Significance
Natural killer (NK) cells undergo a licensing (or education) process through interactions with their MHC class I-specific receptors and self-MHC class I to become functionally competent. Previously it was not possible to study the acute consequences of these interactions. We developed transgenic mice to induce MHC class I acutely in an otherwise MHC class I-deficient environment. In these mice, acute NK cell licensing is dependent on interactions between NK-cell receptors and other hematopoietic cells; the acute process did not appear to induce detectable differences in the licensed NK cells themselves.
Keywords: tolerance, immunity, lymphocytes
Abstract
Mouse natural killer (NK) cells acquire effector function by an education process termed “licensing” mediated by inhibitory Ly49 receptors which recognize self-MHC class I. Ly49 receptors can bind to MHC class I on targets (in trans) and also to MHC class I on the NK-cell surface (in cis). Which of these interactions regulates NK-cell licensing is not yet clear. Moreover, there are no clear phenotypic differences between licensed and unlicensed NK cells, perhaps because of the previously limited ability to study NK cells with synchronized licensing. Here, we produced MHC class I-deficient mice with inducible MHC class I consisting of a single-chain trimer (SCT), ovalbumin peptide-β2 microgloblin-H2Kb (SCT-Kb). Only NK cells with a Ly49 receptor with specificity for SCT-Kb were licensed after MHC class I induction. NK cells were localized consistently in red pulp of the spleen during induced NK-cell licensing, and there were no differences in maturation or activation markers on recently licensed NK cells. Although MHC class I-deficient NK cells were licensed in hosts following SCT-Kb induction, NK cells were not licensed after induced SCT-Kb expression on NK cells themselves in MHC class I-deficient hosts. Furthermore, hematopoietic cells with induced SCT-Kb licensed NK cells more efficiently than stromal cells. These data indicate that trans interaction with MHC class I on hematopoietic cells regulates NK-cell licensing, which is not associated with other obvious phenotypic changes.
The ability of natural killer (NK) cells to be activated by their targets is controlled by their activation and inhibitory receptors (1, 2). Activation receptors can recognize ligands expressed on their targets, triggering NK cells if their inhibitory receptors are not engaged. In mice, most Ly49 molecules are inhibitory receptors that recognize MHC class I molecules on target cells (3). Engagement of Ly49 receptors by MHC class I leads to signals that block NK-cell triggering during effector responses. These receptors explain the “missing-self” hypothesis, which postulates that NK cells survey tissues for normal levels of the ubiquitously expressed MHC class I molecule (4). In the absence of MHC class I, inhibition is released, and NK cells then can be activated through their activation receptors to kill and produce cytokines. Thus, the NK-cell inhibitory receptors control NK-cell responses at the effector level.
NK cells in MHC class I-deficient mice, such as β2-microglobulin (β2m)−/− and TAP−/− mice, do not receive inhibitory signals and should be overactive (5–7). Instead, however, NK cells from these animals are hyporesponsive to activation receptor cross-linking. Moreover, NK cells apparently lacking known MHC-specific receptors also are hyporesponsive (8). Previously, we reported that the NK-cell inhibitory receptors have a second function that can explain these paradoxical findings (7). Through recognition of self-MHC class I via their inhibitory MHC class I-specific receptors, NK cells become licensed (or educated), resulting in the functional competence of their activation receptors. For example, in C57BL/6 (H2b) mice, Ly49C+ NK cells are licensed by recognition of self-H2Kb and secrete more IFN-γ after NK1.1 stimulation than do Ly49C− NK cells. In contrast, IFN-γ production is comparable in Ly49A+ and Ly49A− NK cells, indicating that Ly49A+ NK cells are not licensed in C57BL/6 mice because Ly49A does not recognize an H2b class I allele. On the other hand, Ly49A+ NK cells are specifically licensed in mice expressing its MHC class I ligand, H2Dd, as self-MHC. Thus, the recognition of self-MHC class I leads to enhanced function of NK-cell activation receptors, and this enhancement is influenced by self-MHC–specific Ly49 receptors in mice.
Other studies suggest that a similar process occurs for human NK cells that preferentially express killer Ig-like receptors (KIRs) for HLA class I recognition and effector inhibition. Although the mouse lectin-like Ly49 receptors and human KIRs are structurally distinct, they share many other features, supporting the concept that each species evolved a different genetic solution for the MHC class I-specific inhibitory receptors; that is, Ly49 receptors and KIRs now are considered to be outstanding examples of convergent evolution (9, 10). As with NK cells bearing self-MHC–specific Ly49 receptors in mice, human NK cells with self-HLA–specific KIRs display enhanced function (11–13). In contrast, NK cells from the same donor but without expression of self-HLA–specific KIRs show decreased function. Moreover, NK cells bearing the same KIR but without its cognate ligand in other donors do not display enhanced function. Thus, licensing (i.e., the education effects of self-MHC class I recognition by relevant NK-cell receptors) is now accepted as being operational in mice and humans (13).
Other than expression of self-MHC–specific Ly49 receptors or KIRs and enhanced capacity to signal through their activation receptors, licensed mouse and human NK cells are not clearly distinguishable from unlicensed NK cells (7, 11, 12). However, this apparent similarity could result from a continuous process in which small numbers of NK cells are educated and then join “older” NK cells, and major phenotypic changes during licensing may be transient. Therefore previous phenotypic studies of licensed NK cells may have been limited by the inability to investigate NK cells in which licensing is synchronized.
