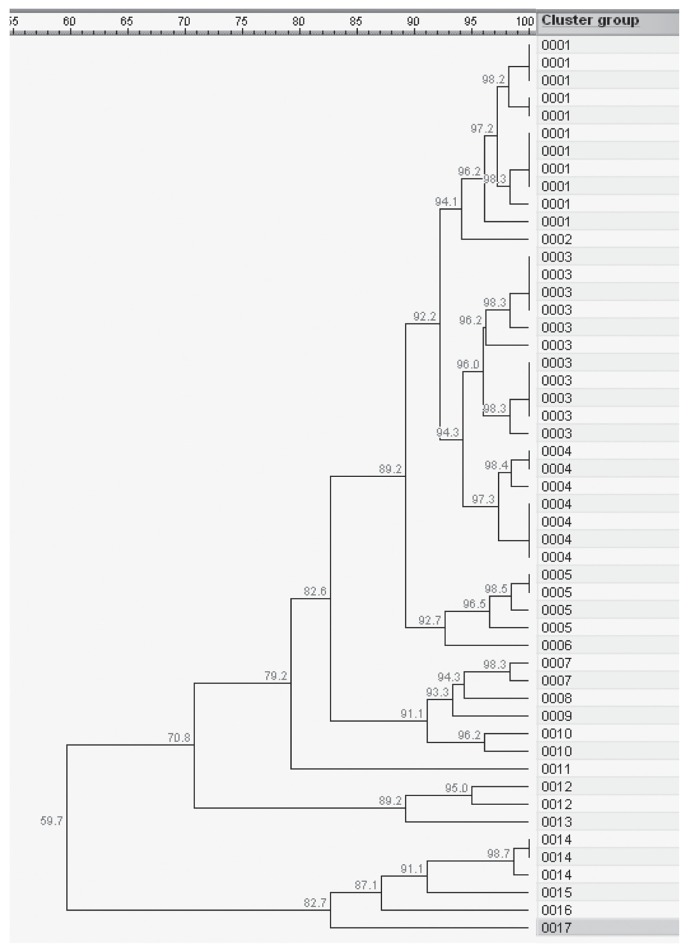
The 51 isolates analyzed with AFLP clustered into 17 profiles. Most profiles (13/17) had ≤ 3 isolates (Figure 1). The two largest profiles each contained 11 isolates and both clusters had 10 isolates originating from 1 study year and a single isolate from the other year. All of the other profiles contained only isolates from a single study year. All profiles with > 2 isolates had representation from ≥ 2 pens with the dominant profiles containing isolates from 4 pens each (Table 6). The 52 isolates came from 36 animals housed in 10 pens (Table 6). Ten calves had multiple M. bovis isolated and, within an animal, isolates had as little as 60% similarity (Table 6). Pairwise comparisons between all isolates found as little as 47% similarity and a mean similarity of 82%. In Table 7, the same 51 isolates were stratified by treatment group and site of swab collection (nasal, lung, or joint) and the mean similarity and range reported.
Figure 1.
Genetic relationships among 51 Mycoplasma bovis isolates collected in 2007 and 2008 from cattle in a Saskatchewan feedlot based on AFLP profiles (60 bp to 600 bp fragment size range) produced by amplification of BglII and MfeI DNA templates with non-selective primers. The dendogram was produced with the UPGMA method with Dice similarity coefficient (SD). Cluster represents the strains with 95% similarity.
Table 6.
