Significance
The current study provides evidence of early and persistent alterations in anxious behavior and amygdala function following the early-life stress of disorganized parental care. These changes in both brain and behavior are not diminished when the stressor is removed nor diminished with the development of prefrontal regulatory regions. The findings underscore the importance of early-life experiences on later development and highlight the need for early intervention for populations at-risk following early-life stressors.
Keywords: anxiety, emotion regulation, infralimbic cortex, c-fos, cross-species
Abstract
Relatively little is known about neurobiological changes attributable to early-life stressors (e.g., orphanage rearing), even though they have been associated with a heightened risk for later psychopathology. Human neuroimaging and animal studies provide complementary insights into the neural basis of problem behaviors following stress, but too often are limited by dissimilar experimental designs. The current mouse study manipulates the type and timing of a stressor to parallel the early-life stress experience of orphanage rearing, controlling for genetic and environmental confounds inherent in human studies. The results provide evidence of both early and persistent alterations in amygdala circuitry and function following early-life stress. These effects are not reversed when the stressor is removed nor diminished with the development of prefrontal regulation regions. These neural and behavioral findings are similar to our human findings in children adopted from orphanages abroad in that even following removal from the orphanage, the ability to suppress attention toward potentially threatening information in favor of goal-directed behavior was diminished relative to never-institutionalized children. Together, these findings highlight how early-life stress can lead to altered brain circuitry and emotion dysregulation that may increase the risk for psychopathology.
Early-childhood adversity (e.g., abuse, neglect) accounts for over 30% of all anxiety disorders (1) and is associated with later emotional and behavioral dysregulation (2–6). One form of early-life stress (ELS) in humans that has received significant attention is that of orphanage rearing (7–11). It is estimated that eight million children live in orphanages worldwide. Children adopted from these orphanages provide a unique opportunity to assess the effects of ELS with a discrete timing and offset (12, 13). However, it is unclear to what extent emotional and behavioral dysregulation reported in this population is the result of the orphanage experience of disorganized care or attributable to preexisting conditions (e.g., prenatal exposure to substances, maternal malnutrition, and/or congenital disorders) (12). Moreover, we know little about the long-term effects of such early-life experiences and whether they reverse after the stressor is removed. The current study examines these issues using a rodent model of ELS (14, 15) and an outcome measure that uniquely parallels human paradigms to test for immediate and long-term effects of stress across development while controlling for preexisting environmental and genetic factors.
To date, most animal studies of stress have either focused on the effects of adult stress or on how early stress impacts later adult brain and behavior. The findings have been mixed depending on the type and timing of the stressor and the specific task and age of testing. Adult-restraint stress leads to reversible decreases in dendritic arborization and volume in prefrontal and hippocampal brain regions (16–18) but lasting amygdaloid neuronal hypertrophy and anxiety-like behavior (19). In contrast, studies of ELS (20–22) (e.g., removal of the dam) show deficits in adult hippocampal-dependent memory but inconsistent effects on anxiety-like behaviors (15, 23, 24), with the specific type of deficit varying as a function of task and age of testing. For example, Raineki et al. showed that ELS leads to social behavioral deficits in preweaned rodents but later depressive-like symptoms in adolescent rodents (25). It, therefore, remains unclear to what extent these rodent studies of stress parallel human experiences and outcomes.
The current study manipulates the type and timing of a stressor and the specific task and age of testing to parallel ELS in humans reared in orphanages. Children who were adopted from orphanage care experienced impoverished caregiving (type of stressor: poor caregiver-to-child ratios, inconsistent caregiver and caregiving), which was temporally restricted to the early postnatal period (26) and ended at the moment of adoption by families.
To parallel the orphanage experience, we adapted a paradigm that limits the nesting material provided to the dams (see Methods and Fig. S1A for details), disrupting maternal care of their pups (14, 15, 27). To simulate the human condition of children being adopted within the first few years of life, the stressor was limited to the preweaning period of postnatal days (P) 2 to P21. In addition, we adapted a fear regulation task for the mouse that paralleled our prior human experiments of ELS (8), showing emotion dysregulation following orphanage rearing. Rodent experiments uniquely inform our understanding of human development. However, differences in regional brain development make it difficult to compare precise ages across species (28). The current mouse experiments were conducted during time periods that approximate the developmental stages of preadolescence, adolescence, and adulthood in humans based on prior comparative developmental studies linking rodent and human postnatal brain development (29–31).