Crystallographic studies revealed two potential interaction sites on MHC class I at which interactions with Ly49 receptors might take place: Site 1 is on the left side of the peptide-binding cleft (when viewed from above with the α1 helix shown on top); site 2 is below the peptide-binding cleft (14). Mutational studies demonstrated that the Ly49 receptors engage site 2 in trans to inhibit effector functions, because point mutations at site 2 abrogated the capacity of transfected MHC class I molecules to inhibit NK cells in an Ly49-dependent manner (15–17). Furthermore, we recently showed that these same residues are involved in conferring Ly49-dependent licensing effects because transgenic (Tg) expression of site 2-mutant MHC class I molecules failed to induce licensed NK cells, whereas wild-type MHC class I alleles allowed licensing (18). Thus, Ly49 receptors interact with the same site on their MHC class I ligands for effector inhibition and licensing, providing another link of these two processes.
Because NK cells also express MHC class I molecules, prior studies suggested that Ly49 receptors also can interact with their ligands on the same NK-cell surface, i.e., in cis as well as in trans with their target cell (19). Site 2 on MHC class I molecules is involved in both cis and trans interactions of Ly49 receptors with their MHC class I ligands. Studies of Tg mice expressing a mutated Ly49A receptor that cannot engage in in cis interactions but retains the ability to engage in in trans interactions showed that NK cells expressing mutated Ly49A were not licensed, suggesting that NK cells are licensed via cis interactions (20). However, the possibility that the mutated Ly49A disrupted other unknown signals or interactions required for NK-cell licensing cannot be ruled out completely. Moreover, we and others recently identified the importance of trans interactions in NK-cell licensing because unlicensed splenic β2m−/− NK cells acquired the licensed phenotype when adoptively transferred to wild-type hosts (21, 22). On the other hand, non–H2Dd-expressing Ly49A+ NK cells can acquire H2Dd through contact with H2Dd-expressing cells in an Ly49A-dependent manner in vitro and in vivo (23, 24), suggesting that the transferred NK cells could have acquired host MHC class I, allowing potential interactions with MHC class I in cis as well as in trans. Thus, it remains unclear whether cis or trans interactions are required for NK-cell licensing.
Previously, we used mice in which an MHC class I single-chain trimer (SCT) consisting of ovalbumin (OVA) peptide (SIINFEKL), β2m, and H2Kb heavy chain (SCT-Kb) is expressed in an MHC class I-deficient environment (7). The SCT-Kb molecule is specifically and solely recognized by Ly49C when stably expressed as a transgene in C57BL/6 mice, conferring the licensed phenotype to Ly49C+ NK cells. Here we were able to monitor Ly49C+ NK cells after acute induction of SCT-Kb and found that unlicensed Ly49C+ NK cells became licensed without changes in NK-cell maturation or activation markers or localization in spleen. Furthermore, our data showed trans interaction with hematopoietic cells is required for NK-cell licensing, but cis interaction is not.
Results
Generation of Inducible MHC Class I (SCT-Kb) Mice.
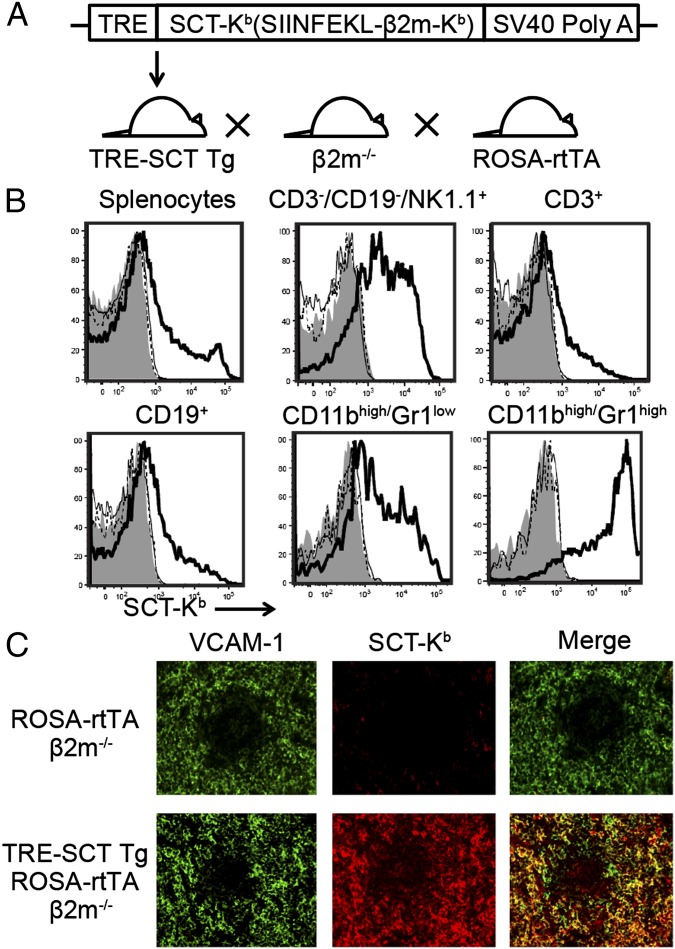
The SCT-Kb construct was modified to produce a more stable SCT-Kb in which the SIINFEKL peptide sequence was covalently linked to the α1 helix of Kb (25, 26). Tetramers of this SCT-Kb molecule bound specifically to Ly49C+ NK cells. The SCT-Kb construct was placed in a cassette for tetracycline-response element (TRE) control of expression [TRE-SCT-Kb-SV40 poly(A)] when the reverse tetracycline transactivator (rtTA) was expressed and doxycycline was administered. The construct was microinjected to generate TRE-SCT Tg mice (Fig. 1A). Then, the mice were crossed with β2m−/− mice and Rosa26-rtTA (ROSA-rtTA) mice, which express inactive rtTA under the control of the Rosa26 locus. Doxycycline then was administered to induce active rtTA and SCT-Kb expression in all cells in an MHC class I-deficient background. In experiments with (TRE-SCT Tg × ROSA-rtTA) β2m−/− mice administered doxycycline by the i.p. route, we found that SCT-Kb was detected acutely by the 25-D1.16 mAb, which reacts specifically with SIINFEKL bound to H2Kb. At 1 d after i.p. injection of doxycycline, all subsets of splenocytes, including NK cells, T cells, B cells, monocytes/macrophages, and granulocytes, expressed SCT-Kb (Fig. 1B). Consistent with variable tissue expression of reporter genes under control of the Rosa26 locus (27), SCT-Kb expression was variable. SCT-Kb expression on vascular cell adhesion protein 1 (VCAM-1)-positive splenic stromal cells also was confirmed by immunohistochemistry (Fig. 1C). Thus, doxycycline administration acutely induced SCT-Kb expression on a broad variety of cells in (TRE-SCT Tg × ROSA-rtTA) β2m−/− mice.
