Significance
Although the response of the Plant Kingdom to climate change is acknowledged as one of the fundamental feedback mechanisms of environmental changes on the Earth, until now, the response of plant species to in situ climate warming has been described at the level of a few fixed plant functional types (i.e. grasses, forbs, shrubs etc.). This approach is very coarse and inflexible. Here, we show that plant functional traits (i.e., plant features) can be used as predictors of vegetation response to climate warming. This finding enlarges possibilities for forecasting ecosystem responses to climate change.
Keywords: alpine plant community, root traits, plant traits, seed mass, specific leaf area
Abstract
Predicting climate change impact on ecosystem structure and services is one of the most important challenges in ecology. Until now, plant species response to climate change has been described at the level of fixed plant functional types, an approach limited by its inflexibility as there is much interspecific functional variation within plant functional types. Considering a plant species as a set of functional traits greatly increases our possibilities for analysis of ecosystem functioning and carbon and nutrient fluxes associated therewith. Moreover, recently assembled large-scale databases hold comprehensive per-species data on plant functional traits, allowing a detailed functional description of many plant communities on Earth. Here, we show that plant functional traits can be used as predictors of vegetation response to climate warming, accounting in our test ecosystem (the species-rich alpine belt of Caucasus mountains, Russia) for 59% of variability in the per-species abundance relation to temperature. In this mountain belt, traits that promote conservative leaf water economy (higher leaf mass per area, thicker leaves) and large investments in belowground reserves to support next year’s shoot buds (root carbon content) were the best predictors of the species increase in abundance along with temperature increase. This finding demonstrates that plant functional traits constitute a highly useful concept for forecasting changes in plant communities, and their associated ecosystem services, in response to climate change.
Climate change is affecting the structure and composition of vegetation worldwide. Increasing temperatures are considered to be a key driver of recent tundra greening (1, 2) and upward migration of vascular plant species in mountains (3–6). Predicting climate change impact on ecosystem structure and services is one of the most important challenges in ecology (7). Previous studies of plant response to warming used concepts of growth form or functional type as predictors of plant response to warming and demonstrated that evergreen and deciduous (dwarf) shrubs and rushes increase their abundance in response to experimental warming in cold biomes (1, 8). However, the categorization of plants into fixed functional types has been criticized for being imprecise and too coarse for accurate prediction of plant response to climate change (9).
Considering a plant species as a set of functional traits instead of an entity belonging to a fixed functional type greatly increases our possibilities for analysis of ecosystem functioning (10), enabling generalization of our knowledge on plant functioning at ecosystem, landscape, or regional scale. When linked to directional changes in the abundance of plant species, variation in traits involved in plant effects on biogeochemical cycling can be applied in estimates of changes in ecosystem carbon and nutrient turnover (9). This provides a powerful tool in environmental assessment and policy development. Moreover, recently assembled large-scale databases, such as TRY (11) hold comprehensive per-species data on plant functional traits. Such data, collected using standardized protocols (12), accompanied by detailed metadata (11) and accessible for the broad scientific community, allows detailed description of many Earth plant communities in terms of plant functional traits (11).
Here, we tested whether and which plant functional traits representing key aspects of plant adaptive life strategy (13–16) could be used as predictors of plant response to climate warming under natural conditions. We hypothesized that traits associated with resource capture efficiency and, thereby, growth rate, would have predictive power of temperature–abundance relationships, because warmer temperature regimes were expected to promote species of faster potential growth rate.