Two basic predictions emerged from the human and animal literatures. The first was that that ELS would alter fear regulation, as measured by the ability to suppress fear responses in favor of goal oriented behavior. The second was that this behavioral pattern would be paralleled by heightened activity in the amygdala. Finally, we tested to what extent development of the infralimbic cortex, a region within the prefrontal cortex implicated in regulation of fear expression, would diminish these effects.
Results
Behavioral Results.
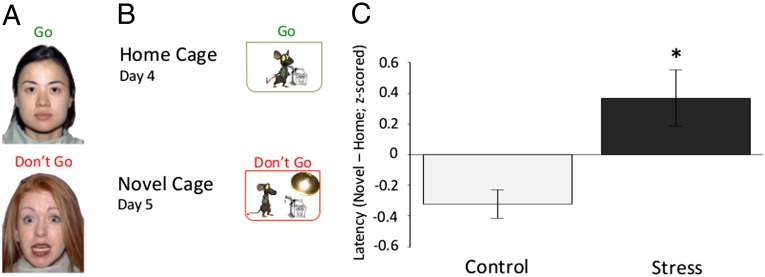
To investigate the effects of ELS in mice, we used an analog of our human emotional “go/no-go” task (8, 32) by modifying the novelty-induced hypophagia paradigm (33). To engage the mice in goal-oriented behavior, we trained the mice on where they could obtain sweetened condensed milk (i.e., a nozzle) in their home cage over the course of 3 d. On the fourth day, we recorded the time it took mice to approach and drink the milk from the nozzle in their home cage (e.g., baseline). On the fifth day, we recorded the time it took mice to approach and drink the milk from a nozzle placed in a barren novel cage devoid of odor cues and illuminated by bright light, a context of potential threat for rodents. The primary measure of interest was the response latency of the mouse to approach the nozzle for milk while anticipating potential threat in the well-lit novel cage. We tested male mice raised under the stressful or standard laboratory conditions, at ages that approximated preadolescence, adolescence, and early adulthood (30, 31, 34) (P26 to P27, n = 32; P33 to P34, n = 29; P67 to P68, n = 31; Fig. 1B). A three-factor (experimental group, age, condition) mixed-effects model was used to test for main effects of age or experimental group on response latencies to approach the sweetened milk in different environments. There was no main effect of age on response latencies (F = 1.56; P = 0.11). Compared with their standard reared counterparts, stressed mice of all ages (see Fig. S2 for specific ages) had longer approach times in the potential threat condition (novel cage) compared with baseline [home cage (Fig. 1C); F = 3.29; P = 0.001]. A similar effect of experimental group on approach latency was observed in female mice (Fig. S3; F = 2.90; P < 0.01)]. These results suggests that ELS impacts the ability to suppress fear responses in favor of goal oriented behavior and that these effects persist into adulthood.
Fig. 1.
Behavioral paradigm and results. (A) Human paradigm. Subjects were instructed to detect frequently presented neutral targets embedded among rare threat nontarget cues. (B) Mouse paradigm. Mice were trained where to obtain sweetened milk in their home cage for 3 consecutive days, and then latency to approach the milk was measured in the home cage on the fourth day and in an odorless, brightly lit novel cage on the fifth day. (C) Behavioral results. The difference in time that control and stressed mice take across development to approach a cue in a novel cage compared with their home cage (F = 3.40; P < 0.01; nc = 49; nels = 43). Data are z-scored and expressed as means ± SEM.
Neural Correlates.
To investigate the neural correlates of the observed stress effects, we examined neural activity in two regions: (i) the basolateral amygdala (BLA), a region shown earlier to be sensitive to ELS (7, 8, 35); and (ii) the infralimbic cortex (IL), a region implicated in regulating fear (36) across development (37) and for which deep brain stimulation has been shown to decrease approach latency to appetitive cues in novel open fields (38, 39). Neuronal activity has been shown to correlate with the expression of the early immediate gene, c-fos (40, 41). Further, c-Fos expression in the BLA is selectively down-regulated by both acute benzodiazepine and chronic antidepressant treatment in rats performing similar behavioral paradigms (42), suggesting that c-Fos is a reliable marker for activity in circuits involved in anxiety-related phenomena. We used immunohistochemical techniques to measure c-Fos expression in the amygdala 90 min after the 10-min epoch spent approaching and avoiding the milk in the threatening context (i.e., the novel cage) of the novelty-induced hypophagia paradigm (43).