Fig. 1.
Inducible SCT-Kb mice. (A) A schematic figure of inducible SCT-Kb Tg mice. (B) At day 1 after i.p. injection of doxycycline or PBS, SCT-Kb expression on splenocyte subsets was assessed by flow cytometry in ROSA-rtTA β2m−/− mice treated with doxycycline (thin line), TRE-SCT Tg β2m−/− mice treated with doxycycline (dotted line), and (TRE-SCT Tg × ROSA-rtTA) β2m−/− mice treated with PBS (gray shading) and doxycycline (thick line). (C) Immunohistochemistry of spleen for SCT-Kb and VCAM-1 expression as indicated 1 d after i.p. injection of doxycycline. VCAM-1 is a marker of splenic stromal cells.
Unlicensed Ly49C+ β2m−/− NK Cells Become Licensed After SCT-Kb Induction.
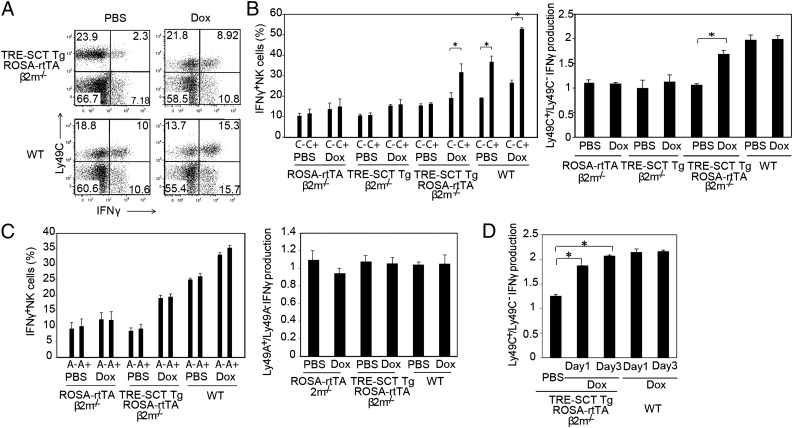
We next examined effect of SCT-Kb induction on NK-cell licensing in the β2m−/− background. We injected doxycycline into ROSA-rtTA β2m−/−, TRE-SCT Tg β2m−/−, (TRE-SCT Tg × ROSA-rtTA) β2m−/−, and wild-type mice on days 0 and 2, and at day 3 splenocytes were stimulated by anti-NK1.1 cross-linking. Upon SCT-Kb induction, Ly49C+ NK cells produced significantly more IFN-γ than did Ly49C− NK cells (Fig. 2 A and B), whereas IFN-γ production by Ly49A+ NK cells and Ly49A− NK cells was unchanged (Fig. 2C). The licensing ratio for a given Ly49 receptor was determined by assessing the percentage of IFN-γ–producing cells in receptor-positive and receptor-negative NK-cell populations. Accordingly, the licensing ratio for Ly49C but not for Ly49A was increased significantly by SCT-Kb induction (Fig. 2 B and C). Similar results were obtained with (TRE-SCT Tg × ROSA-rtTA) β2m−/−;Kb−/−;Db−/− mice. A partial licensing effect on Ly49C+ NK cells was observed even on day 1 of doxycycline treatment (Fig. 2D). Unlicensed splenic β2m−/− NK cells become licensed when adoptively transferred to wild-type hosts (21, 22). However, what happened in the spleen to licensed NK cells, regarding NK cell localization, was not studied, and 3 or 4 d were required for β2m−/− NK cells to acquire the licensed phenotype in a wild-type host. During the acute induction of NK-cell licensing studied here, almost all the NK cells remained in the red pulp (Fig. S1). Collectively, these data suggested that induced SCT-Kb specifically licensed Ly49C+ β2m−/− NK cells, but not Ly49A+ β2m−/− NK cells, within 1 d and without major change in splenic localization.
