Significance
Schwann cells (SCs) are the glial cells of the peripheral nervous system essential for nerve ensheathment and myelination. Deregulation in SC development is often associated with diseases in humans. Around birth, immature SCs segregate axons, a process called “axonal radial sorting.” Here we unravel the mechanism of Wnt/β-catenin signaling in axonal sorting of SCs in mice. Loss- and gain-of-function mutations of β-catenin in SCs impede and accelerate radial sorting of axons, respectively. Moreover, down- or up-regulated Wnt/β-catenin signaling inhibits or promotes cell spreading and lamellipodia formation of cultured SCs. Our research contributes to a better understanding of the mechanisms of SC development and disease.
Keywords: Axin2-LacZ reporter mice, paracrine mechanism, lamellipodia, myelination, Lgr5 receptor
Abstract
During late Schwann cell development, immature Schwann cells segregate large axons from bundles, a process called “axonal radial sorting.” Here we demonstrate that canonical Wnt signals play a critical role in radial sorting and assign a role to Wnt and Rspondin ligands in this process. Mice carrying β-catenin loss-of-function mutations show a delay in axonal sorting; conversely, gain-of-function mutations result in accelerated sorting. Sorting deficits are accompanied by abnormal process extension, differentiation, and aberrant cell cycle exit of the Schwann cells. Using primary cultured Schwann cells, we analyze the upstream effectors, Wnt and Rspondin ligands that initiate signaling, and downstream genetic programs that mediate the Wnt response. Our analysis contributes to a better understanding of the mechanisms of Schwann cell development and fate decisions.
Development of the neural crest-derived Schwann cells (SCs) proceeds through several embryonic and postnatal stages (1). Around birth, SCs perform radial sorting of axons. During this process, immature SCs that envelop axonal bundles project cytoplasmic processes into the bundles to segregate individual large-diameter axons and to establish a one-to-one relationship with the axons. Subsequently, large sorted axons become myelinated, whereas multiple small axons remain unsorted and surrounded by nonmyelinating SCs (Remak bundles). The precise timing of radial sorting is instrumental for correct nerve development and homeostasis and is achieved through signaling cues provided by axons and by the extracellular environment (1–3). Axonal Neuregulin-1 type III acts through the ErbB/Shp2 signaling system to control SC proliferation, migration along nerves, and terminal differentiation (4–6). Axonal Notch signals promote the formation of immature SCs from precursors (7). Progression of the SC lineage beyond the immature SC stage and radial sorting are controlled by signals from both axons and the extracellular matrix, e.g., laminin/integrin β1 and the small GTPase Rac1, and by transcription regulators, e.g., Sox10 and Sox2 (8–11).
Canonical Wnt signaling is evolutionarily conserved and takes over critical roles in development, organ maintenance, and regeneration (12, 13). The role of Wnt/β-catenin signals in specification of the early neural crest and in formation of the sensory lineage of the peripheral nervous system has been defined (13–17). Work performed in zebrafish embryos and in SC lines implicated Wnt/β-catenin signals in SC proliferation and apoptosis and as positive regulators of myelination (18–21). In contrast, conditional mouse mutants of β-catenin using Dhh-cre suggested that Wnt signaling does not affect SC functions in adult mice (22, 23). To date, it is unclear which role Wnt/β-catenin signals play in late SC development. Recently, Rspondin (Rspo) growth factors were identified as previously undescribed coactivators of Wnt signaling that bind to Lgr4, 5, and 6. Lgr4–6 receptors associate with the Lrp/Frizzled complex to enhance Wnt signaling and control progenitor cell maintenance in different developing organs (24–27). A role of Rspondins and Lgr4–6 receptors in SC development has not been studied.
Here we define a temporal window of Wnt/β-catenin activity and its role in SC lineage progression using mouse genetics and cell culture techniques. Conditional loss-of-function (LOF) and gain-of-function (GOF) mutations of β-catenin in mouse SCs produce converse phenotypes: a delay and an acceleration of axonal sorting, respectively. Using cultured primary SCs and established SC lines, we delineate the roles of ligands, e.g., axonal Rspondins and Wnts, and of the downstream target genes that mediate the Wnt response.
Results
Expression of Wnt Pathway Components and Activity of Wnt/β-Catenin Signaling in Mouse Schwann Cell Development.