A summary of the average and range of Dice similarity coefficients between 51 isolates of Mycoplasma bovis collected from nasal, joint, and lung swabs at arrival, treatment, and postmortem of cattle at a southern Saskatchewan feedlot during the fall of 2007 and 2008. The pairwise comparisons are summarized by calf, pen, and study year. Cattle were allocated to receive oxytetracycline metaphylaxis on arrival to the feedlot (META) or no antimicrobials (NO-META) on arrival and by first BRD treatment of two 20 mg/kg body weight (BW) IM doses of florfenicol administered 48 h apart (HOLD) or a single 40 mg/kg BW SQ dose of florfenicol on first diagnosis (HOME)
| Isolates | Average (range) of pairwise comparisons between isolates | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Year | Pen | Animal | Treatment group | Sites and DOFa | Calf | Pen | Year |
| 2007 | 1 | 21 | META | Arth-76 | 78 (62–95) | 83 (48–100) | |
| 148 | NO-META: HOME | BRD-19, PM-lung-31 | 95 | ||||
| 227 | META: HOLD | Arth-56 | |||||
| 2 | 98 | META: HOME | PM-lung-57 | 100 | |||
| 3 | 114 | META: HOME | BRD-79, PM-lung-91 | 95 | 82 (70–95) | ||
| 130 | META: HOME | PM-joint-24, PM-lung-24 | 76 | ||||
| 4 | 91 | NO-META: HOLD | BRD-35, BRD-37 | 93 | 93 (93–93) | ||
| 150 | META: HOME | PM-lung-23 | |||||
| 6 | 211 | NO-META: HOLD | BRD-18, PM-lung-37 | 98 | 98 | ||
| 7 | 5 | META | Arth-34 | 87 (56–100) | |||
| 22 | META: HOME | BRD-22 | |||||
| 23 | Control-arrival | ||||||
| 77 | META | Arth-24 | |||||
| 110 | META: HOME | PM-lung-22 | |||||
| 144 | Control-arrival | ||||||
| 159 | META: HOLD | BRD-4, BRD-6, PM-lung-57, PM-joint-57 | 80 (60–100) | ||||
| 257 | NO-META: HOLD | BRD-1, BRD-3, BRD-13, PM-lung-21 | 95 (92–98) | ||||
| 2008 | 11 | 21 | META: HOLD | BRD-27 | 86 (49–100) | 84 (49–100) | |
| 37 | META | PM-lung-71 | |||||
| 106 | META: HOME | PM-lung-31 | |||||
| 175 | NO-META: HOLD | BRD-54 | |||||
| 193 | NO-META: HOLD | BRD-23 | |||||
| 243 | NO-META: HOLD | Arth-23, BRD-49, PM-lung-64 | 90 (86–100) | ||||
| 246 | NO-META: HOME | PM-lung-64 | |||||
| 13 | 173 | META | Arth-41 | 100 | |||
| 218 | META: HOME | Arth-34 | |||||
| 14 | 135 | META | Arth-58 | 77 (54–97) | |||
| 200 | META: HOME | Arth-31, Arth-43 | 97 | ||||
| 220 | META: HOME | BRD-36 | |||||
| 15 | 55 | NO-META | PM-lung-98 | 96 (90–100) | |||
| 111 | NO-META | PM-lung-35 | |||||
| 160 | NO-META | Arth-16 | |||||
| 179 | NO-META: HOLD | BRD-13, BRD-21 | 97 | ||||
| 186 | NO-META: HOME | PM-lung-29 | |||||
| 206 | META: HOME | BRD-19 | |||||
| 231 | META: HOLD | BRD-15 | |||||
Sites: deep nasal swab taken at treatment of BRD or arthritis; swab collected from lung or joint at postmortem; followed by days on feed (DOF) of sample collection.
Table 7.
Average and range of Dice similarity coefficients between 51 Mycoplasma bovis isolates collected from calves in a Saskatchewan feedlot by treatment, study year, and site of collection. Cattle were allocated to receive oxytetracycline metaphylaxis on arrival at the feedlot (META) or no antimicrobials (NO-META) on arrival and by first BRD treatment of two 20 mg/kg body weight (BW) IM doses of florfenicol administered 48 h apart (HOLD) or a single 40 mg/kg BW SQ dose of florfenicol on first diagnosis (HOME)
| Year | Site | N | Average similarity | Min | Max |
|---|---|---|---|---|---|
| 2007 | Joint | 2 | 76 | ||
| 2007 | Lung | 9 | 65 | 48 | 98 |
| 2007 | Nasal | 17 | 89 | 59 | 100 |
| 2008 | Lung | 7 | 94 | 92 | 96 |
| 2008 | Nasal | 16 | 81 | 49 | 100 |
| Treatment | |||||
| 2007 | META-HOLD | 9 | 84 | 59 | 100 |
| META-HOME | 9 | 81 | 67 | 95 | |
| NO-META-HOLD | 8 | 92 | 83 | 100 | |
| NO-META-HOME | 2 | 96 | |||
| 2008 | META-HOLD | 5 | 83 | 61 | 97 |
| META-HOME | 6 | 77 | 54 | 98 | |
| NO-META-HOLD | 9 | 85 | 49 | 100 | |
| NO-META-HOME | 3 | 96 | 93 | 97 | |
| Both years | META-HOLD | 14 | 81 | 56 | 100 |
| META-HOME | 15 | 75 | 47 | 98 | |
| NO-META-HOLD | 17 | 86 | 49 | 100 | |
| NO-META-HOME | 5 | 92 | 85 | 97 |
N — total number; Min — minimum; Max — maximum.