Fig. 2.
SCT-Kb induction specifically licensed Ly49C+ NK cells but not Ly49A+ NK cells. Doxycycline or PBS was i.p. injected into the indicated mice on days 1 and 3. Splenocytes were stimulated with plate-bound anti-NK1.1 antibody and analyzed for IFN-γ production by CD3−/CD19−/NKp46+ cells. (A) Representative dot plots demonstrating IFN-γ production by Ly49C+ or Ly49C− NK cells. Data are representative of three independent experiments. (B and C) Frequency of IFN-γ–producing NK cells in the Ly49C+ and Ly49C− populations (B, Left) and Ly49A+ and Ly49A− populations (C, Left) and the ratio of IFN-γ–producing Ly49C+ NK cells/Ly49C− NK cells (B, Right) and IFN-γ–producing Ly49A+ NK cells/Ly49A− NK cells (C, Right). (D) The ratio of IFN-γ–producing Ly49C+ NK cells to Ly49C− NK cells on days 1 and 3 of doxycycline treatment. Dox, doxycycline. Data in B–D are shown as mean ± SEM (n = 3). *P < 0.01 by unpaired Student t test.
Licensed NK-Cell Phenotype Is Not Altered After SCT Induction.
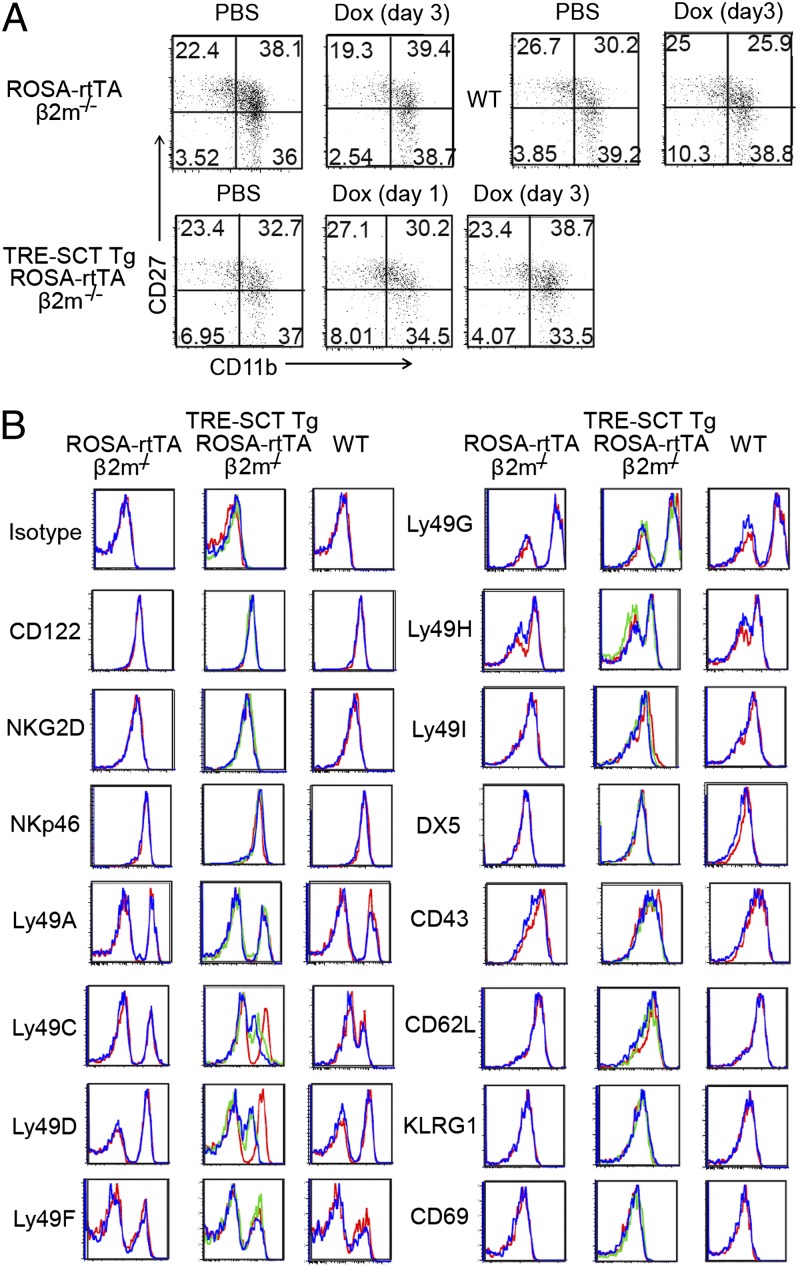
To investigate further the effects of SCT-Kb induction on NK-cell maturation, we next examined NK cells from ROSA-rtTA β2m−/−, (TRE-SCT Tg × ROSA-rtTA) β2m−/−, and wild-type mice for a comprehensive set of markers on day 3 after i.p. doxycycline injection. In particular, normal splenic NK cells undergo a process of maturation defined by CD27 and CD11b, from CD27highCD11blow to CD27highCD11bhigh and then to CD27lowCD11bhigh. Unlicensed β2m−/− NK cells express CD27, CD11b, and other maturation markers comparable to licensed wild-type NK cells, indicating that the licensed NK-cell phenotype is not normally accompanied by changes of NK-cell maturation or activation (7, 28, 29). Here, doxycycline treatment itself and SCT-Kb induction did not change the maturation status of NK cells as determined by CD27 and CD11b expression (Fig. 3A) and the expression of other markers such as CD122, NKG2D, NKp46, DX5, CD43, CD62L, KLRG1, and CD69 (Fig. 3B). Thus, doxycycline treatment and induction of SCT-Kb had minimal effect on NK-cell maturation and apparently did not activate NK cells.