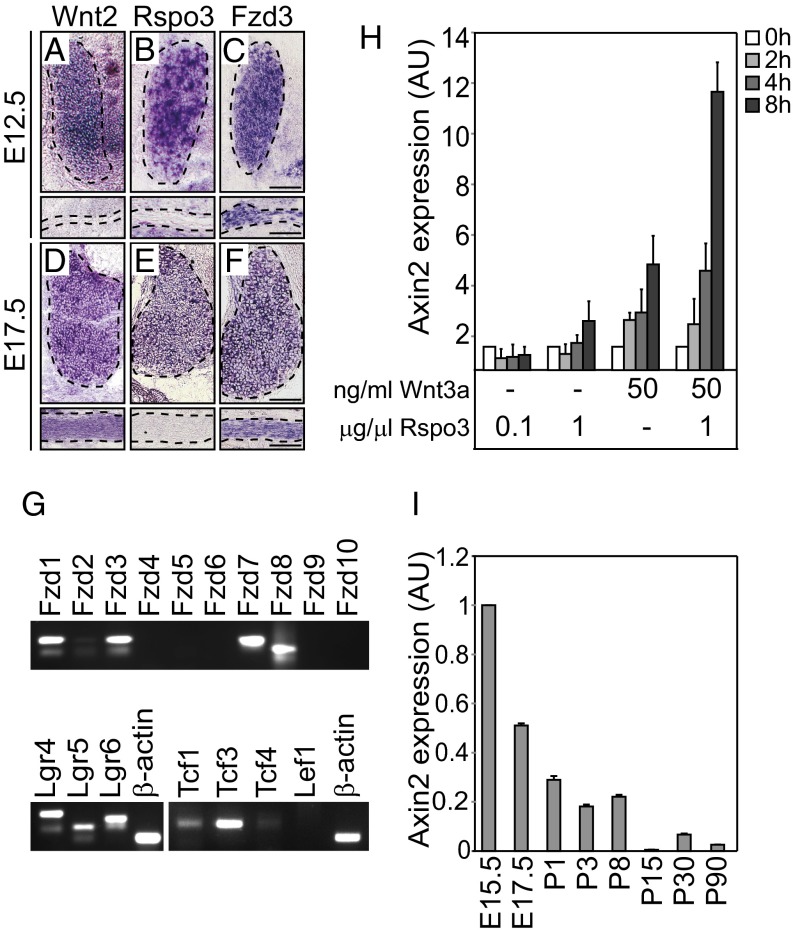
We first determined the expression of Wnt signaling components in the developing SCs and neurons by in situ hybridization of dorsal root ganglia (DRG) and spinal and sciatic nerves and by quantitative real-time PCR (qRT-PCR) of primary embryonic SCs. Genes encoding the ligands Wnt2, 6, and 9b and Rspondin 1–4 were expressed in DRG neurons starting at E12.5; Wnt2 was also expressed in SCs of the spinal and sciatic nerves starting at E14.5 (Table S1, Fig. 1 A–F, and Fig. S1A). Genes encoding the receptors Fzd1, 3, 7, and 8 and Lgr4–6 as well as the transcription factors Tcf1, 3, and 4 were expressed in SCs from E12.5 on (Table S1 and Fig. 1 A–G; see also Fig. 1 A–F, Lower). Thus, in the peripheral nervous system, neurons express several Wnt and Rspondin ligands that can signal to SCs in a paracrine manner; in addition, SCs might receive autocrine signals by Wnt2.
Fig. 1.
Expression of the components and activity of Wnt signaling during SC development. Analysis of the expression of canonical Wnt signaling components (A–F) by in situ hybridization at two different stages of SC development (in each panel, Upper shows dorsal root ganglia, and Lower shows nerves) and (G) by qRT-PCR in primary SC cultures. Canonical Wnt signaling is synergistically activated by Wnt and Rspondin ligands in cultured SCs. (H) Dose-dependent coactivation of canonical Wnt signals by Wnt3a and Rspo3, determined by increase in the expression of the classical Wnt target gene Axin2. (I) Endogenous expression of Axin2 in mouse sciatic nerves measured by qRT-PCR is highest at E15.5 and decreases postnatally. Error bars represent SD (n = 5); the value at E15.5 is from embryonic sciatic nerves from 10 embryos pooled as a single probe. AU, arbitrary units. (Scale bars: 100 μm.)
We found that Wnt3a and Rspondin ligands synergistically activated transcription of the classical Wnt target gene Axin2 in mouse primary SCs and, similarly, the Wnt-responsive luciferase gene (Topflash assay) in rat RT4 cells, an established SC line (Fig. 1H and Fig. S1B) (28, 29). Endogenous Wnt signaling in developing SCs was assessed by analyzing two independent Wnt-reporter mouse strains, Axin2-LacZ (Fig. S1 C–E) (30) and Topgal (31), and by measuring the expression of the classical Wnt target gene Axin2 in developing sciatic nerves by qRT-PCR (Fig. 1I). These analyses indicate that endogenous Wnt signaling activity in SCs peaks in the late fetal period (E15.5–E17.5) and declines subsequently.
Schwann Cell-Specific β-Catenin Loss- and Gain-of-Function Mutations in Mice Lead to Disrupted Axonal Sorting.
To genetically analyze canonical Wnt signaling in the SC lineage, we generated β-catenin LOF and GOF mutations using Cnp-cre in mice (32). Two different alleles of β-catenin were used: an allele with “floxed” exons 3–6 (β-catfl) whose recombination leads to a frameshift mutation that prevents production of functional protein and an allele with “floxed” exon 3 (β-catEx3fl) whose recombination leads to the expression of an N-terminally truncated, nondegradable, and constitutively active β-catenin (33, 34). Cre-mediated recombination was assessed using a YFP-reporter transgene (Fig. S2A). β-Catenin LOF mutations led to a strong reduction of full-length β-catenin protein (86% reduction), whereas GOF mutations resulted in formation of a stable, activated protein (140% of wild-type level) at postnatal day (P) 1 (Fig. S2 B–D). Residual full-length protein in mutants was likely due to the presence of perineural fibroblasts and other cell types that do not express Cnp-cre and/or of SCs that escaped recombination (35).