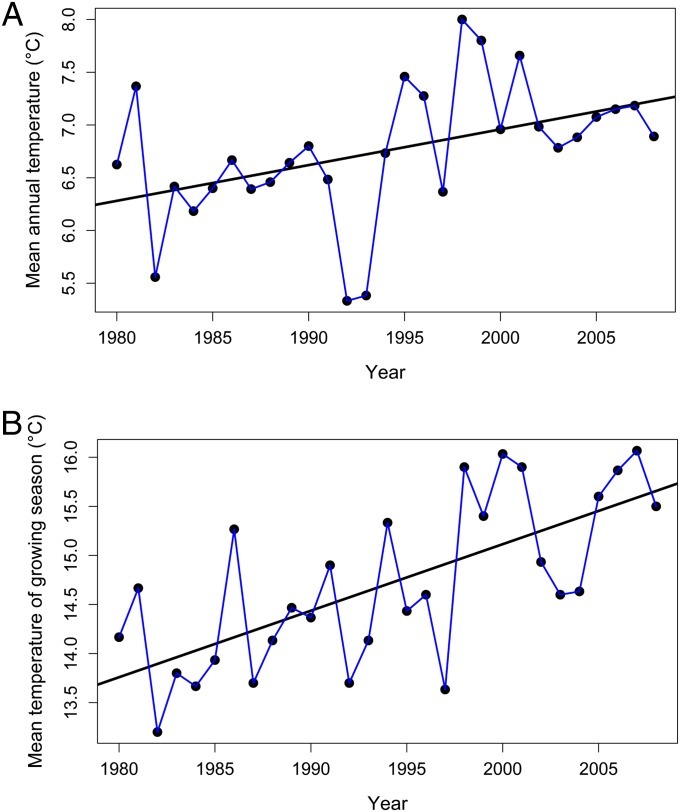
We monitored long-term (23–28 y) annual shoot abundance dynamics of 50 alpine species, growing in four widespread alpine communities of the Northwestern Caucasus, Russia. During this study period, mean annual temperature increased by 0.6 °C and mean growing season temperature by 2 °C (Fig. 1 A and B), whereas precipitation did not show any pattern. In a series of autocorrelation-corrected, scaled regression analyses conducted for each species individually, we assessed for each species the relationship between its annual abundance (number of shoots per square meter) and temperature. This analysis yielded for each species a regression coefficient describing a slope of the abundance vs. temperature regression, hereafter referred to as the abundance–temperature slope. We tested whether the character of the relationship between plant abundance and temperature could be predicted by plant functional traits (10), using weighted regression analyses, with traits as predictors, abundance–temperature slopes as response variables, and the inverse of per-species coefficient of variation (CV) of temperature–abundance slope as data-point weights, the latter allowing the data points for species exhibiting a tighter abundance vs. temperature relationship to have larger weight in the analysis. Furthermore, we examined variation in the following traits among species: specific leaf area (SLA) (area/dry mass), area of individual leaf, mass-based leaf, and root nitrogen (N) content and specific root length, together representing a species’ resource capture efficiency; leaf thickness, leaf dry matter content, leaf and root carbon (C) content, and C:N ratio, representing its resource protection and conservation strategy; and seed mass, representing reproductive strategy (13–16).
Fig. 1.
Interannual dynamic of the temperature during the period of 1980–2008, based on the Teberda meteorological station data (Russia, Teberda, Mt. Malaya Khatipara, 43°27′N, 41°44′E, 1.313 m above sea level). (A) Mean annual temperature. (B) Mean temperature of the growing season (June to September).
Results and Discussion
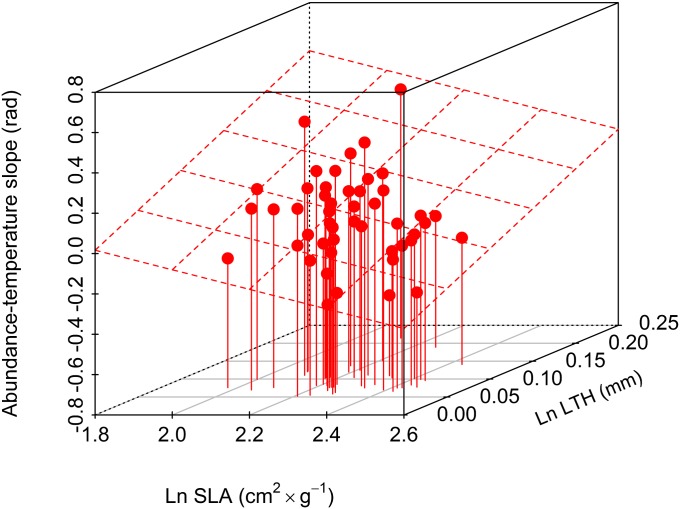
Two traits were good individual predictors of the relationship between species abundance and temperature: leaf thickness explained 25% of variance in the shoot abundance over temperature coefficient, and SLA explained 13% (Table 1 and Fig. 2). The best model based on multiple traits explained 59% of the variance and included leaf thickness, SLA, seed mass, and root carbon content.