Fig. 3.
NK-cell maturation is not enhanced after doxycycline treatment or SCT-Kb induction. The mice were i.p. injected with doxycycline on days 1 and 3. Markers of NK cell maturation were assessed by flow cytometry. (A) Representative dot plots of CD27 and CD11b expression on CD3−/CD19−/NK1.1+ cells. (B) Representative histograms depicting markers of NK-cell maturation. PBS treatment (red line) and doxycycline exposure on day 1 (green line) and day 3 (blue line) are shown. Data are representative of three independent experiments.
When we examined expression of Ly49 receptors, we surprisingly found that the Ly49D-activation receptor was down-regulated on SCT-Kb-expressing NK cells, but this down-regulation was not observed on wild-type NK cells. However, Ly49D expression did not affect the licensed phenotype by Ly49C+ NK cells after doxycycline treatment (Fig. S2). As expected, we found Ly49C expression was specifically down-regulated on NK cells from SCT-Kb–expressing and wild-type mice as compared with β2m−/− NK cells (Figs. 2A and 3B), as previously shown (7). However, there was no change in the expression of other inhibitory Ly49 receptors. Thus, there is evidence for a productive physical interaction between Ly49C and induced SCT-Kb, but otherwise there is no change in the phenotype of acutely licensed NK cells.
NK-Cell Licensing Requires trans Interaction with Hematopoietic Cells.
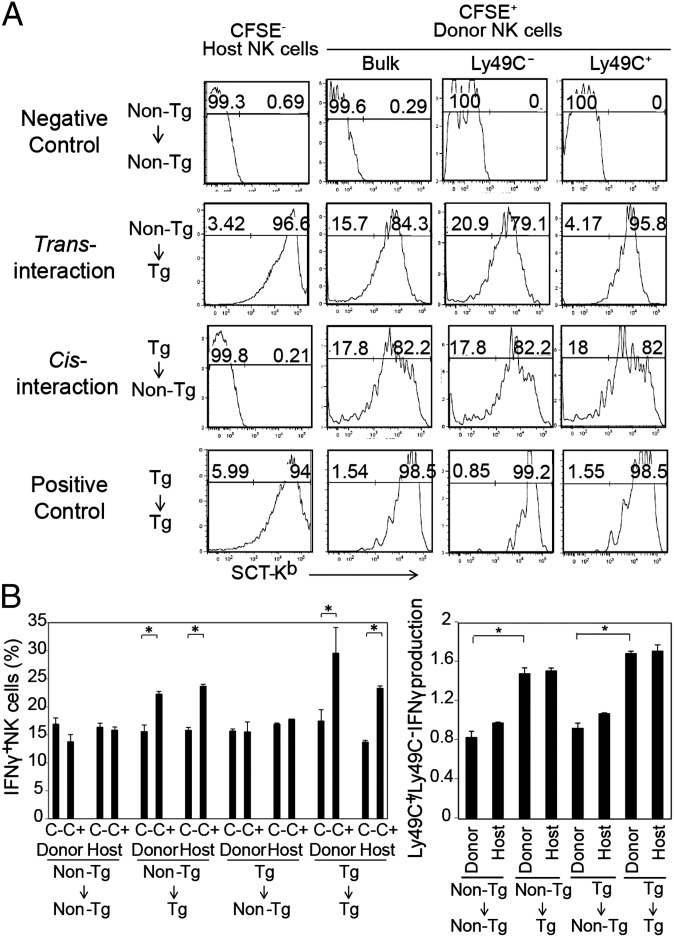
We next addressed whether only cis interaction or also trans interaction of Ly49 receptors and MHC class I licenses NK cells. We transferred carboxyfluorescein succinimidyl ester (CFSE)-labeled (TRE-SCT Tg × ROSA-rtTA) β2m−/− (denoted Tg) or ROSA-rtTA β2m−/− (denoted non-Tg) NK cells into non-Tg or Tg mice (Fig. 4). On day 7 and day 9 after transfer, doxycycline was injected i.p. into the mice, and on day 10 splenocytes were harvested to determine SCT-Kb expression and IFN-γ production after anti-NK1.1 cross-linking in host and recipient NK cells. Donor non-Tg NK cells were licensed in SCT-Kb–expressing hosts, whereas donor Tg NK cells were not licensed in non-Tg hosts (Fig. 4). Interestingly, SCT-Kb was detected on donor non-Tg NK cells in Tg hosts, apparently because of the acquisition of SCT-Kb as previously described (23, 24). However, unlike previous reports, SCT-Kb acquisition was independent of the relevant NK-cell receptor (Ly49C), because the donor Ly49C− β2m−/− NK cells expressed SCT-Kb as well as the donor Ly49C+ β2m−/− NK cells in the SCT-Kb–expressing host (Fig. 4A). In any case, expression of SCT-Kb on Ly49C+ NK cells was not associated with licensing, because donor Tg Ly49C+ NK cells were not licensed even though they expressed high levels of SCT-Kb comparable to those expressed by licensed non-Tg NK cells in the SCT-Kb–expressing host (Fig. 4). Taken together, these data indicate that the level of SCT-Kb expression on NK cells and hence cis interaction are not associated with NK-cell licensing, whereas NK cells having contact with MHC class I in trans are licensed.