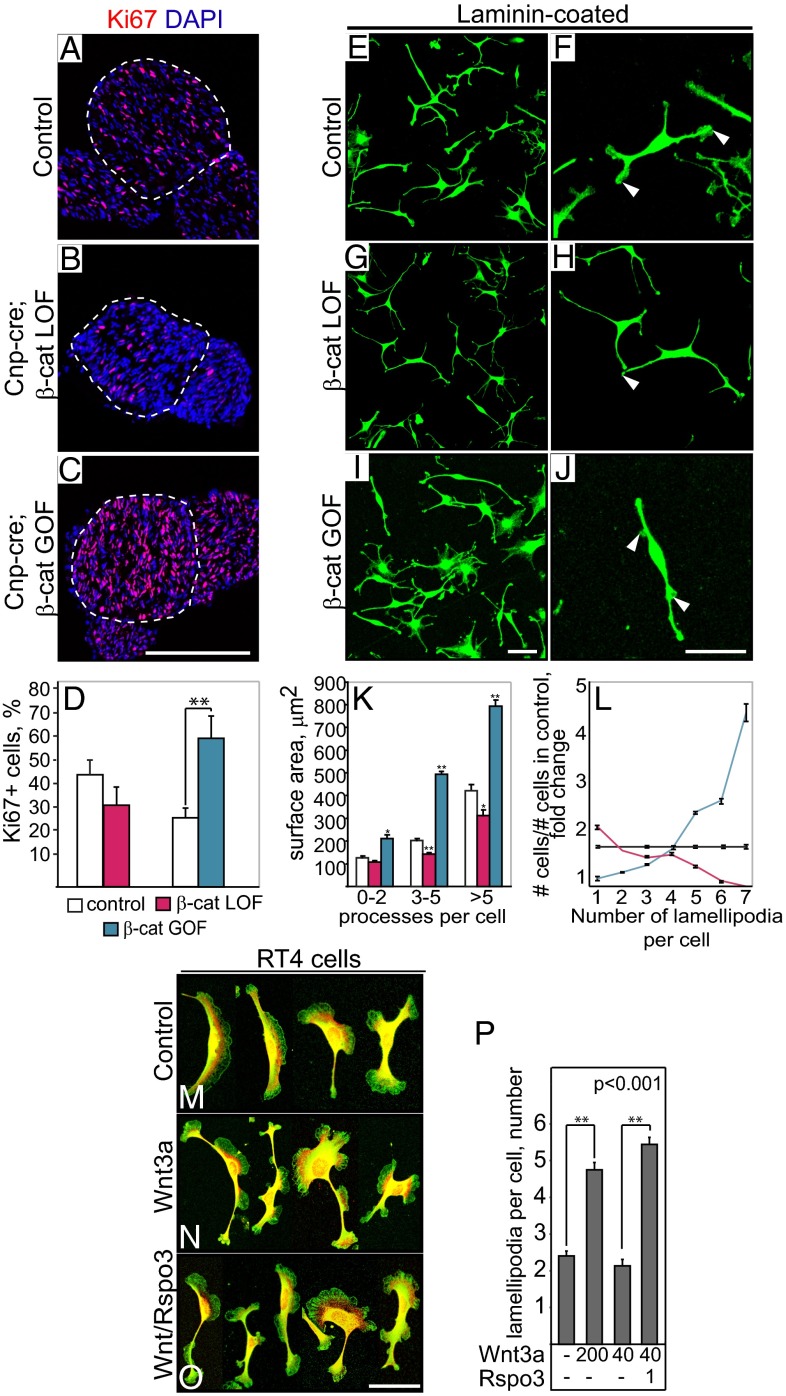
Cnp-cre; β-catfl/fl and Cnp-cre; β-catEx3fl/fl mutant mice were born at the expected Mendelian ratios; however, ∼90% of LOF and ∼30% of GOF mutants died within 1 wk after birth, likely due to defects in the central nervous system where Cnp-cre is also expressed (23, 32). Light microscopy of toluidine blue-stained semithin sections, and electron micrographs of sciatic nerves were used to define axonal sorting. In control mice, the majority of large-diameter axons were sorted from bundles and engaged in 1:1 relationship with SCs, and many SCs had begun to myelinate at P3 (Fig. 2 A, D, and G). Remarkably, nerves of the Cnp-cre; β-cat LOF mutants contained very large axonal bundles with more than 100 axons per bundle, including large-caliber axons that were unsorted (Fig. 2 B, E, and H, marked by brackets, and quantification in Fig. 2J). In addition, SCs often failed to fully envelope the bundles and to extend processes between axons (Fig. 2 E and H). Conversely, in nerves of Cnp-cre; β-cat GOF mutants, Remak bundles were smaller and contained fewer axons compared with controls (Fig. 2 C, F, and I and quantification in Fig. 2K), and the majority of large-diameter axons were sorted (Fig. 2F). Thus, LOF mutations of β-catenin in SCs impede and GOF mutations promote axonal radial sorting. Comparison of the g ratios (ratio between axonal diameter and outer diameter of the myelinated fiber) indicated a minor myelination deficit in β-cat LOF mutants compared with control littermates (Fig. 2L). However, Cnp-cre; β-catenin GOF mutants showed an unexpected paucity of myelination at P3, with 96% of axons displaying g ratios between 0.95 and 1 (Fig. 2 C, F, and M). Even at P25, axons exhibited markedly thinner myelin, indicating that sustained β-catenin activity interferes with (but does not fully block) myelination, possibly by delaying myelination onset (Fig. S2 E–J). Thus, the sustained β-catenin activity in GOF mutants enhances sorting and additionally impedes late SC differentiation and myelination.
Fig. 2.