The pair-wise dice similarity coefficient was significantly associated with the year the isolates were collected and whether the isolates were collected from the same or different calf (Table 8). Isolates from the same year or calf were more similar (higher dice coefficient) than isolates from different years or calves, respectively. Isolates from calves receiving metaphylaxis or antimicrobial treatment had equivalent dice coefficients. Isolates from the same pen or site (nasal, lungs or joint) were not significantly different.
Table 8.
Results of a multiple membership model to evaluate factors (isolate source and antimicrobial exposure) significantly associated (P < 0.05) with the pair-wise Dice similarity coefficient using Markov Chain Monte Carlo estimation to account for the relationship between isolate pairs and pens of calves. The model includes 1275 pair-wise comparisons from 51 Mycoplasma bovis isolates from nasal, joint, and lung swabs from cattle at a southern Saskatchewan feedlot during the fall of 2007 and 2008. All self-comparisons were removed. The beta-coefficient, 95% confidence interval, P-value, and intra-class correlation coefficient (ICC) are reported
| Factor | Coefficient | 95% Confidence interval | P-value | ICC | |
|---|---|---|---|---|---|
| Intercept | 89.4 | 82.4–96.4 | < 0.01 | 0.35 | |
| Calf | Different | −2.4 | −4.4 to −0.3 | 0.013 | |
| Same | ref | — | — | ||
| Year | Different | −3.5 | −4.1 to −3.0 | < 0.01 | |
| Same | ref | — | — |
As expected, all 51 isolates were resistant to ampicillin (MIC ≥ 32) and ceftiofur (MIC ≥ 64). No variation was observed in susceptibility to enrofloxacin, erythromycin, or tilmicosin. The MICs were within a single dilution for all remaining antimicrobials except florfenicol and tulathromycin (Table 1). In the case of florfenicol, a single isolate from postmortem tissues had an MIC of 8 while the remaining 50 isolates had MICs of 1 or 2. Hence, tulathromycin was the only antimicrobial tested for which the MIC values had notable variation.
Discussion
Two notable findings arose from this study of CPPS and antimicrobial use in feedlot animals. First, OTC on arrival protected against BRD morbidity but possibly raised the risk of arthritis morbidity while having no effect on overall mortality or BRD mortality. Secondly, the genetic diversity of M. bovis isolates from these feedlot animals was not affected by antimicrobial exposure (metaphylaxis and treatment), but isolates remained largely susceptible to tested antimicrobials except tilmicosin. This study was conducted with commercial animals sourced through auction markets, comingled in a southern Saskatchewan feedlot, reflective of all animals in this feedlot. Finding that prophylactic OTC had no significant effect on overall mortality or BRD mortality contradicts the most recent study that compared oxytetracycline to a negative control (10). This contradiction is not likely explained by the administration route or OTC formulation, as these perform equivalently (8,10). Rather, the difference is most likely attributable to the disease challenge. In the previous trial the BRD morbidity rate was 52% with an overall mortality rate of 10%, while the current trial reported 7% and 2%, respectively. This discrepancy illustrates the risk in extrapolating results to situations with different disease challenges and emphasizes the value in examining therapeutic dogmas when disease ecology changes.
This trial was designed on the premise that BRD incidence would be approximately 25%, providing 812 animals to study the effects of treatment regimen on BRD relapse rate and mortality. Instead, only 269 animals were diagnosed with BRD which limited the likelihood of detecting significant differences if they existed. Seventy percent of the calves dying from BRD were untreated and suggests that treatment rates were lower than they should have been, further reducing the power of the study. This was a newer feedlot with a less experienced crew at the time of the study.
Exposure to animals persistently infected with BVDV is a well-documented risk for BRD, yet in this trial pen mates of PI animals did not have elevated health problems when evaluated at the individual animal level (16,17,24,25). Deleterious effects on performance and health have been shown in cattle penned adjacent to PI animals and in pens from which a PI animal was removed (26). As it was not possible to test all calves for BVDV that were housed adjacent to our study pens, it is possible that the true effect of BVDV in the study animals was not accounted for.
The most obvious link between these 2 study questions was finding that calves receiving metaphylaxis had an increased risk of being treated for arthritis. This finding may simply be due to chance, given that swabs from animals that received metaphylaxis were not more likely to be positive for M. bovis. Still, the strength of the relationship indicates that the potential for prior antimicrobial exposure to contribute to CPPS should continue to be investigated (1,3).