Fig. 4.
Cis interaction is dispensable for NK-cell licensing. (A and B) ROSA-rtTA β2m−/− (non-Tg) NK cells or (TRE-SCT Tg × ROSA-rtTA) β2m−/− (Tg) NK cells were labeled with CFSE and adoptively transferred into Tg or non-Tg mice. At days 7 and 9 posttransfer, doxycycline was injected i.p.. At day 10, CSFE-labeled (donor) and unlabeled (host) CD3−/CD19−/NK1.1+ cells were assessed for SCT-Kb expression and IFN-γ production by plate-bound anti-NK1.1 stimulation. (A) Histograms depicting expression of SCT-Kb by donor and host NK cells. Data are representative of three independent experiments. (B) Frequency of IFN-γ–producing NK cells in the Ly49C+ or Ly49C− population (Left) and the ratio of IFN-γ–producing Ly49C+ NK cells/Ly49C− NK cells (Right) for donor and host NK cells, as indicated. Data in B are shown as mean ± SEM (n = 2 samples measured in duplicate). *P < 0.01 by unpaired Student t test.
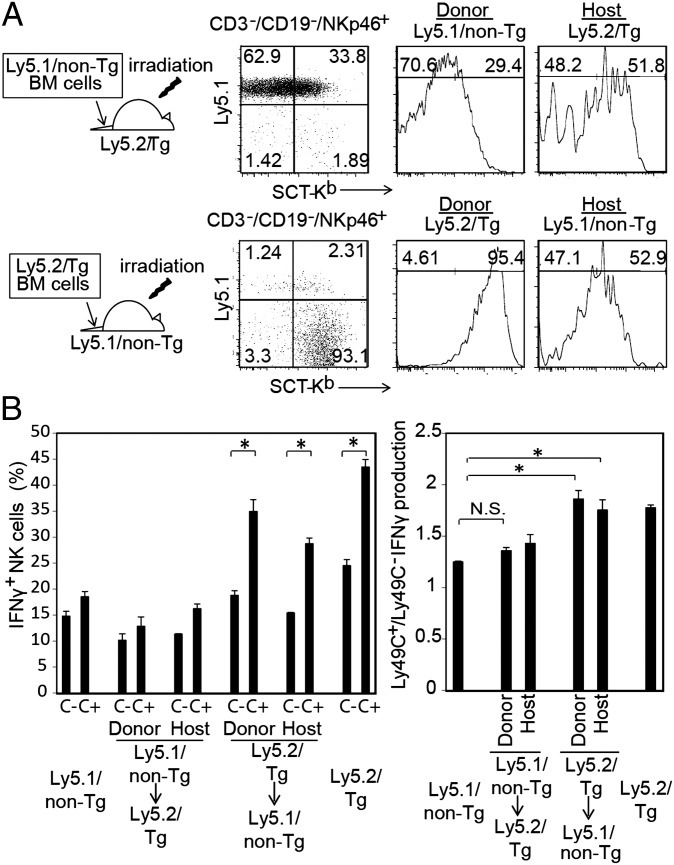
To determine if hematopoietic or stromal cells license NK cells in trans, we generated bone marrow (BM) chimeras by transferring Ly5.1+ non-Tg BM cells into irradiated Ly5.2+ Tg hosts. Alternatively, Ly5.2+ Tg BM cells were transferred into irradiated Ly5.1+ non-Tg hosts. Six weeks after transfer of BM cells, the mice were treated with doxycycline on days 1 and 3 and were analyzed for expression of SCT-Kb and its licensing effect on Ly49C+ NK cells. Nearly 97% of NK cells in the chimeras were derived from the donor (Fig. 5A). Ly49C+ NK cells from Ly5.1/non-Tg and Ly5.2/Tg were equally licensed if hematopoietic cells expressed SCT-Kb, but they were unlicensed if SCT-Kb was expressed on stromal cells even if they had SCT-Kb on their cell surface (Fig. 5B). Moreover, the residual host-derived Ly49C+ NK cells in the BM chimeric mice showed evidence of licensing only if hematopoietic cells expressed SCT-Kb, but they remained unlicensed if SCT-Kb was largely restricted to stromal cells (Fig. 5B). Thus, hematopoietic cells license NK cells in trans.
Fig. 5.
NK cells were licensed through trans interaction with hematopoietic cells. Ly5.1/β2m−/− (Ly5.1/non-Tg) and Ly5.2/(TRE-SCT Tg × ROSA-rtTA)β2m−/− (Ly5.2/Tg) BM cells were i.v. injected into irradiated (9.5 Gy) Ly5.2/Tg mice and Ly5.1/non-Tg mice, respectively. At 6 wk after transfer, the mice were treated with doxycycline on days 1 and 3. Then splenocytes were stimulated with plate-bound anti-NK1.1 to analyze the frequency of IFN-γ–producing NK cells among the Ly49C+ or Ly49C− population. (A) SCT-Kb expression of donor and host CD3−/CD19−/NK1.1+ cells in the BM chimeras. (B) Frequency of IFN-γ–producing NK cells in the Ly49C+ or Ly49C− population (Left) and the ratio of IFN-γ–producing Ly49C+ NK cells/Ly49C− NK cells (Right) for donor and host NK cells, as indicated. Data in B are shown as mean ± SEM (n = 2 samples measured in duplicate). *P < 0.01 by unpaired Student t test.