Table 1.
Results of the regression analysis on plant functional traits and strength of the relationship between temperature and abundance of alpine plant species (abundance–temperature slope), expressed as the coefficient of the increase in abundance over temperature increase
| Models | N | B | SE B | R2 | P |
| Leaf thickness + SLA + seed mass + root carbon | 33 | 0.585 | 0.001 | ||
| Leaf thickness | 0.530 | 0.147 | 0.001 | ||
| SLA | −0.536 | 0.235 | 0.030 | ||
| Root carbon concentration | −0.509 | 0.215 | 0.025 | ||
| Seed mass | 0.364 | 0.244 | 0.147 | ||
| Individual plant traits that were significant predictors of the abundance–temperature slope | |||||
| Leaf thickness | 50 | 0.503 | 0.125 | 0.252 | <0.001 |
| Specific leaf area | 50 | −0.538 | 0.205 | 0.125 | 0.012 |
| Individual plant traits that were not significant predictors of the abundance–temperature slope | |||||
| Root nitrogen concentration | 43 | 0.149 | 0.288 | 0.006 | 0.606 |
| Root carbon concentration | 43 | −0.231 | 0.244 | 0.021 | 0.348 |
| Root C:N concentration ratio | 43 | −0.167 | 0.276 | 0.009 | 0.548 |
| Specific root length | 43 | −0.227 | 0.215 | 0.022 | 0.297 |
| Leaf nitrogen concentration | 50 | −0.065 | 0.240 | 0.002 | 0.786 |
| Leaf carbon concentration | 50 | 0.0537 | 0.201 | 0.001 | 0.791 |
| Leaf C:N concentration ratio | 50 | 0.068 | 0.238 | 0.002 | 0.771 |
| Leaf dry matter content | 50 | −0.129 | 0.225 | 0.007 | 0.570 |
| Leaf area | 50 | −0.379 | 0.221 | 0.057 | 0.093 |
| Seed mass | 38 | −0.415 | 0.255 | 0.068 | 0.113 |
B, regression coefficient; N, number of species; SE B, SE of the regression coefficient. For the multiple regression model, P value is shown for the whole model and for each parameter.
Fig. 2.
The relationship between SLA, leaf thickness, and abundance–temperature slope (n = 50 species).
Our findings suggest that plant traits may be used as powerful predictors of correlations between plant abundance and climate change. We show that alpine species with high resource input into structural traits, such as thick leaves, low SLA, and, at the same time, high carbon content in roots, increase in abundance at warmer climate by forming more buds or producing seeds of higher quality, which allow them to increase shoot number in the next season.
The two leaf traits included into the best model (SLA, leaf thickness) represent a species’ resource (including light) capture efficiency (SLA) and nutrient and water-conservation strategy (15). Often, these traits have been found to be highly correlated (13, 16), but in our case, the correlation between these traits was negligible (Pearson correlation coefficient, 0.191; P = 0.18; n = 50). The SLA is a function of leaf thickness and leaf tissue density (17). Between these two components of SLA, leaf thickness tends to be linked more strongly to variation in light regime, including sun-imposed stress at high light regimes, whereas the leaf tissue-density component of SLA tends to be associated with variation in soil resources (17). Thus, we propose that in harsh alpine conditions, where competition for light plays a lesser role but sun-imposed stress is important (18), variation in leaf thickness may be mostly decoupled from variation in leaf density and, therefore, from SLA.
Remarkably, addition of seed mass into the best model improved its predictive power, despite the fact that this parameter in itself was not a significant predictor of temperature–abundance slope (Table 1). Although many alpine vascular plant species feature clonal reproduction (18), reproduction by seeds remains important in the long-term, enabling long-distance dispersal and genetic flexibility of a population (19, 20). The importance of seed mass as a predictive trait for the relationship between plant abundance and warming alerts to the potential for these processes to be affected by climate change.
We expected to detect the most pronounced relationships between temperature and plant abundance among dominant species. However, this was not the case: temperature-associated changes in abundances were observed both in dominant and in subordinate plant species (Dataset S1). Also, the opposite was true: some dominant, as well as subordinate, species had very high temperature-slope CV (and, therefore, lower weight in the regression analysis), indicating that the abundance dynamic of these species was not related to temperature regime.