Few weaned calves arrived at the feedlot harboring M. bovis. However, isolation of M. bovis from the nasal swabs collected at arrival was not attempted until 4 to 6 months post-collection which likely reduced our success. In retrospect, polymerase chain reaction (PCR) may have been more sensitive than culture, although isolates were required for antimicrobial sensitivity tests. Following placement, M. bovis appears to have spread among the study animals. Isolates were predominantly cultured from animals treated for, or dying of, chronic pneumonia and poly-arthritis as these were the animals evaluated. Unfortunately, the healthy calves could only be sampled upon arrival at the feedlot as they were selected retrospectively. A case-control study cannot describe prevalence in a population, but similar infection dynamics were noted in 2 prospective studies conducted in commercial feedlots in North America (27,28). Since 3 trials in separate locations across North America have reported similar findings, we suggest that a next step could be to determine if M. bovis clusters within beef-cow herds. This would assist in identifying potential intervention points to control the spread of M. bovis.
Little is known about the genetic similarity of M. bovis isolates in western Canadian feeder cattle. The degree of genetic heterogeneity was comparable to the 13 profiles and 73% similarity described in 62 isolates from a single Ontario feedlot sourcing cattle from 4 cow-calf farms in 1 year (28). In the United Kingdom, 54 isolates from 1996 to 2002 resulted in 35 profiles with 79% similarity (29). A Danish study of 42 field strains from multiple disease processes collected from 1981 to 1998 distributed into 18 profiles with > 90% similarity (30). Both European studies found isolates from outbreaks were identical (29,30). This is in agreement with the current study’s observation that isolates were more similar within an animal or year. Further investigation is needed to better understand the impact of sourcing, management, and treatment of Canadian feeder cattle on the genetic similarity of BRD pathogens.
The antimicrobial susceptibility of the isolates could not be definitively stated because approved resistance thresholds are not available. However, the MIC distributions and CLSI breakpoints for other bovine respiratory pathogens have been previously used to guide interpretation (22,23). Given the observed MIC values, tilmicosin should not be used to treat M. bovis without prior susceptibility testing. In contrast, oxytetracycline, florfenicol, tulathromycin, and danofloxacin had high in vitro activity with minimal variation in the MIC values, providing no evidence of acquired resistance to these antimicrobials. Few other descriptions of M. bovis antimicrobial susceptibility exist. Isolates from Europe have shown regional variation in antimicrobial susceptibility while those from the United States have not (23,31). These susceptibility results were similar to the previous American description of 223 isolates from 4 tissues and 5 geographical regions between 2002 and 2003 (23).
The small number of isolates cultured prevented a statistical assessment of the effects of metaphylactic and therapeutic antimicrobials on the MICs. It was notable that isolates were uniformly susceptible to oxytetracycline, florfenicol, and enrofloxacin which were all administered to cattle in this population. We measured genetic heterogeneity using AFLP and did not evaluate any of the genetic elements known to confer antibiotic resistance in other bovine respiratory pathogens. Mycoplasma bovis is highly pleomorphic (3) and antimicrobial exposure may result in genetic heterogeneity, but may not select for genetic resistance to antimicrobials. Minimum inhibitory concentrations may not reflect how the antimicrobials function in vivo. Treatment failure should not be attributed to acquired resistance without laboratory confirmation.
Four design features of this study could be criticized; all 4 were due to the logistics of following many animals in a commercial setting. Animals were mixed rather than penned by their META allocation. This could have biased results towards a null finding because antimicrobial use could influence disease transmission. The use of antimicrobials at arrival to any extent will have some impact on the disease dynamics within the pen. Ultimately, treatment should be assigned by pen or even feedlot to avoid issues associated with “herd immunity” when assessing the effect of a prophylactic treatment in a mixed pen of animals. Second, the animals were followed from placement until re-implantation. This period varied between pens and years so animals were not at risk for equal amounts of time. Thirdly, despite the lower sensitivity, nasal swabs were used instead of bronchoalveolar lavage because feedlot personnel collected all samples during the labor intense fall season (32). Finally, the study was completed over 2 years and the conclusions were slightly different between years. However, this may not be a weakness given that results in a commercial feedlot may vary each year because of differences in management and environment.