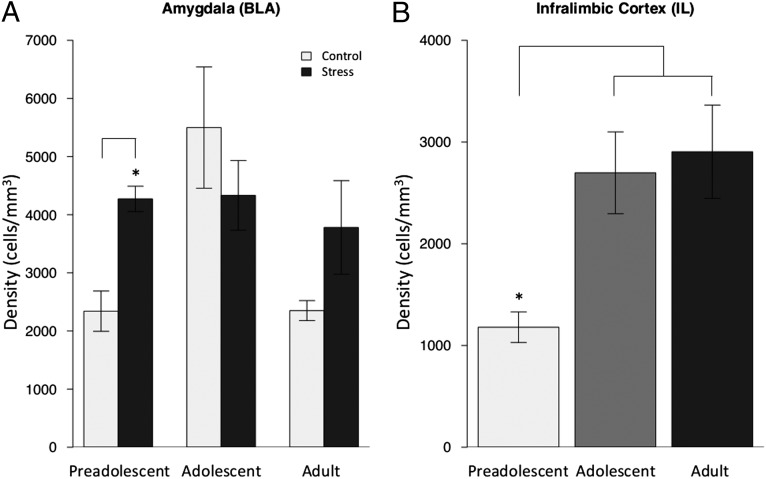
Two-factor (experimental group, age) mixed-effects models showed that ELS mice had persistently elevated levels of c-Fos in the BLA across development relative to control animals (Fig. 2A; F = 2.13; P = 0.04). c-Fos expression in the IL increased across development (F = 2.466; P = 0.02; Fig. 2B), with no main effect of experimental group (F = 0.164; P = 0.87; Fig. 2B and Fig. S4B).
Fig. 2.
c-Fos activity by group and age. (A) The density of c-Fos protein in the amygdala following exposure to the threatening context (i.e., novel cage) was elevated in ELS mice across development, similar to adolescent controls. The age by experimental group interaction [F = 2.132; P < 0.05; n = 5–8 mice per experimental group (10–15 slices per animal)] shows the most robust differences were between the preadolescent ELS and control groups (t = 4.71; P < 0.001). Data are expressed as means ± SEM. (B) The density of c-Fos protein in the IL increases with age [F = 2.466; P < 0.05; n = 3–8 mice per experimental group (10–15 slices per animal)]. All data expressed as means ± SEM.
To test the specificity of c-Fos expression, measurements were made in ventral cornu ammonis 1 (vCA1), a region highly networked with the BLA and IL and sensitive to stress that also undergoes high rates of change during the early postnatal period (29), and in the nonlimbic region of the paraventricular thalamus, a region that is more developed at birth (44). c-Fos expression was equivalent across experimental groups in both vCA1 (Fig. S5A; t = 0.38; P = 0.71) and the paraventricular nucleus of the thalamus (Fig. S5B; t = 0.21; P = 0.83).
Mouse and Human Parallels.