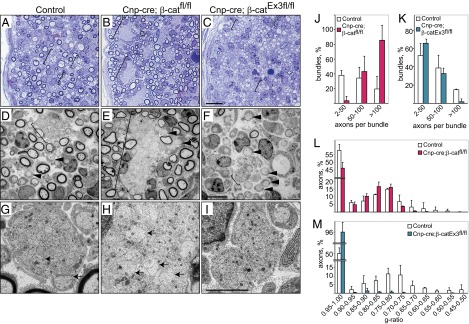
Impaired Wnt signaling in SCs causes defects in the sorting of axons and delays myelination. (A–C) Transverse semithin sections and (D–I) electron micrographs of peripheral nerves of control, Cnp-cre; β-catfl/fl, and Cnp-cre; β-catEx3fl/fl mice at P3. Note the large size of the axonal bundles in the Cnp-cre; β-catfl/fl nerves and the relatively small size of bundles in the Cnp-cre; β-catEx3fl/fl nerves. Sorted axons are marked with arrowheads, axonal bundles are marked with brackets, and unsorted large axons within the bundles are marked with arrows. (Scale bars: 10 μm in A–C and 5 μm in D–I.) Quantification of the axonal bundle size in control mice along with (J) Cnp-cre; β-catfl/fl (red) and (K) Cnp-cre; β-catEx3fl/fl (blue) mice. Shown is the percentage of bundles that contain a particular range of numbers of axons. Quantification of the g ratio in control mice along with (L) Cnp-cre; β-catfl/fl (red) and (M) Cnp-cre; β-catEx3fl/fl (blue) mice. Shown is the percentage of axons that display a particular g ratio. Errors bars indicate SD calculated from at least three mice per group.
Mechanisms of Axonal Sorting Deficits in β-Catenin Mutant Schwann Cells.
The radial sorting deficit in β-cat LOF and GOF mutant mice might be caused by aberrant SC proliferation, migration, process extension, or differentiation (9, 10, 36, 37). At early developmental stages (E14.5–17.5), no significant changes in proliferation or migration of SCs were observed in β-cat LOF or GOF mice compared with controls (Fig. S3 A–H). In contrast, at P3, proliferation of β-catenin LOF SCs was reduced, and conversely, in the GOF sciatic nerves, proliferation was increased (Fig. 3 A–D). To study the mechanisms further, we inhibited Wnt signaling by introducing LOF mutations in isolated primary β-catfl/fl SCs using the membrane-penetrable cre protein, His-Tat-NLS-cre (HTN-cre; refs. 4 and 38); this resulted in a 76% reduction of β-catenin protein after 4 d culture (Fig. S3I). To activate canonical Wnt signaling in primary SC cultures, we used a small-molecule chemical inhibitor of GSK3β, Chir98014 (39), or recombinant Wnt3a together with Rspo3. No significant changes were observed in proliferation or migration of cultured SC (Fig. S3 J–V). However, both HTN-cre and Chir 98014 treatments markedly affected cell shape and process extension, as visualized by immunolabeling of focal adhesions by anti-vinculin antibody and staining of polymerized actin by phalloidin. HTN-cre–treated SCs (β-cat LOF) showed reduced cell spreading, whereas treatment with Chir98014 (mimicking β-cat GOF) increased spreading (Fig. 3 E–J and quantification in Fig. 3K). Moreover, the number of peripheral lamellipodia was significantly reduced in β-cat LOF and increased in Chir98014-treated cells (Fig. 3 E–J, quantification in Fig. 3L, and enlargements in Fig. S4 A–C″). Combined treatment with Wnt3a and Rspo3 reproduced the effect of Chr98014: the number of lamellipodia in RT4 cells and in primary mouse SCs increased upon addition of recombinant Wnt3a in concentration-dependent manners, and Rspo3 acted synergistically (Fig. 3 M–O, quantification in Fig. 3P, and Fig. S4 D–P). Wnt-induced changes in spreading and lamellipodia formation in cultured primary cells and SC lines were independent of β1 integrin or of signaling through Rac1, Rho, Fak, and p38, as assessed by culturing control and β-catenin mutant SCs on the β1 integrin-independent substrate vitronectin, or in the presence of pharmacological inhibitors of Rac1, Rho-kinase, Fak, or p38 (Figs. S4 Q–Y and S5). Changes in axonal sorting in β-cat LOF mutant mice were not accompanied by alterations in adherens junctions. Main components of the adherens junctions, E-cadherin and N-cadherin, were detectable in myelinating fibers starting at P3 and persisted into adulthood in regions of noncompact myelin such as Schmidt–Lantermann incisures (Fig. S6 A–D″). Conditional ablation of E-cadherin in SCs did not lead to abnormal bundle size or abnormal myelination (Fig. S6 F–M). Thus, β-catenin exerts its role in SC sorting by mediating Wnt signals, rather than stabilizing cadherin-dependent adhesion.
Fig. 3.
Defects in cell cycle exit and lamellipodia formation in β-catenin mutant SCs in vivo and in vitro. (A–C) Proliferating SCs at P3, visualized by immunostainings using antibodies against proliferation marker Ki67. Dotted outlines surround axons. (Scale bar: 100 μm.) (D) Quantification of the proliferating cells. Shown is the percentage of the Ki67+ cells per nerve cross-section. (E–J) SC shape and lamellipodia formation were analyzed by immunostaining using antibodies against vinculin. SCs carrying β-catenin LOF mutations fail to produce terminal lamellipodia, whereas SCs mimicking β-catenin GOF mutations produce more lamellipodia. (Scale bars: 40 μm.) (K) Measurement of surface area of control, β-catenin LOF, and GOF SCc on laminin. Shown is the surface area quantified separately for cells containing zero to two, three to five, and more than five processes per cell. (L) Quantification of the number of terminal lamellipodia per cell in the β-catenin LOF and GOF cells, shown as fold changes, normalized to controls. (M–O) Lamellipodia formation in RT4 cells in the absence and presence of 40–200 ng/mL Wnt3a and 1 μg/μL Rspo3 ligands as assessed by immumostaining using Phalloidin (red) and antibodies against vinculin (green). (Scale bar: 40 μm.) (P) Quantification of lamellipodia in M–O. Error bars in D indicate SD (n = 3), and error bars in K, L, and P indicate SEM. t test: **P < 0.01.