This trial is the first to evaluate the effects of metaphylactic oxytetracycline compared to a negative control in over 20 years. Veterinarians should review treatment protocols for CPPS given that all tested isolates were resistant to tilmicosin. This study was unable to conclusively state if metaphylactic OTC use had any effect on subsequent M. bovis infections. However, finding a significant positive association between metaphylaxis and treatment for arthritis indicates that further study of this potential relationship is justified.
Acknowledgments
We acknowledge the contribution made to this study by the staff at Border Line Feeders Inc., Ceylon, Saskatchewan and by Dr. Cheryl Waldner for her assistance with the multiple membership model. CVJ
Footnotes
Funds were provided by the Beef Cattle Research Council, Ontario Cattlemens’ Association, Saskatchewan Agriculture Development Fund, and Merck Animal Health.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Caswell JL, Bateman KG, Cai HY, Castillo-Alcala F. Mycoplasma bovis in respiratory disease of feedlot cattle. Vet Clin North Am Food Anim Pract. 2010;26:365–379. doi: 10.1016/j.cvfa.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Griffin D, Chengappa MM, Kuszak J, McVey DS. Bacterial pathogens of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2010;26:381–394. doi: 10.1016/j.cvfa.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas RA. Bovine Mycoplasmosis: Silent and deadly. Vet Rec. 2011;168:459–462. doi: 10.1136/vr.d2468. [DOI] [PubMed] [Google Scholar]
- 4.Maunsell FP, Donovan GA. Mycoplasma bovis infections in young calves. Vet Clin North Am Food Anim Pract. 2009;25:139–77. doi: 10.1016/j.cvfa.2008.10.011. , vii. [DOI] [PubMed] [Google Scholar]
- 5.Compendium of Veterinary Products. [Last accessed August 20, 2013]. Available from: http://cdmv.naccvp.com/product/view/1234274.
- 6.Booker C, Abutarbush S, Schunicht O, et al. Evaluation of the efficacy of tulathromycin as a metaphylactic antimicrobial in feedlot calves. Vet Ther. 2007;8:183–200. [PubMed] [Google Scholar]
- 7.Stanton AL, Kelton DF, Leblanc SJ, et al. The effect of treatment with long-acting antibiotic at postweaning movement on respiratory disease and on growth in commercial dairy calves. J Dairy Sci. 2010;93:574–581. doi: 10.3168/jds.2009-2414. [DOI] [PubMed] [Google Scholar]
- 8.Guichon PT, Booker CW, Jim GK. Comparison of two formulations of oxytetracycline given prophylactically to reduce the incidence of bovine respiratory disease in feedlot calves. Can Vet J. 1993;34:736–741. [PMC free article] [PubMed] [Google Scholar]
- 9.Schunich OC, Guichon PT, Booker CW, et al. A comparison of prophylactic efficacy of tilmicosin and a new formulation of oxytetracycline in feedlot calves. Can Vet J. 2002;43:355–362. [PMC free article] [PubMed] [Google Scholar]
- 10.Harland RJ, Jim GK, Guichon PT, Townsend HG, Janzen ED. Efficacy of parenteral antibiotics for disease prophylaxis in feedlot calves. Can Vet J. 1991;32:163–168. [PMC free article] [PubMed] [Google Scholar]
- 11.Booker CW, Jim KG, Guichon T, Schunicht OC, Thorlakson BD, Lockwood PW. Evaluation of florfenicol for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1997;38:555–560. [PMC free article] [PubMed] [Google Scholar]
- 12.Van Donkersgoed J, Merrill J, Hendrick S. Comparative efficacy of florfenicol versus tulathromycin for the treatment of undifferentiated fever in Alberta feedlot calves. Vet Ther. 2008;9:275–281. [PubMed] [Google Scholar]
- 13.Perrett T, Abutarbush S, Widman B, et al. A comparison of florfenicol and tulathromycin for the treatment of undifferentiated fever in feedlot calves. Vet Ther. 2008;9:128–140. [PubMed] [Google Scholar]
- 14.Jim GK, Booker CW, Guichon PT, et al. A comparison of florfenicol and tilmicosin for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1999;40:179–184. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoar BR, Jelinski MD, Ribble CS, Janzen ED, Johnson JC. A comparison of the clinical field efficacy and safety of florfenicol and tilmicosin for the treatment of undifferentiated bovine respiratory disease of cattle in western Canada. Can Vet J. 1998;39:161–166. [PMC free article] [PubMed] [Google Scholar]