Discussion
Licensing effects caused by the acute induction of self-MHC class I provide strong evidence that licensing is not strictly a developmental process, because here we demonstrate that NK cells already out in the periphery can become licensed. Other than doxycycline administration, there was no further manipulation of the NK cells themselves. Moreover, we found that induction of MHC class I on non-NK cells was sufficient to confer licensing. Our studies are consistent with studies in which NK cells were adoptively transferred to another host (21, 22). At least 3 or 4 d were required for the conversion of the NK-cell licensing status in the adoptive transfer experiments, but it was unclear if cell trafficking from the adoptive transfer approach affected licensing so that licensing potentially could occur faster. Indeed, our current experiments indicate that licensing requires a shorter period of only a day or so. It is difficult to determine the time course of the licensing process in our studies more precisely because of the dependence on doxycycline pharmacokinetics, MHC class I induction, contact with NK cells, and the apparent signaling events necessary for licensing, no matter whether licensing occurs through arming or disarming (30, 31). Furthermore, we show here that acutely licensed NK cells do not undergo major changes in tissue distribution within the spleen. Nevertheless, the current studies, along with the adoptive transfer studies (21, 22), indicate that the licensing process occurs relatively quickly and can “educate” peripheral NK cells.
Additionally, the synchronized process studied here allowed us to determine the acute consequences of licensing that normally is a summation of NK cells that likely underwent licensing at different times in wild-type mice. Consistent with previous findings on nonsynchronized NK cells (7, 11, 12), licensed NK cells are phenotypically similar to unlicensed NK cells except for their expression of self-MHC–specific receptors and their capacity to be triggered more readily through their activation receptors. Thus, we show here that the acute licensing process apparently does not otherwise affect the phenotype of the licensed NK cells. However, we cannot deny the possible existence of unknown markers that could discriminate licensed NK cells from unlicensed NK cells or of undetected changes in immunological synapses after the induction of licensing.
Down-regulation of Ly49D after SCT-Kb induction suggests that SCT-Kb binds to Ly49D in cis as it does to Ly49C. However, there is no prior evidence to indicate this interaction. Moreover, Ly49D expression on wild-type NK cells was comparable to that on β2m−/− NK cells, and Ly49D-specific IFN-γ production was not affected in SCT-Kb–expressing mice. Thus, our data indicate that Ly49D alteration did not affect NK-cell function, whereas Ly49D engagement should have diminished its function as extrapolated from studies of hypofunctional Ly49H+ NK cells in mice expressing its ligand (32, 33).
Our data indicating that licensing occurs by trans interactions between Ly49 receptors on NK cells and self-MHC class I on hematopoietic cells are consistent with previous studies that used an adoptive transfer approach to study NK-cell licensing. In such studies, unlicensed NK cells from MHC class I-deficient mice became licensed when transferred into MHC class I-sufficient mice (21, 22). Licensing required the appropriate expression of an Ly49 receptor on the NK cell and its cognate ligand in the host, although which tissue is required to express MHC class I was not examined previously (21). Moreover, licensed NK cells from MHC class I-sufficient mice lost the licensing effect when transferred into MHC class I-deficient mice, even though the transferred NK cells themselves still retained MHC class I expression (22). Thus, inducible MHC class I expression and adoptive transfer studies indicate that licensing requires trans but not cis interactions between the relevant NK-cell receptor and self-MHC class I.
These studies are in contrast to the conclusions of investigations using a mutant Ly49A receptor in which the stalk region was replaced by that of another type II integral membrane receptor (20). In Tg mice, NK cells expressing this receptor failed to show a licensing effect even though effector inhibition was retained in target-killing experiments in vitro. Given the evidence from multiple approaches summarized above supporting trans but not cis interactions, other interpretations should be considered. One possibility is that the signaling required for licensing is not exactly the same as that required for effector inhibition. Emerging evidence indicate that effector inhibition is not only the recruitment of a tyrosine phosphatase but also the recruitment of a macromolecular complex that potentially could give rise to different outcomes depending on the context (34). Indeed, other studies suggest that signal strength through a given receptor could modulate the ability of an NK-cell receptor to give rise to the licensing effect and that signal strength required for the licensing effect is different from that required to produce inhibition (35). Therefore it is possible that Ly49A receptors with mutant stalks could alter the ability of the receptor to deliver the appropriate signal strength necessary for licensing to occur. In further support of this possibility, older studies suggested that the stalk region of Ly49 receptors contributes to MHC class I binding (36). Our current studies differ from previous studies with mutant Ly49 receptors in that we did not alter the endogenous expression of the relevant Ly49 receptors. Therefore, Ly49A receptors with mutant stalk regions could have altered MHC class I binding, thereby changing their capacity for normal licensing outcomes.
Our studies appear to be relevant to human KIRs, which also show licensing effects on human NK cells (11, 12). KIRs have no apparent capacity to bind their HLA ligands in cis (37), as is consistent with the relevance of trans rather than cis interactions in NK-cell licensing in mice. Licensed (or unlicensed) human NK cells appear to be relevant to several clinical conditions and for adoptive immunotherapy (for recent examples, see refs. 38–41). Of particular relevance, human NK cells with self-specific KIRs can be educated after BM transplantation in a manner related to the studies presented here (42), suggesting that relevant trans interactions between KIRs on NK cells and HLA class I on hematopoietic cells should be considered in the appropriate clinical context.