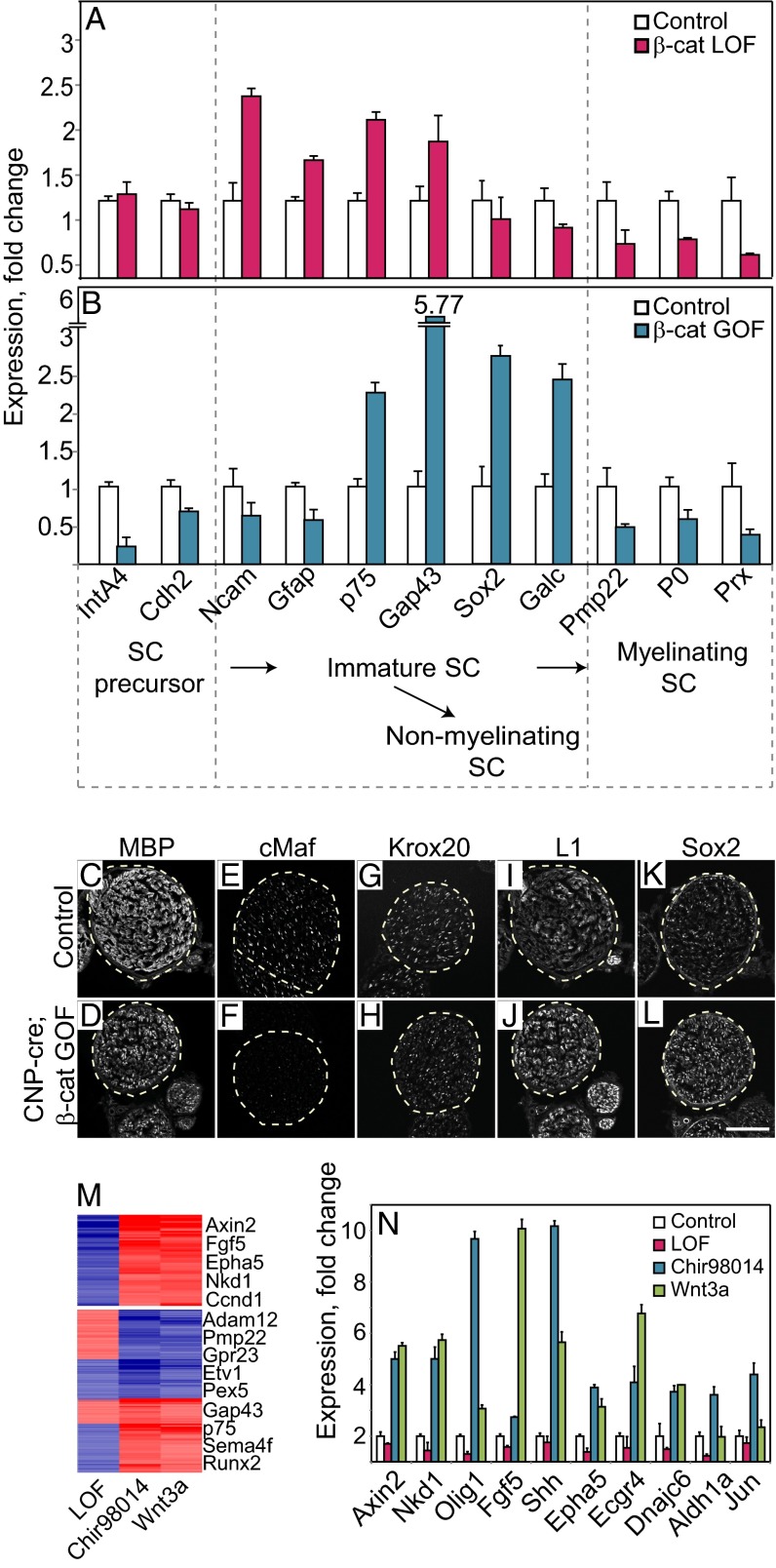
We next examined the differentiation of cultured SCs in vitro by measuring with qRT-PCR the mRNA levels of genes known to be differentially expressed at various stages of SC differentiation. Compared with control cells, β-cat LOF mutant SCs up-regulated Ncam, Gfap, p75, and Gap43 after 6 d culture; these genes are known to be expressed at high levels in the immature SCs (Fig. 4A) (1). This indicates that the β-cat LOF mutation interferes with the maturation of cultured SC. β-Catenin GOF cells showed up-regulated expression of p75 and Gap43 but also of Sox2, a marker of late immature SCs, and of GalC, a marker of differentiated SCs in culture (Fig. 4B) (1, 40). Similarly, addition of recombinant Wnt3a to primary SCs enhanced the expression of those late immature SC markers in a dose-dependent manner (Fig. S7A). Thus, activation of Wnt signaling drives the differentiation from an early to a late immature SC stage. The expression of the myelin genes Pmp22, P0, and Prx was reduced in both LOF and GOF cells in culture (Fig. 4 A and B).
Fig. 4.
Gene expression signature imposed by β-catenin LOF and GOF mutations in SCs. (A and B) qRT-PCR analysis of mRNA expression in SCs of (A) β-catenin LOF mutations (HTN-cre–treated) and (B) β-catenin GOF mutations (Chir98014 treatment), using primers directed against the indicated marker genes. The time course of SC development is shown at the bottom. (C–L) Immunostainings on transverse sections of the sciatic nerves of (C, E, G, I, and K) control and (D, F, H, J, and L) Cnp-cre;β-cateninEx3fl/fl mice using antibodies against MBP, cMaf, Krox20, L1, and Sox2. Dotted lines surround axons. (Scale bar: 100 μm.) (M) Gene expression profiling of SCs upon down-regulation or overactivation of canonical Wnt signaling. Heat map of k-means clustering comparing gene expression in HTN-cre–treated or Chir98014- and Wnt3a-treated cells with their respective controls. Shown is a cluster of genes that were significantly down-regulated in HTN-cre–treated cells and up-regulated in Chir98014- and Wnt3a-treated cells (Upper), and clusters of genes that were significantly modulated in Chir98014- and Wnt3a-treated cells only (Lower). (N) qRT-PCR analysis of mRNA expression using primers against the genes indicated. In all qRT-PCR experiments, transcript levels in each sample were normalized to β-actin. Transcript levels of controls were set to 1. Error bars represent SD from at least three independent experiments.