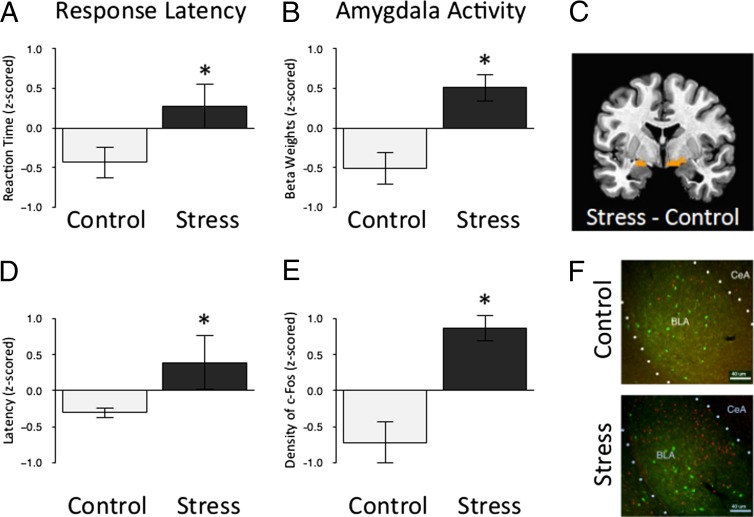
To compare the current results in the mouse with the previous data collected from humans adopted from orphanages abroad (8), we reanalyzed the largest subset of data, the preadolescent group (ages 5–10 y; n = 26; Table S1). In the human paradigm, children were instructed to “go” to a neutral cue, and “not go” to a rare threat cue (Fig. 1A). The dependent variable of interest was the response latency to approach recurring stimuli in the context of knowing that a rare aversive stimulus may occur at anytime. This aspect of the task, in part, parallels the mouse approaching the nozzle for milk while anticipating potential threat in the well-lit novel cage. In both tasks, the mouse and human have to ignore a potential threat in the environment to engage in goal-directed behavior. Independent samples t tests showed that ELS-exposed preadolescent humans (Fig. 3A; t = 2.08; P < 0.05) and mice (Fig. 3D; t = 2.06; P < 0.05) took longer to approach a cue when anticipating a potential threat. Blood oxygen level-dependent (BOLD) activity, shown to be correlated with neuronal activity (45, 46), was examined in frontolimbic regions including the amygdala, ventromedial prefrontal cortex, and hippocampus. Only activity in the amygdala differentiated the ELS-exposed children from the comparison group in the context of anticipating an aversive stimulus (Fig. 3B; F = 4.43; P < 0.05), similar to the higher c-Fos expression observed in preadolescent mice (Fig. 3E; t = 4.71; P < 0.001) following exposure to the threatening context (see Fig. 3 C and F for images). Ventromedial prefrontal activity was diminished in the ELS-exposed children but did not significantly differ from non–ELS-exposed children (Fig. S4; F = 0.14; P > 0.05).
Fig. 3.
Greater amygdala activity in humans and mice following ELS. (A) Stressed preadolescent humans take longer than their standard reared counterparts to detect frequently presented neutral targets embedded among rare threat nontarget cues that they were instructed to ignore (t = 2.08; P < 0.05; nc = 10; nels = 16). Data are z-scored and expressed as means ± SEM. (B) Parameter estimates of amygdala activity in response to the threat cue (i.e., fearful face) were greater in stressed preadolescent humans than their standard-reared counterparts (F = 4.43; P < 0.05; nc = 10; nels = 16). (C) Bilateral region of the amygdala identified as more reactive to threat (i.e., fear face stimuli) in stressed preadolescent humans than their standard reared counterparts. (D) The difference in time that control and stressed preadolescent mice take to approach a cue in a novel cage compared with their home cage (F = 2.06; P < 0.05; nc = 18; nels = 14). Data are z-scored and expressed as means ± SEM. (E) The density of c-Fos protein in the amygdala following exposure to the threatening context (i.e., novel cage) was greater in stressed preadolescent mice than their standard-reared counterparts [t = 4.71; P < 0.001; nc = 6; nels = 5 (10–15 slices per animal)]. Data are z-scored and expressed as means ± SEM. (F) An individual slice cut through the amygdala taken from each mouse was stained for c-Fos (red) and PVA (green) and used for quantification of c-Fos following exposure to the threatening context, clustered by experimental group and at 10x magnification.
Discussion
Our mouse and human studies, together, provide converging evidence for alterations in amygdala development and function following ELS. The mouse experiments mimic human conditions of ELS while uniquely controlling for environmental and genetic confounds in human studies to isolate effects of stress on brain and behavior. As such, our results strengthen previous human findings (7, 8, 47) that the disorganized and noncontingent care of the orphanage experience can alter emotional and behavioral regulation regardless of preexisting conditions (e.g., prenatal exposure to substances, maternal malnutrition, and/or congenital disorders).
The results provide evidence of both early and persistent alterations in amygdala circuitry and function following ELS in rodents. These effects do not appear to be reversed when the stressor is removed nor diminished with the development of prefrontal emotion regulation regions. Specifically, group differences in fear regulation and amygdala activity persist into adulthood even though regulatory regions in the prefrontal cortex showed increased activity with age for both stressed and nonstressed groups. This finding is similar to human findings in children adopted from orphanages abroad. Specifically, their ability to suppress attention toward potentially threatening information in favor of goal-directed behavior is diminished relative to never-institutionalized children even after the stressor is removed (7, 8) and paralleled by heightened amygdala activity (7, 8, 47).