Our method of analysis presumes that plants do not alter their traits in response to climatic changes. Although this is generally not true [i.e., because of plasticity, plants alter their traits in response to environmental changes (21)], we consider that the changes in per-species mean trait values induced by a temperature increase of 0.5–2 °C will be insignificant in comparison with the interspecific trait differences [e.g., Aerts et al. (22) have shown, at comparable experimental temperature ranges, that warming has little impact even on relatively responsive traits such as nutrient contents, thus justifying our analysis]. To test whether this is true for the plants examined in this study, we examined whether mean shoot abundance at our experimental site differed between years of high and low vapor pressure deficit (VPD) [calculated using Penman–Monteith equation (23)]. On the one hand, VPD is known to be a very good predictor of annual plant biomass and, on the other hand, to be correlated to plant traits (24, 25). Decrease in shoot abundance during years of low VPD would provide indirect evidence of high plasticity of the plants examined of this study. However, we found that mean shoot abundance in years of high VPD versus those in years of low VPD did not differ significantly (F1,6 = 0.829; P = 0.398; results of ANOVA with shoot number as dependent variable and high or low VPD as a predictor), providing additional evidence of insignificant plasticity of traits of alpine plant species, examined in this study, in response to warming by 1–2 °C.
We had expected that enhanced mineralization attributable to increased temperature would promote the abundance of nutrient-acquisitive species (26) that typically have large leaves with high SLA (26). In contrast, we found no links with leaf area and detected a positive association with temperature increase in species with low SLA. Low SLA (sclerophylly) is usually associated with low leaf water content and conservative water economy (27). In temperate alpine areas, increasing temperatures without concurrent increase in precipitation will put a premium on water conservation and, thus, on low SLA. Because low SLA species tend to have lower litter decomposability (28), slower decomposition, in turn, is expected to slow ecosystem nutrient cycling and to provide a negative feedback to global warming by reducing carbon release from more recalcitrant leaf litter (29).
We demonstrate that relationships between plant abundance and temperature could be predicted by plant traits. The causality between changes in plant abundance and temperature trends could undoubtedly be established exclusively via experimental manipulations. However, experiments manipulating environmental factors might suffer from short-term artifacts (30), which might becloud the results. In contrast, our long-term observations in natural conditions, with no environmental factors except temperature being changed, are clear from such artifacts. Indeed, our study site is situated on the territory of the Teberda reserve, which has enjoyed strict protection virtually without human interference for about 70 y and where vegetation and animal dynamics have been monitored scrupulously every year by staff of the reserve and the university researchers. During the study period, no natural disasters, animal outbreaks, or changes in landscape management took place, suggesting that temperature is the only possible factor to explain the long-term trends of abundance change in vascular plants. The path from temperature to plant behavior is unidirectional (at least the scale of this study), although the underlying mechanisms are not necessarily straightforward. For instance, not only direct (physiological) effects of the temperature on plant-shoot dynamics may play a role but also indirect effects of temperature, such as changes in soil nutrient dynamics, competitive relationships within communities (31), or fluctuations in the natural herbivore population abundance (32). Notwithstanding such uncertainties about the exact mechanisms of the temperature effect, our data demonstrate that plant functional traits bear great promise for predicting relationships between temperature trends and plant abundance and, therefore, create a powerful tool for forecasting changes in vegetation structure attributable to climatic changes.
Material and Methods
Sampling.
Plant abundance dynamic.
We studied long-term (1981–2009) dynamics of alpine plants on Mt. Malaya Khatipara at 2,750 m above sea level in the Northwestern Caucasus (Russia). Data from the Teberda weather station (Russia, Teberda, Mt. Malaya Khatipara, 43°27′N, 41°44′E, 1.313 m above sea level), closest to our permanent plots, were used to estimate direction of climatic changes during the study period. During this study period, mean annual temperature increased by 0.6 °C and mean growing season temperature by 2 °C (Fig. 1 A and B), whereas precipitation did not show any pattern. Although alpine temperature regimes might vary among plant communities because of microtopology (33), interannual climate dynamics should be highly correlated within the same area. Because alpine habitats and their plant communities are strongly shaped by climatic drivers, particularly temperature (18), one could expect a considerable response of alpine plants to changing temperature.