- 16.Haines DM, Martin KM, Clark EG, Jim GK, Janzen ED. The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. Can Vet J. 2001;42:857–860. [PMC free article] [PubMed] [Google Scholar]
- 17.Booker CW, Abutarbush SM, Morley PS, et al. Microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada. Can Vet J. 2008;49:473–481. [PMC free article] [PubMed] [Google Scholar]
- 18.Gagea MI, Bateman KG, van Dreumel T, et al. Diseases and pathogens associated with mortality in Ontario beef feedlots. J Vet Diagn Invest. 2006;18:18–28. doi: 10.1177/104063870601800104. [DOI] [PubMed] [Google Scholar]
- 19.Bradbury JM, Oriel CA, Jordan FT. Simple method for immunofluorescent identification of Mycoplasma colonies. J Clin Microbiol. 1976;3:449–452. doi: 10.1128/jcm.3.4.449-452.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokotovic B, Friis NF, Jensen JS, Ahrens P. Amplified-fragment length polymorphism fingerprinting of Mycoplasma species. J Clin Microbiol. 1999;37:3300–3307. doi: 10.1128/jcm.37.10.3300-3307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island: AVC Inc; 2003. pp. 502–504. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute (CLSI) CLSI Document M31-A3. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard — third edition. [Google Scholar]
- 23.Rosenbusch RF, Kinyon JM, Apley M, Funk ND, Smith S, Hoffman LJ. In vitro antimicrobial inhibition profiles of Mycoplasma bovis isolates recovered from various regions of the United States from 2002 to 2003. J Vet Diagn Invest. 2005;17:436–441. doi: 10.1177/104063870501700505. [DOI] [PubMed] [Google Scholar]
- 24.Shahriar FM, Clark EG, Janzen E, West K, Wobeser G. Coinfection with bovine viral diarrhea virus and Mycoplasma bovis in feedlot cattle with chronic pneumonia. Can Vet J. 2002;43:863–868. [PMC free article] [PubMed] [Google Scholar]
- 25.Gagea MI, Bateman KG, Shanahan RA, et al. Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest. 2006;18:29–40. doi: 10.1177/104063870601800105. [DOI] [PubMed] [Google Scholar]
- 26.Hessman BE, Fulton FR, Sjeklocha DB, Murphy TA, Ridpath JF, Payton ME. Evaluation of economic effects and the health and performance of the general cattle population after exposure to cattle persistently infected with bovine viral diarrhea virus in a starter feedlot. Am Vet J Res. 2009;70:73–85. doi: 10.2460/ajvr.70.1.73. [DOI] [PubMed] [Google Scholar]
- 27.White BJ, Hanzlicek G, Sanderson MW, Anderson DE, Larson RL. Mollicutes species and Mycoplasma bovis prevalence and association with health outcomes in beef feeder calves at arrival and initial treatment for bovine respiratory disease. Can Vet J. 2010;51:1016–1018. [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo Alcala F. Molecular Characterization of Respiratory Infection with Mycoplasma bovis in Feedlot Cattle. Guelph, ON: University of Guelph; 2009. [Google Scholar]
- 29.McAuliffe L, Kokotovic B, Ayling RD, Nicholas RA. Molecular epidemiological analysis of Mycoplasma bovis isolates from the United Kingdom shows two genetically distinct clusters. J Clin Microbiol. 2004;42:4556–4565. doi: 10.1128/JCM.42.10.4556-4565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusiluka LJ, Kokotovic B, Ojeniyi B, Friis NF, Ahrens P. Genetic variations among Mycoplasma bovis strains isolated from Danish cattle. FEMS Microbiol Lett. 2000;192:113–118. doi: 10.1111/j.1574-6968.2000.tb09368.x. [DOI] [PubMed] [Google Scholar]
- 31.Ayling RD, Baker SE, Peek ML, Simon AJ, Nicholas RA. Comparison of in vitro activity of danofloxacin, florfenicol, oxytetracycline, spectinomycin and tilmicosin against recent field isolates of Mycoplasma bovis. Vet Rec. 2000;146:745–747. doi: 10.1136/vr.146.26.745. [DOI] [PubMed] [Google Scholar]
- 32.Thomas A, Dizier I, Trolin A, Mainil J, Linden A. Comparison of sampling procedures for isolating pulmonary Mycoplasmas in cattle. Vet Res Commun. 2002;26:333–339. doi: 10.1023/a:1016291208229. [DOI] [PubMed] [Google Scholar]