We also assessed the differentiation of mutant SCs in vivo. Immunohistological analysis of Cnp-cre; β-cat GOF mice showed that protein levels of myelin basic protein (MBP) and the transcription factor cMaf were reduced compared with the controls, in line with the observed myelin disruption (Fig. 4 C–F). Another marker of myelinating SCs, Krox20, was unaffected (Fig. 4 G and H). In contrast, markers of immature SCs, L1 and Sox2, were up-regulated in the β-cat GOF cells (Fig. 4 I–L). This confirmed that SCs reached a late immature stage, but their entry into the myelination program was impaired when Wnt signaling was activated in a sustained manner. Cnp-cre; β-cat LOF mice showed a mild reduction in MBP and cMaf, in line with the slightly reduced myelination, as well as a decrease in Sox2 and an increase in L1 (Fig. S7 B–I). We also used gene expression profiling to identify sets of genes that are controlled by Wnt/β-catenin signaling in SCs. First, we focused on genes that were regulated in opposing manners by reduction or stimulation of canonical Wnt signaling, i.e., that were significantly down-regulated in β-cat LOF and up-regulated in Chir98014- or Wnt3a-treated SCs. Among these were the known Wnt target and regulator genes, e.g., Axin2, Nkd1, and Ccnd1 (Fig. 4M and refs. 30 and 41); genes involved in cytoskeletal rearrangement and actin-binding, like Arpc1a, Syn1, Fyn, Afap1, and Fez1; and potential glial differentiation genes, like Olig1, Shh, Jun, and Fgf5 (Fig. 4N and Table S2). The mRNA levels of differentiation-associated genes were confirmed by qRT-PCR (Fig. 4N). Second, we analyzed a group of genes that were significantly deregulated upon Chir98014 or Wnt3a treatment only. Among these, Gap43 and p75 were up-regulated, whereas myelin genes such as Pmp22 and Pmp2 and genes encoding enzymes that control lipid and fatty acid metabolism, e.g., Pex5, Cpt2, and Cpt1c, were down-regulated (Fig. 4M and Table S3).
Discussion
Here we show that conditional LOF and GOF mutations of β-catenin in mouse SCs impede and accelerate radial sorting of axons, respectively. We further show that down- or up-regulated Wnt/β-catenin signaling inhibits or promotes cell spreading and lamellipodia formation in cultured SCs. A comprehensive expression analysis of the components of Wnt/β-catenin signaling suggests that during axonal sorting, paracrine signal exchange between axons and SCs may occur. Analysis of in vivo Wnt-reporter activity and target gene expression indicated that perinatal SCs transiently receive Wnt signal during radial sorting. Wnts and Rspondins are produced by neurons and potentially released along axons, where they could interact with Lgr4–6 and Lrp/Frizzled receptors on SCs. We suggest a model where Wnt and Rspondin signals are essential to promote axonal sorting and SC lineage progression beyond the immature stage.
Several ligands of canonical Wnt signaling are expressed in developing peripheral ganglia and in SCs: Wnt2, 6, and 9b and Rspondins 1–4 in neurons and Wnt2 also in SCs. Addition of exogenous Wnt ligands to primary cultured SCs activated the canonical Wnt pathway in a dose-dependent manner, demonstrating that the downstream Wnt cascade was functional. SCs express the three Lgr4–6 receptors, and Rspondins synergistically promote Wnt signaling and lamellipodia formation in SC culture. This suggests that Rspondins that are expressed neuronally during the sorting process could contribute to the sorting also in vivo. The expression of Wnts and Rspondins in nerves and their receptors in SCs was observed starting at E12.5, but Wnt reporter activity was detected in the peripheral nerves starting at E15.5. The absence of Wnt responses at earlier stages may indicate the existence of specific regulatory mechanisms that suppress precocious Wnt activity downstream of Wnt/Rspondin receptors in vivo. Alternatively, a more complex interaction may exist; that is, Wnt2 that is expressed late in SCs may initiate, whereas axonal Rspondins may modulate Wnt signaling in SCs. Postnatally, no Wnt reporter activity could be detected in SCs. Thus, canonical Wnt signaling is active during a small window in the late fetal period of SC development.
Conditional LOF and GOF mutations of β-catenin in mouse SCs were introduced in vivo and in vitro, respectively, using Cnp-cre and HTN-cre. In both systems, β-catenin was not required for SC early proliferation or migration; instead, mutant cells showed abnormal extension of cytoplasmic processes. Electron microscopic analyses showed that conditional β-catenin LOF mutant SCs failed to fully envelope axonal bundles and to extend processes between axons, leading to abnormal radial sorting and accumulation of large unsorted bundles containing more than 100 axons. Conversely, in β-catenin GOF nerves, bundles were smaller than in controls and contained fewer axons, pointing to an accelerated sorting. We could exclude Wnt-independent roles of β-catenin, for instance, in cadherin-mediated cell adhesion, because axonal sorting was normal in Cnp-cre; E-cadherin LOF mutant mice. Previous studies demonstrated that axonal sorting largely depends on signals from the extracellular matrix, e.g., of Fak, Rac1, β1 integrin, and Integrin-linked kinase (9, 10, 42). However, Wnt-dependent changes of the cultured SCs persisted on β1-integrin–independent substrates. Moreover, pharmacological inhibitors of Rac1, Fak, and p38 signaling could not rescue Wnt-induced phenotypes. Together, this suggests that the SC phenotypes induced by Wnt are independent of the matrix.