Four typical alpine communities were included in the study: alpine lichen heath (ALH), Festuca varia grassland (FVG), Geranium–Hedysarum meadow (GHM), and alpine snowbed (SBC). Detailed descriptions of these plant communities were published earlier (25). In 1981, in each plant community, we randomly established 40 permanent plots of 25 × 25 cm where we annually counted per-species shoot number of all vascular plants during 1981–2009 (ALH), 1986–2009 (GHM and SBC), and 1987–2009 (FVG). The shoots were counted in late July to August (i.e., peak growing season). For further analysis, we selected plant species forming at least 15 shoots per year on a sampling plot (240 shoots per square meter). If a species was found in several plant communities, we used in the analysis the data on dynamics of such species in a community where the species was most abundant. In total annual dynamics of 50 species were assessed. Two Carex species (Carex umbrosa and Carex sempervirens) and two Pedicularis species (Pedicularis comosa and Pedicularis caucasica) were assessed together as Carex spp. and Pedicularis spp, respectively. We opted for this because these species pairs are difficult to distinguish in the field when they do not have reproductive shoots.
Although shoot number might respond to variations in climate differently than biomass of separate shoots if assessed over a short time period (34), because larger number of shoots per area might lead to smaller-sized shoots in case of a resource tradeoff, generally for herbaceous plant species, shoot numbers per area are well correlated with biomass per area (35). We verified this for our communities (see SI Text for details).
Plant traits measurements.
For all studied species, we measured the following functional traits: SLA (square centimeters per gram), leaf area (square centimeters), mass-based leaf and root nitrogen content (percentage of dry weight), specific root length (meters per gram), leaf thickness (millimeters), leaf dry matter content (milligrams per gram), leaf and root carbon content (percentage of dry weight), leaf and root C:N ratio, and seed mass (grams). The trait measurements were conducted following standardized protocols (12). Morphological traits were assessed in 10 replicates (i.e., individual plants), and chemical traits were assessed in 5 replicates. If a plant species under scrutiny was found in multiple communities, its traits were measured in the community where it was most abundant.
Data Analysis.
To test whether plant functional traits can be used to predict the relationship between plant species abundance and temperature, we conducted two-step statistical analysis. First, we assessed per-species annual abundance dynamic in relation to temperature. Subsequently, we tested whether per-species relation between annual abundance and temperature could be predicted by plant functional traits.
Step 1: Assessment of per-species annual abundance dynamic in relation to temperature.
For each plant species, we conducted a generalized linear model analysis with mean yearly shoot abundances as response variables, year and temperature as predictors, and autoregressive model of order 1 (AR-1) or autoregressive moving average model (ARMA) autocorrelation correction for sampling interdependency attributable to repeated measures at the same plots. All data were scaled, enabling direct comparison between species differing in abundance. Such analysis described in detail by Zuur et al. (36) allows separation of temperature versus year correlations with the shoot abundance and yields per-species regression coefficients for year and temperature (the latter coefficient hereafter referred to as “abundance–temperature slope”). Because all data were scaled, the abundance–temperature slopes and their CVs are directly comparable among species. Larger slopes reflect stronger relation between species abundance and temperature. Slope CVs characterize the confidence boundaries of the relationship. Larger slope CV reflects lower confidence.
To represent “temperature,” we tested mean temperature over four periods: June 1 of the previous year until June 1 of the current year, July 1 of the previous year until July 1 of the current year, June to August of the previous year, and the entire previous year. Abundance of distinct plant species was best predicted by different temperature predictors. However, to conduct interspecific analysis on predictive power of plant traits for relation between plant abundance and temperature, we needed to select a single temperature predictor to be used across all species (allowing, however, individual type of autocorrelation correction for each plant species). Based on Akaike Information Criterion (AIC), for all species, mean temperature from June 1 of the previous year until June 1 of the current year was either the best or very close to the best temperature predictor (ΔAIC < 3 for 66% of the plant species; for the other 34%, 3 < ΔAIC < 11). This period covers the entire growing season of the previous year (with some excess for snowbed communities, where growing season starts only in late July) and early spring of the current year. Mean temperature over such period was the best predictor of the plant abundance for the great majority of alpine species for a mountain grassland in North Bohemia (34). Indeed, the number of shoots of alpine plants is known to be determined by weather conditions of the previous year and early spring of the current year, because perennial alpine plants mostly form buds at the end of the growing season, whereas early spring is critical for bud break and shoot extension, as well as for germination and seedling recruitment in annual species and species with intensive seed propagation (18).