Our findings are similar to recent rodent stress studies (25) in showing that ELS [e.g., reduced bedding (23)] can lead to early and/or persistent alterations in behavior well into adulthood (15). There are differences in the type and duration of stress effects, however. These dissimilarities may stem, in part, from differences in the behavior measured and ages tested. Such mixed effects are consistent with epidemiological studies showing heterogeneous effects of different forms of ELS on psychopathology, with depression and anxiety disorders as common outcomes and resilience in others (2, 5, 48). Importantly, however, our results show translation of early chronic stress effects across species when using parallel stress manipulations and outcome measures, thus providing further translatability and validity of rodent to human findings.
Whether the effects of ELS are ameliorated in humans following adoption is unclear. Few human studies follow children adopted from orphanages beyond adolescence. What appears to be more evident from these studies is that the earlier the child is adopted, the better the outcome (7, 26, 49). This effect may be attributable to a sensitive period for emotional development (50) such that beyond this window, the underlying circuitry is less plastic or more resistant to change. The current study cannot specifically address this issue because although the early-life stressor was removed after P21 and the pups were housed with their littermates (as opposed to single-caged), any caged living conditions for mice lack ethological validity. Nonetheless, the current study provides a model for future experiments that could examine specific timing and duration of ELS and for testing potential pharmacologic or behavioral interventions.
Together, these findings suggest that ELS results in both early and persistent alterations in amygdala development, circuitry, and function. Even after the removal of the stressor, the development of prefrontal regulatory regions is not enough to dampen fearful behavior. These results parallel human findings of ELS effects and underscore the importance of translational approaches that may explain the increased risk of psychopathology in such populations, which call for early identification and intervention for these children.
Methods
Mouse.
Animals.
Male C57BL6/J mice were used for all experiments (see Fig. S3 for similar results in females). Breeding pairs of C57BL6/J wild-type mice from Charles River were set up in the colony and monitored daily to reduce developmentally sensitive shipping-induced stress effects. Litters were culled to six pups and assigned to early stress or control conditions (described below) at P2 and weaned at P21. Mice had ad libitum access to food and water in a temperature- and humidity-controlled vivarium maintained on a 12-h reverse light/dark cycle. All procedures regarding animal care and treatment were approved and in compliance with guidelines established by the Institutional Animal Care and Use Committee of the Weill Cornell Medical College and the National Institutes of Health.
ELS.
On P2, litters were transferred with their dam to either a standard laboratory cage with bedding and two square nestlets or to a cage with a false mesh metal bottom resting above the bedding and one square nestlet. Cages were changed on P9 and P16, before all pups were weaned at P21 and transferred to standard laboratory cages with bedding and two nestlets. Between P2 and P9, litters were digitally recorded between 1800 hours and 0600 hours for a minimum of 3 nights to confirm validity of the ELS model in mice (Fig. S1A). Pups were weighed at P2, P9, P21, P29, and P63, with mice used for preadolescent and adolescent experiments sampled only for the first three or four time points listed, respectively (Fig. S1B).
Behavioral paradigm.
Before testing, mice were housed two to five per cage and received 3 consecutive days of training (day 1–3) in a dark room (∼5 lx). Training consisted of presenting mice with a standard dual bearing sipper tube (6-ounce bottle) inserted between the wire bars of the cage roof and containing undiluted sweetened condensed milk. On day 4, mice underwent home cage testing. Before testing, mice were placed in a novel cage containing some home cage nesting material for 30 min. For testing, the setup was identical to that used in training, with a camera focused on the cage to record each trial. For each trial, a mouse was removed from their holding cage and placed into their home cage. The trial began as soon as the cage top containing the sipper bottle was placed back on the home cage base. The latency to drink and the amount of time spent drinking was recorded with a stopwatch over a 10-min period. Following completion of the 10-min trial, each subsequent animal was rotated into the home cage and tested in the same manner. On day 5, novel cage testing was conducted by placing a single mouse into a clean cage of the same dimensions as its home cage, wiped down with 70% ethanol (700mL/L) to eliminate odors, with no shavings and under bright light conditions (∼4,000 lx). Mice were again presented with a sipper tube containing undiluted sweetened milk, and the latency to drink and duration of drinking were recorded. Our measure of interest was the difference in time that it took a mouse to drink from the nozzle in the novel cage vs. the home cage. Ninety-seven male mice were used for behavioral testing at three developmental time points [preadolescent: 18 control (C), 14 ELS; adolescent: 19 C, 13 ELS; adult: 16 C, 17 ELS]. We used all male mice for our experiments, because during pilot experiments, we did not observe a difference in behavioral effects among female mice (Fig. S3; F = 2.9; P = 0.01; six C and six ELS).