Materials and Methods
C57BL/6 mice were purchased from the National Cancer Institute. C57BL/6 Ly5.1 congenic, β2m−/−, and B6.129S7-Gt(ROSA)26Sor/J (ROSA-rtTA) mice were purchased from The Jackson Laboratory. Ly5.1/β2m−/− mice were generated by crossing β2m−/− mice with Ly5.1 mice. The generation of TRE-SCT Tg mice is described in SI Materials and Methods. TRE-SCT Tg mice were crossed with β2m−/− mice and ROSA-rtTA mice. Animal studies were approved by the Animal Study Committee at Washington University in St. Louis.
Detailed experimental procedures for flow cytometry, immunohistochemistry, adoptive transfer of NK cells, and BM chimeras are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ted Hansen, Beatrice Plougastel-Douglas, and Dorothy Sojka for helpful discussions, and Jennifer Laurent, Liping Yang, and Stephen Ferris for technical assistance. This study was supported by National Institutes of Health Grant R01-AI033903 and the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318255110/-/DCSupplemental.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama WM. Natural killer cells. In: Paul WE, editor. Fundamental Immunology. 7th Ed. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 395–431. [Google Scholar]
- 3.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358(6381):66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 4.Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 5.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: Susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 6.Höglund P, et al. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: Nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci USA. 1991;88(22):10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gumperz JE, Parham P. The enigma of the natural killer cell. Nature. 1995;378(6554):245–248. doi: 10.1038/378245a0. [DOI] [PubMed] [Google Scholar]
- 10.Kelley J, Walter L, Trowsdale J. Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet. 2005;1(2):129–139. doi: 10.1371/journal.pgen.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci USA. 2008;105(8):3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tormo J, Natarajan K, Margulies DH, Mariuzza RA. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature. 1999;402(6762):623–631. doi: 10.1038/45170. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto N, Mitsuki M, Tajima K, Yokoyama WM, Yamamoto K. The functional binding site for the C-type lectin-like natural killer cell receptor Ly49A spans three domains of its major histocompatibility complex class I ligand. J Exp Med. 2001;193(2):147–158. doi: 10.1084/jem.193.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto N, Yokoyama WM, Kojima S, Yamamoto K. The NK cell MHC class I receptor Ly49A detects mutations on H-2Dd inside and outside of the peptide binding groove. J Immunol. 2001;166(7):4422–4428. doi: 10.4049/jimmunol.166.7.4422. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, et al. Binding of the natural killer cell inhibitory receptor Ly49A to its major histocompatibility complex class I ligand. Crucial contacts include both H-2Dd AND beta 2-microglobulin. J Biol Chem. 2002;277(2):1433–1442. doi: 10.1074/jbc.M110316200. [DOI] [PubMed] [Google Scholar]
- 18.Choi T, Ferris ST, Matsumoto N, Poursine-Laurent J, Yokoyama WM. Ly49-dependent NK cell licensing and effector inhibition involve the same interaction site on MHC ligands. J Immunol. 2011;186(7):3911–3917. doi: 10.4049/jimmunol.1004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doucey MA, et al. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol. 2004;5(3):328–336. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- 20.Chalifour A, et al. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30(3):337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207(10):2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207(10):2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjöström A, et al. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med. 2001;194(10):1519–1530. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmer J, Ioannidis V, Held W. H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: Implications for NK cell function. J Exp Med. 2001;194(10):1531–1539. doi: 10.1084/jem.194.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truscott SM, et al. Disulfide bond engineering to trap peptides in the MHC class I binding groove. J Immunol. 2007;178(10):6280–6289. doi: 10.4049/jimmunol.178.10.6280. [DOI] [PubMed] [Google Scholar]
- 26.Mitaksov V, et al. Structural engineering of pMHC reagents for T cell vaccines and diagnostics. Chem Biol. 2007;14(8):909–922. doi: 10.1016/j.chembiol.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrowicz BP, et al. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94(8):3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3(6):523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 30.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6(7):520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 31.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32(8):364–372. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathy SK, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205(8):1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205(8):1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonsson AH, Yang L, Kim S, Taffner SM, Yokoyama WM. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J Immunol. 2010;184(7):3424–3432. doi: 10.4049/jimmunol.0904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brennan J, Mahon G, Mager DL, Jefferies WA, Takei F. Recognition of class I major histocompatibility complex molecules by Ly-49: Specificities and domain interactions. J Exp Med. 1996;183(4):1553–1559. doi: 10.1084/jem.183.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8(4):269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarek N, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest. 2012;122(9):3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jennes W, et al. Inhibitory KIR/HLA incompatibility between sexual partners confers protection against HIV-1 transmission. Blood. 2013;121(7):1157–1164. doi: 10.1182/blood-2012-09-455352. [DOI] [PubMed] [Google Scholar]
- 40.Cooley S, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKenna DH, Kadidlo DM, Cooley S, Miller JS. Clinical production and therapeutic applications of alloreactive natural killer cells. Methods Mol Biol. 2012;882:491–507. doi: 10.1007/978-1-61779-842-9_28. [DOI] [PubMed] [Google Scholar]
- 42.Foley B, et al. NK cell education after allogeneic transplantation: Dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118(10):2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.