In contrast to temperature, precipitation pattern did not show any directional changes during the study period. However, to ensure that the relationship between temperature and per-species abundance was not affected by precipitation, we derived a number of precipitation-based predictors: amounts of precipitation calculated over the same periods as temperature periods; and we inspected Bayesian information criterions (BICs) of all possible models including combinations of any temperature-based predictor and any precipitation-based predictor. For all species, all such models yielded BICs higher than the BIC of the model, including only year and temperature over the period of June 1 of the previous year until June 1 of the current year. Therefore, we focused further testing on the relationship between species abundance and temperature.
Step 2: Test whether per-species abundance–temperature relationships could be predicted by plant traits.
To analyze whether the per-species relationships between abundance and temperature, derived at step 1, could be explained by functional traits, we performed weighted least-squares regressions with per-species abundance–temperature slope as the response value and per-species trait means as predictors. The data were weighted by the inverse of per-species CV of the abundance–temperature slope, allowing species with more significant abundance–temperature relationship to have larger weight in the analysis. For this analysis, we combined trait data of C. umbrosa and C. sempervirens and of P. comosa and P. caucasica, because in the assessment of annual dynamics, each of these species pairs was considered as one species (see under Plant abundance dynamic). Thus, we used mean trait values of these species pairs. Data used in this analysis are provided in Dataset S1.
All trait data were centered, scaled, and log10-transformed, the latter to comply with normality assumptions for analysis. We used BIC statistic for best-model selection (combination of predictors and their interactions). Only models including noncorrelated predictors (with variance inflation factor < 3) were considered. All analyses were conducted in R language and environment for statistical computing (R Foundation for Statistical Computing).
Supplementary Material
Acknowledgments
We thank all members of the Teberda expeditions during 1981–2009 for help with fieldwork. We thank Will Cornwell and James Weedon for discussions on statistical analysis. This work was supported by Russian Foundation for Basic Research Grant 11-04-01215 (to V.G.O.) and Netherlands Organization for Scientific Research Grant 047.018.003 (to J.H.C.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310700110/-/DCSupplemental.
References
- 1.Elmendorf SC, et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol Lett. 2012;15(2):164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 2.Elmendorf SC, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim Chang. 2012;2(6):453–457. [Google Scholar]
- 3.Walther GR, Beissner S, Burga CA. Trends in the upward shift of alpine plants. J Veg Sci. 2005;16(5):541–548. [Google Scholar]
- 4.Holzinger B, Hulber K, Camenisch M, Grabherr G. Changes in plant species richness over the last century in the eastern Swiss Alps: Elevational gradient, bedrock effects and migration rates. Plant Ecol. 2008;195(2):179–196. [Google Scholar]
- 5.Gottfried M, et al. Continent-wide response of mountain vegetation to climate change. Nat Clim Chang. 2012;2(2):111–115. [Google Scholar]
- 6.Pauli H, et al. Recent plant diversity changes on Europe’s mountain summits. Science. 2012;336(6079):353–355. doi: 10.1126/science.1219033. [DOI] [PubMed] [Google Scholar]
- 7.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15(4):365–377. doi: 10.1111/j.1461-0248.2011.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker MD, et al. Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci USA. 2006;103(5):1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Bodegom PM, et al. Going beyond limitations of plant functional types when predicting global ecosystem-atmosphere fluxes: Exploring the merits of traits-based approaches. Glob Ecol Biogeogr. 2012;21(6):625–636. [Google Scholar]
- 10.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct Ecol. 2002;16(5):545–556. [Google Scholar]
