Significance
Essential activities such as feeding and reproduction as well as social, emotional, and mental behavior are reinforced by the brain’s reward system. Pleasure status directly correlates with dopamine levels released in the brain. Because dopamine leaks into the bloodstream via the sympathetic nervous system, brain and blood dopamine levels are interrelated. We designed a synthetic dopamine sensor-effector device that enables engineered human cells, insulated by immunoprotective microcontainers and implanted into the abdomen of mice, to monitor blood-dopamine levels and drive dopamine-dependent secretion of product proteins in pleasure situations associated with palatable food, drugs, or sexual arousal. Hypertensive animals treated with this device, which produces a clinically licensed antihypertensive peptide, had their high blood pressure corrected when exposed to sexual arousal.
Keywords: synthetic gene circuits, prosthetic networks, gene regulation, gene switch, designer cells
Abstract
Synthetic biology has significantly advanced the design of synthetic trigger-controlled devices that can reprogram mammalian cells to interface with complex metabolic activities. In the brain, the neurotransmitter dopamine coordinates communication with target neurons via a set of dopamine receptors that control behavior associated with reward-driven learning. This dopamine transmission has recently been suggested to increase central sympathetic outflow, resulting in plasma dopamine levels that correlate with corresponding brain activities. By functionally rewiring the human dopamine receptor D1 (DRD1) via the second messenger cyclic adenosine monophosphate (cAMP) to synthetic promoters containing cAMP response element-binding protein 1(CREB1)-specific cAMP-responsive operator modules, we have designed a synthetic dopamine-sensitive transcription controller that reversibly fine-tunes specific target gene expression at physiologically relevant brain-derived plasma dopamine levels. Following implantation of circuit-transgenic human cell lines insulated by semipermeable immunoprotective microcontainers into mice, the designer device interfaced with dopamine-specific brain activities and produced a systemic expression response when the animal’s reward system was stimulated by food, sexual arousal, or addictive drugs. Reward-triggered brain activities were able to remotely program peripheral therapeutic implants to produce sufficient amounts of the atrial natriuretic peptide, which reduced the blood pressure of hypertensive mice to the normal physiologic range. Seamless control of therapeutic transgenes by subconscious behavior may provide opportunities for treatment strategies of the future.
The constant iteration between memory, providing consolidated information on past experience, and reward, confirming success of current activities, represents the central learning cycle of higher organisms that programs essential processes such as drinking, eating, and reproduction and reinforces complex somatic, social, emotional, mental, and cognitive behavior (1–4). The neurotransmitter dopamine is a key endogenous molecule that coordinates memory consolidation with learning, controls emotions, and motivates individuals to perform reward- and pleasure-seeking behavior and excel and improve fitness (4–6). The dopaminergic reward system is finely tuned, and major imbalances have severe pathologic consequences. Excessive reward-seeking behavior may lead to obesity (eating), addiction (alcohol, nicotine), drug abuse (cocaine, methylamphetamine), schizophrenia, or depression, whereas decreased dopamine availability has been associated with Parkinson’s disease (6–11). Although the precise dynamics of memory/learning processing remains a little-understood process, it involves dopaminergic neurons of the mesolimbic pathway that release the neurotransmitter into the presynaptic space in response to a motivation-related action potential (12). Dopamine then binds and activates dopamine receptors, such as the dopamine receptor D1 (DRD1), a G-protein–coupled receptor (GPCR) that modulates the cyclic adenosine monophosphate (cAMP) second messenger to produce a cellular response ultimately triggering an action potential by opening plasmalemmal ion channels (13, 14). In addition to the brain, dopamine receptors are also expressed in peripheral tissues such as kidney, adrenal glands, or blood vessels, and serum dopamine has been found to regulate respiration and glucose homeostasis, suggesting that central and peripheral actions of dopamine converge to mediate homeostasis in central metabolic pathways (15–17). For example, palatable food stimuli engage dopamine in the reward circuits and increase dopamine levels in circulation where it regulates pancreatic endocrine function including insulin release and modulation of insulin impact on adipocytes (17, 18). Because the liver removes and metabolizes virtually all catecholamines including dopamine delivered from the gut via the portal vein and because dopamine does not cross the blood–brain barrier, peripheral dopamine in the blood is predominantly derived from networks of sympathetic nerves enmeshing blood vessels throughout the body (17, 19–21). Because sympathetic neurons synthesize norepinephrine from dopamine in rapid-release vesicles containing the dopamine-β-hydroxylase, unconverted neurotransmitter may reach the bloodstream at concentrations that correlate with the reward system in the brain (20, 22).
To demonstrate a possible correlation of dopamine levels between the central and peripheral systems, we have created designer cells containing a synthetic dopamine-responsive sensor-effector device that taps into the peripheral dopaminergic network and can be controlled by motivational stimuli such as food, addictive drugs, and sexual arousal, which are known to trigger the central reward system.
Results
Design and Functionality of a Synthetic Mammalian Reward-Based Biosensor.