Altered Wnt signaling apparently leads to aberrant differentiation, as shown by the expression of genes characteristic for specific stages of SC development. In spontaneously differentiating SC cultures, activation of Wnt signaling led to an up-regulation of markers of the late immature and differentiated SCs, such as Sox2 and GalC (1, 40, 43), whereas LOF cells failed to up-regulate these genes, indicating a delay of differentiation. To identify directly regulated genes controlled by Wnt signals in SCs, we mapped a cluster of genes significantly down-regulated in β-catenin LOF mutants and up-regulated in cells with activated Wnt signaling. Within this cluster were several previously known Wnt target genes, genes encoding components and regulators of the cytoskeleton, and genes encoding growth and transcription factors. The identification of regulators of actin polymerization suggests a direct effect of Wnt signaling on SC process extension and lamellipodia formation. In particular, Fyn kinase has been shown to control cytoskeletal modifications and lamellae extension in oligodendrocytes (44, 45). The identified transcription factors could also directly affect SC differentiation: Jun is a known negative regulator of SC differentiation and myelination (2, 46). Olig1 promotes oligodendrocyte differentiation (47), but a role in SCs has not been addressed.
Shortly after birth, β-catenin GOF SCs showed an increase whereas LOF cells showed a decrease in number of proliferating cells, compared with control SCs that are progressively exiting the cell cycle at this stage. The question arises as to how the precise coordination between cell cycle exit and the differentiation in SCs may be controlled. It is generally believed that cell cycle exit precedes differentiation, but new data question this hierarchy (48). Wnt signaling has been shown to be essential in cell cycle progression but also in promoting specific lineage decisions independently of proliferation (13). We have observed cell cycle exit defects in sciatic nerves but not in cultured β-catenin mutant SCs, whereas defects in lamellipodia formation and the expression of differentiation genes were observed consistently in vivo and in vitro. This suggests that changes in lamellipodia formation and expression of differentiation genes are likely the direct effects of deregulated Wnt/β-catenin signaling.
We also observed a myelination deficit in β-catenin GOF mutants after birth that was transient: at later stages, axons were myelinated; however, myelin was thinner compared with that of controls. This indicates that the β-catenin GOF mutation delays the onset of myelination, i.e., retains cells for prolonged periods in a late immature stage. Transcriptional profiling identified a group of genes that showed significant deregulation in the β-catenin GOF but not in β-catenin LOF SCs. This group may represent potential Wnt target genes that suppress myelination. Sox2 up-regulation has been previously shown to cause peripheral demyelination in mice and to prevent myelin gene expression in cultured SCs (11). Up-regulation of Sox2 upon Chir98014 and Wnt3a treatment in cultured SCs and an increase in Sox2 protein in the Cnp-cre; β-catenin GOF sciatic nerves could therefore account for the delayed myelination in the β-catenin GOF mutant mice. The fact that Sox2 was strongly down-regulated in the β-catenin LOF mice supports the notion that the LOF mutant SCs proceed beyond the early immature stage in a delayed manner. It was previously suggested that Sox2 might control the levels of Egr2/Krox20 (11). However, in Cnp-cre; β-catenin GOF sciatic nerves, Krox20 protein levels were not affected, indicating that Sox2 acts downstream of Krox20 to prevent myelination, potentially at the level of other myelin-regulating transcription factors (49).
Methods
Mice with floxed β-catenin alleles to produce LOF and GOF mutants and RosaSTOP-YFPfl, Cnp-cre, and Axin2-LacZ mice were previously described (30, 33, 34, 50, 51).
Isolated sciatic nerves were fixed overnight, treated with 1% osmium tetroxide, dehydrated, and embedded into PolyBed resin (Polysciences). For light microscopy, 250-nm sections were stained with toluidine blue. For electron microscopy, 60-nm sections were contrasted with lead citrate and examined using a LEO 906E transmission electron microscope. Axon diameters and g ratios were determined from electron micrographs. Immunofluorescence was performed as described (4).
Primary SCs were isolated from E13.5 DRGs as described (52). HTN-cre protein was produced as described (38). To induce homologous recombination, homozygous floxed β-catenin SCs were incubated with 2 μM HTN-cre for 20 h and subsequently cultured for 3 d to deplete the β-catenin protein. RT4 cells (28) were cultured in high-Glucose DMEM (Gibco) supplemented with 10% (vol/vol) FBS and antibiotics.
For the microarray, total RNA was isolated from cultured primary SCs using TRIzol (Invitrogen), purified by RNeasy kit (Qiagen), biotin-labeled using Illumina TotalPrep DNA amplification kits (Ambion) according to the manufacturer’s instructions, and hybridized to Illumina MouseRef-8 whole genome expression bead chips. Microarray data were analyzed using Partek Genomics Suite. Normalization was performed using the gcRMA method; subsequently, probe sets with expression levels <64 were eliminated as not expressed. Genes showing differential expression using Benjamini Hochberg false discovery rate (FDR) < 0.05 in ANOVA were selected. Functional analysis and gene ontology classification were performed using the Database for Annotation, Visualization, and Integrated Discovery v6.7 (53).