- 11.Kattge J, et al. TRY - a global database of plant traits. Glob Change Biol. 2011;17(9):2905–2935. [Google Scholar]
- 12.Perez-Harguindeguy N, et al. New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot. 2013;61(3):167–234. [Google Scholar]
- 13.Diaz S, et al. The plant traits that drive ecosystems: Evidence from three continents. J Veg Sci. 2004;15(3):295–304. [Google Scholar]
- 14.Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J Ecol. 2010;98(2):362–373. [Google Scholar]
- 15.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 16.Grime JP, et al. Integrated screening validates primary axes of specialisation in plants. Oikos. 1997;79(2):259–281. [Google Scholar]
- 17.Hodgson JG, et al. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann Bot (Lond) 2011;108(7):1337–1345. doi: 10.1093/aob/mcr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Körner C. Alpine Plant Life. Functional Plant Ecology of High Mountain Ecosystems. Berlin: Springer; 2003. [Google Scholar]
- 19.Welling P, Laine K. Regeneration by seeds in alpine meadow and heath vegetation in sub-arctic Finland. J Veg Sci. 2002;13(2):217–226. [Google Scholar]
- 20.Alsos IG, et al. Frequent long-distance plant colonization in the changing Arctic. Science. 2007;316(5831):1606–1609. doi: 10.1126/science.1139178. [DOI] [PubMed] [Google Scholar]
- 21.Akhmetzhanova AA, Onipchenko VG, El’kanova MK, Stogova AV, Tekeev DK. Changes in ecological morphological parameters of alpine plant leaves upon application of mineral nutrients. Biol Bull Rev. 2012;1(2):1–12. [Google Scholar]
- 22.Aerts R, Cornelissen JHC, van Logtestijn RSP, Callaghan TV. Climate change has only a minor impact on nutrient resorption parameters in a high-latitude peatland. Oecologia. 2007;151(1):132–139. doi: 10.1007/s00442-006-0575-0. [DOI] [PubMed] [Google Scholar]
- 23.Allen RG, Pereira LS, Raes D, Smith M. Crop Evapotranspiration - Guidelines for Computing Crop Water Requirements, FAO Irrigation and Drainage. Rome, Italy: Food and Agriculture Organization of the United Nations; 1998. [Google Scholar]
- 24.DeLucia EH, Maherali H, Carey EV. Climate-driven changes in biomass allocation in pines. Glob Change Biol. 2000;6(5):587–593. [Google Scholar]
- 25.Reich PB, Wright IJ, Lusk CH. Predicting leaf physiology from simple plant and climate attributes: A global GLOPNET analysis. Ecol Appl. 2007;17(7):1982–1988. doi: 10.1890/06-1803.1. [DOI] [PubMed] [Google Scholar]
- 26.Grime JP. Plant Strategies, Vegetation Processes, and Ecosystem Properties. 2nd Ed. Chichester, UK: John Wiley & Sons; 2006. p. 456. [Google Scholar]
- 27.Vendramini F, et al. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytol. 2002;154(1):147–157. [Google Scholar]
- 28.Freschet GT, Aerts R, Cornelissen JHC. A plant economics spectrum of litter decomposability. Funct Ecol. 2012;26(1):56–65. [Google Scholar]
- 29.Cornelissen JHC, et al. M.O.L. Team Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett. 2007;10(7):619–627. doi: 10.1111/j.1461-0248.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 30.Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. Responses of arctic tundra to experimental and observed changes in climate. Ecology. 1995;76(3):694–711. [Google Scholar]
- 31.Namba T. Competetive co-existence in a seasonally fluctuating environment. J Theor Biol. 1984;111(2):369–386. [Google Scholar]
- 32.Speed JDM, Austrheim G, Hester AJ, Mysterud A. Elevational advance of alpine plant communities is buffered by herbivory. J Veg Sci. 2012;23(4):617–625. [Google Scholar]
- 33.Scherrer D, Schmid S, Körner C. Elevational species shifts in a warmer climate are overestimated when based on weather station data. Int J Biometeorol. 2011;55(4):645–654. doi: 10.1007/s00484-010-0364-7. [DOI] [PubMed] [Google Scholar]
- 34.Herben T, Krahulec F, Hadincova V, Pechackova S. Climatic variability and grassland community composition over 10 years - separating effects on module biomass and number of modules. Funct Ecol. 1995;9(5):767–773. [Google Scholar]
- 35.Bergamini A, Pauli D, Peintinger M, Schmid B. Relationships between productivity, number of shoots and number of species in bryophytes and vascular plants. J Ecol. 2001;89(6):920–929. [Google Scholar]
- 36.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York: Springer; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.