c-Fos immunohistochemistry.
All experiments were carried out at room temperature unless otherwise specified. Ninety minutes after exposure to experimental factors, the mice were killed by i.p. injection of Sleepaway (Fort Dodge) and perfused transcardially with 30 mL of saline, followed by 90 mL of 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.4) at a flow rate of 30 mL/min. Brains were removed and postfixed overnight in 4% paraformaldehyde before transfer to 30% sucrose in 0.1 M sodium phosphate (pH 7.4) for 48 h at 4 °C. Brains were frozen in powdered dry ice and stored at −20 °C until sectioning. Coronal sections (40 μm) of whole brain were cut by using a sliding microtome frozen by powdered dry ice. Six sets of serial sections were collected in Eppendorf tubes each containing 2 mL of cryoprotectant (30% glycerol and 30% ethylene glycol in 0.1 M sodium phosphate; pH 7.4) and stored at −20 °C. Free-floating serial sections (every third section) were washed (three times for 10 min each) in Tris-buffered saline (TBS), incubated for 30 min in a blocking solution containing 4% normal horse serum and 1% BSA in TBS with 0.2% Triton X-100 (TBS-Tx), and incubated overnight at 4 °C with rabbit anti–c-Fos primary antibody (c-Fos sc-52; Santa Cruz Biotechnology; sc-52 antibody was raised against amino acids 3–16 of human c-Fos: SGFNADYEASSSRC) diluted 1:1,000 mixed with goat anti-parvalbumin (1:2,000; Swant) in the blocking solution mentioned above. Sections were then washed in TBS and incubated for 2 h with Alexa Fluor-labeled donkey anti-rabbit and anti-goat IgG secondary antibodies (Alexa Fluor 555 and 488) diluted 1:500 in TBS-Tx. Sections were again washed, mounted on chrome-alum/gelatin-coated slides, and air-dried for 2 h in the dark. Slides were cover-slipped by water-soluble glycerol-based mounting medium containing DAPI and sealed with nail polish. Estimation of cell density of c-Fos–positive neurons in prelimbic and infralimbic cortices was performed with StereoInvestigator 9.0 (Microbrightfield). Briefly, serial sections (every third section; 120 μm) were numbered by rostra-caudal order, and contours of PL, IL BLA, PVT, and ventral hippocampus were traced by referring to the Allen Brain Map (Allen Co.). To identify the boundary of prefrontal cortex, we used DAPI-counterstained sections, whereas in the amygdala, thalamus, and hippocampus, we used parvalbumin-counterstained sections combined with DAPI to yield a clear boundary for subnuclei or layers. All cells across all sections per animal were counted. Individual cell density was calculated for each mouse by dividing the total sampled cell numbers by the total volume of the region. Eleven preadolescent mice (6 C, 5 ELS) were used for measuring c-Fos protein levels in amygdala and IL/PL. Twelve mice (6 C, 6 ELS) were used for measuring c-Fos protein levels in ventral hippocampus and PVT.
Human.
Subjects.
Twenty-six children less than 11 y of age were included in the current analyses from a larger sample of 44 [see Tottenham et al. (8) for details on the full sample]. This sample included 16 previously orphanage-reared (mean age, 8.5 y old; 13 female; mean time in orphanage care, 15.3 mo; SD = 10) and 10 never-institutionalized children (9.2 y old; 7 female; see demographics in Table S1).
Behavioral paradigm.