For more details, see also SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Christof Niehrs (Institute of Molecular Biology, Mainz, Germany) for providing Rspondin expression constructs, Carola Griffel and Bettina Barby [Max Delbrück Center for Molecular Medicine (MDC), Berlin] for part of the EM analyses, and Thomas Mueller (MDC) for discussions and help with the preparation of the figures.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Microarray data have been deposited in the ArrayExpress database, www.ebi.ac.uk/arrayexpress (accession no. E-MTAB-1943).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310490110/-/DCSupplemental.
References
- 1.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 2.Mirsky R, et al. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J Peripher Nerv Syst. 2008;13(2):122–135. doi: 10.1111/j.1529-8027.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- 3. Newbern J, Birchmeier C (2010) Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol 21(9):922–928. [DOI] [PMC free article] [PubMed]
- 4.Grossmann KS, et al. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci USA. 2009;106(39):16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47(5):681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56(14):1491–1497. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- 7.Woodhoo A, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12(7):839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feltri ML, et al. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156(1):199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nodari A, et al. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177(6):1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finzsch M, et al. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189(4):701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le N, et al. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci USA. 2005;102(7):2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22(17):2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 15.Gammill LS, Bronner-Fraser M. Neural crest specification: Migrating into genomics. Nat Rev Neurosci. 2003;4(10):795–805. doi: 10.1038/nrn1219. [DOI] [PubMed] [Google Scholar]
- 16.Hari L, et al. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159(5):867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee HY, et al. (2004) Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science 303(5660):1020–1023. [DOI] [PubMed]
- 18.Makoukji J, et al. Interplay between LXR and Wnt/β-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J Neurosci. 2011;31(26):9620–9629. doi: 10.1523/JNEUROSCI.0761-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawk M, et al. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 2011;31(10):3729–3742. doi: 10.1523/JNEUROSCI.4270-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gess B, et al. Inhibition of N-cadherin and beta-catenin function reduces axon-induced Schwann cell proliferation. J Neurosci Res. 2008;86(4):797–812. doi: 10.1002/jnr.21528. [DOI] [PubMed] [Google Scholar]
- 21.Jacob C, et al. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat Neurosci. 2011;14(4):429–436. doi: 10.1038/nn.2762. [DOI] [PubMed] [Google Scholar]
- 22.Lewallen KA, et al. Assessing the role of the cadherin/catenin complex at the Schwann cell-axon interface and in the initiation of myelination. J Neurosci. 2011;31(8):3032–3043. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye F, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12(7):829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmon KS, Lin Q, Gong X, Thomas A, Liu Q. LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/β-catenin signaling. Mol Cell Biol. 2012;32(11):2054–2064. doi: 10.1128/MCB.00272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13(3):242. doi: 10.1186/gb-2012-13-3-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glinka A, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12(10):1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 28.Imada M, Sueoka N. Clonal sublines of rat neurotumor RT4 and cell differentiation. I. Isolation and characterization of cell lines and cell type conversion. Dev Biol. 1978;66(1):97–108. doi: 10.1016/0012-1606(78)90276-2. [DOI] [PubMed] [Google Scholar]
- 29.Molenaar M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86(3):391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 30.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22(4):1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126(20):4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 32.Lappe-Siefke C, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33(3):366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 33.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 34.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goebbels S, et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30(26):8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benninger Y, et al. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177(6):1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grove M, et al. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176(3):277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peitz M, et al. Enhanced purification of cell-permeant Cre and germline transmission after transduction into mouse embryonic stem cells. Genesis. 2007;45(8):508–517. doi: 10.1002/dvg.20321. [DOI] [PubMed] [Google Scholar]
- 39.Ring DB, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52(3):588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- 40.Eccleston PA, Mirsky R, Jessen KR, Sommer I, Schachner M. Postnatal development of rat peripheral nerves: An immunohistochemical study of membrane lipids common to non-myelin forming Schwann cells, myelin forming Schwann cells and oligodendrocytes. Brain Res. 1987;432(2):249–256. doi: 10.1016/0165-3806(87)90049-6. [DOI] [PubMed] [Google Scholar]
- 41.Rousset R, et al. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 2001;15(6):658–671. doi: 10.1101/gad.869201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feltri ML, Suter U, Relvas JB. The function of RhoGTPases in axon ensheathment and myelination. Glia. 2008;56(14):1508–1517. doi: 10.1002/glia.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodhoo A, Sommer L. Development of the Schwann cell lineage: From the neural crest to the myelinated nerve. Glia. 2008;56(14):1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 44.Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J Neurosci. 2009;29(38):11794–11806. doi: 10.1523/JNEUROSCI.0888-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, et al. Myosin II is a negative regulator of oligodendrocyte morphological differentiation. J Neurosci Res. 2012;90(8):1547–1556. doi: 10.1002/jnr.23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkinson DB, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181(4):625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109(1):61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 48.Hindley C, Philpott A. Co-ordination of cell cycle and differentiation in the developing nervous system. Biochem J. 2012;444(3):375–382. doi: 10.1042/BJ20112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wegner M. Transcriptional control in myelinating glia: Flavors and spices. Glia. 2000;31(1):1–14. doi: 10.1002/(sici)1098-1136(200007)31:1<1::aid-glia10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 50.Langseth AJ, et al. Wnts influence the timing and efficiency of oligodendrocyte precursor cell generation in the telencephalon. J Neurosci. 2010;30(40):13367–13372. doi: 10.1523/JNEUROSCI.1934-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodhoo A, Dean CH, Droggiti A, Mirsky R, Jessen KR. The trunk neural crest and its early glial derivatives: A study of survival responses, developmental schedules and autocrine mechanisms. Mol Cell Neurosci. 2004;25(1):30–41. doi: 10.1016/j.mcn.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.