Children completed an emotional face go/no-go task [Hare et al. (32)] while in the MRI scanner. The event-related task required pressing a button (go condition) when target facial expressions appeared and inhibiting this behavioral response when distracter (no-go condition) facial expressions appeared. Face stimuli (51) were presented singly with a fixed random order and an average interstimulus jitter of 5 s (range: 2.5–10 s). Children were instructed to execute the Go response quickly for the named target expression (e.g., “neutral”), which was presented frequently (70% of the trials), while inhibiting the response when the distracter expression (e.g., “fear”) appeared, which was presented infrequently (30% of the trials). The target facial expression changed with each run. There were two conditions separated by run, each presented twice—fear faces as the target with neutral faces as the distracter and neutral faces as the target with fearful faces as the distracter—resulting in 140 target trials (70 fearful, 70 neutral) and 52 distracter trials (26 fearful, 26 neutral). The order of runs was counterbalanced across participants. Each face stimulus was presented for 500 ms, and participants were allowed 1,500 ms to respond by pressing a button with their index finger.
Statistics.
All statistical analyses were conducted using R (release 2.15.2). When two means were compared, Student’s unpaired t test was used to calculate statistical significance. For multiple comparisons, data were analyzed using linear mixed effects models with Bonferroni post hoc t tests used to determine statistical significance between groups.
Supplementary Material
Acknowledgments
This work was supported, in part, by Grants P50 MH079513 (to B.J.C. and F.S.L.), R01 MH73175 (to B.J.C.), and T32 HD055177-01A2 (to M.M.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310163110/-/DCSupplemental.
References
- 1.Merikangas KR, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green JG, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: A meta-analysis. JAMA. 2005;293(20):2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- 4.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology (Berl) 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health Committee on Early Childhood, Adoption, and Dependent Care Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 6.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 7.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tottenham N, et al. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumsta R, et al. III. Deprivation-specific psychological patterns. Monogr Soc Res Child Dev. 2010;75(1):48–78. doi: 10.1111/j.1540-5834.2010.00550.x. [DOI] [PubMed] [Google Scholar]
- 11.Kreppner J, et al. IV. Developmental course of deprivation-specific psychological patterns: Early manifestations, persistence to age 15, and clinical features. Monogr Soc Res Child Dev. 2010;75(1):79–101. doi: 10.1111/j.1540-5834.2010.00551.x. [DOI] [PubMed] [Google Scholar]
- 12.Tottenham N. Risk and developmental heterogeneity in previously institutionalized children. J Adolesc Health. 2012;51(2 Suppl):S29–S33. doi: 10.1016/j.jadohealth.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutter M, O’Connor TG. English and Romanian Adoptees (ERA) Study Team Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Dev Psychol. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15(2):114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149(10):4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31(1):3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Stevens HE, Leckman JF, Coplan JD, Suomi SJ. Risk and resilience: Early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):114–127. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- 22.Cui M, et al. Enriched environment experience overcomes the memory deficits and depressive-like behavior induced by early life stress. Neurosci Lett. 2006;404(1-2):208–212. doi: 10.1016/j.neulet.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 23.Dalle Molle R, et al. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: Relationship to peripheral BDNF levels. Transcult Psychiatry. 2012;2:e195. doi: 10.1038/tp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackowski A, et al. Early-life stress, corpus callosum development, hippocampal volumetrics, and anxious behavior in male nonhuman primates. Psychiatry Res. 2011;192(1):37–44. doi: 10.1016/j.pscychresns.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: Research and policy. Dev Psychopathol. 2000;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- 27.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15(5-6):341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25(10):518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 31.Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35(8):1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hare TA, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 34.Kolb B, et al. Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pattwell SS, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci USA. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamani C, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67(2):117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16(8):486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29(3):261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 42.Bechtholt AJ, Valentino RJ, Lucki I. Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology. 2008;33(9):2117–2130. doi: 10.1038/sj.npp.1301616. [DOI] [PubMed] [Google Scholar]
- 43.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 44.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268(5217):1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 45.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 46.Lee JH, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465(7299):788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gee DG, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLaughlin KA, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: Associations with persistence of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):124–132. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutter M. English and Romanian Adoptees (ERA) Study Team Developmental catch-up, and deficit, following adoption after severe global early privation. J Child Psychol Psychiatry. 1998;39(4):465–476. [PubMed] [Google Scholar]
- 50.Sabatini MJ, et al. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27(12):3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tottenham N, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.