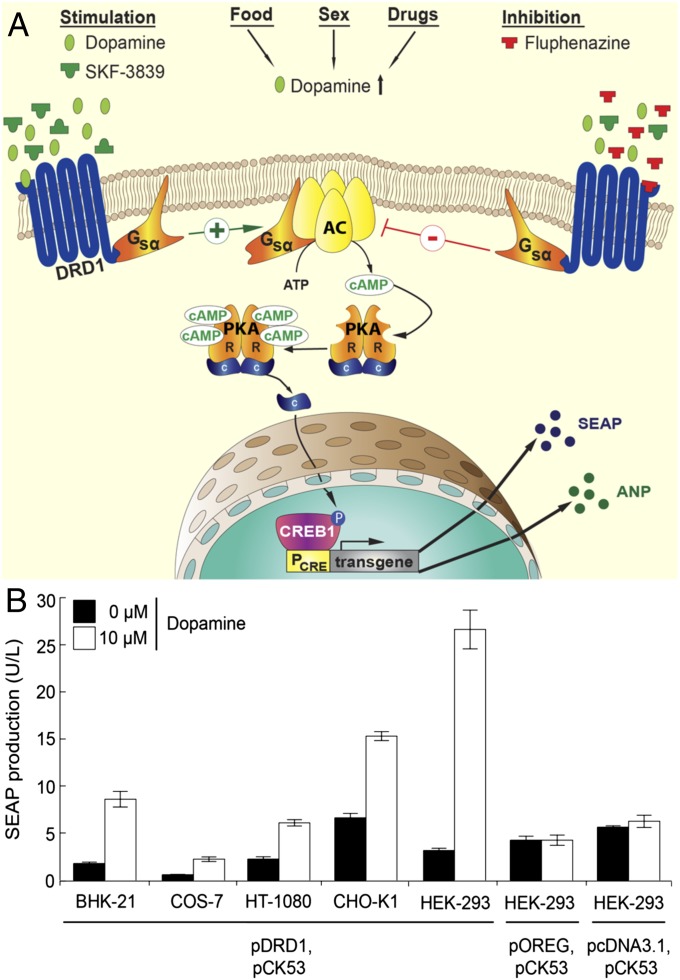
The dopamine sensor device was designed by ectopically expressing the human dopamine receptor D1 in mammalian cells and rewiring its native signaling cascade involving Gsα proteins via the second messenger cAMP to cAMP-responsive protein kinase A (PKA)-mediated phospho-activation of cAMP response element-binding protein (CREB1) that triggers transcription from synthetic promoters containing CREB1-specific cAMP response elements (CRE) (Fig. 1A). Cotransfection of several rodent, primate, and human cell lines with the constitutive human dopamine receptor D1 expression vector pDRD1 (PhCMV-DRD1-pA) (Table S1) and the DRD1-dependent PCRE-driven human placental secreted alkaline phosphatase (SEAP) reporter construct pCK53 (PCRE-SEAP-pA) (23) showed that SEAP was exclusively induced whenever the cells were exposed to the neurotransmitter dopamine, which confirms the broad applicability of the dopamine sensor device as a mammalian gene switch (Fig. 1B). Possible differences in availability and compatibility of endogenous signal-transduction components with dopamine-triggered DRD1-mediated input may in part explain the differences in basal expression and induction profiles among different cell lines (Fig. 1B). Despite capitalizing on the endogenous cAMP-signaling pathway to achieve a most minimalistic all-human design, basal transcription remains very low in the absence of dopamine, which indicates that pleiotropic input into this endogenous signaling cascade is insignificant under standard culture conditions (Fig. 1B). The human dopamine-sensor device combining human DRD1 and human HEK-293 equipped with a synthetic human signaling cascade and transgene showed the best dopamine-triggered transgene expression profile and was therefore used in all follow-up studies. A basic set of control experiments confirmed (i) that HEK-293 cells express endogenous DRD1 (24) but that these DRD1 expression levels were insufficient to drive significant dopamine-responsive PCRE activation (Fig. 1B and Fig. S1A); (ii) that neither ectopic expression of DRD1 nor exposure of cells to excessive dopamine concentrations had a negative impact on HEK-293 viability (Fig. S1B) and protein production capacity (Fig. S1C); (iii) that DRD1 was localized in the cell membrane and activated by dopamine (Fig. S1 D and E) and (iv) that the dopamine-triggered DRD1-mediated signaling was inactivated by the DRD1 antagonist and antipsychotic drug Fluphenazine (Fig. S1F) and the PKA inhibitor H-89 (Fig. S1G).
Fig. 1.
Design and functionality of the synthetic dopamine-triggered signaling cascade. (A) Binding of dopamine to ectopically expressed human dopamine receptor 1 (DRD1) triggers the G protein Gsα to stimulate the adenylyl cyclase (AC), which catalyzes conversion of ATP to cAMP. This surge in the cAMP second messenger pool activates protein kinase A (PKA), the subunit C of which subsequently dissociates and translocates into the nucleus where it activates the cAMP-responsive element binding protein 1 (CREB1) by phosphorylation. Activated CREB1 binds to a synthetic promoter (PCRE) containing consensus cAMP-response elements and triggers specific transgene expression. Rewiring of heterologous human DRD1 via endogenous control components to a chimeric promoter in a human cell produces an all-human synthetic signaling cascade that senses dopamine as well as related inducing (SKF-38393) and inhibitory (Fluphenazine) compounds and converts a transient trigger input to a sustained transcription response. (B) Dopamine-responsive expression of the human glycoprotein SEAP in different mammalian cell lines cotransfected (1:1 ratio) with constitutive DRD1 (pKR22) and PCRE-driven SEAP (pCK53) expression vectors and cultivated for 48 h in the presence (10 µM) or absence of the neurotransmitter dopamine. The parental vector (pcDNA3.1) or an isogenic expression plasmid encoding an unrelated GPCR (pOREG) were cotransfected with pCK53 and served as controls. Data are mean ± SD; n = 4 independent experiments.
Characterization and Specificity of the Reward-Based Biosensor.
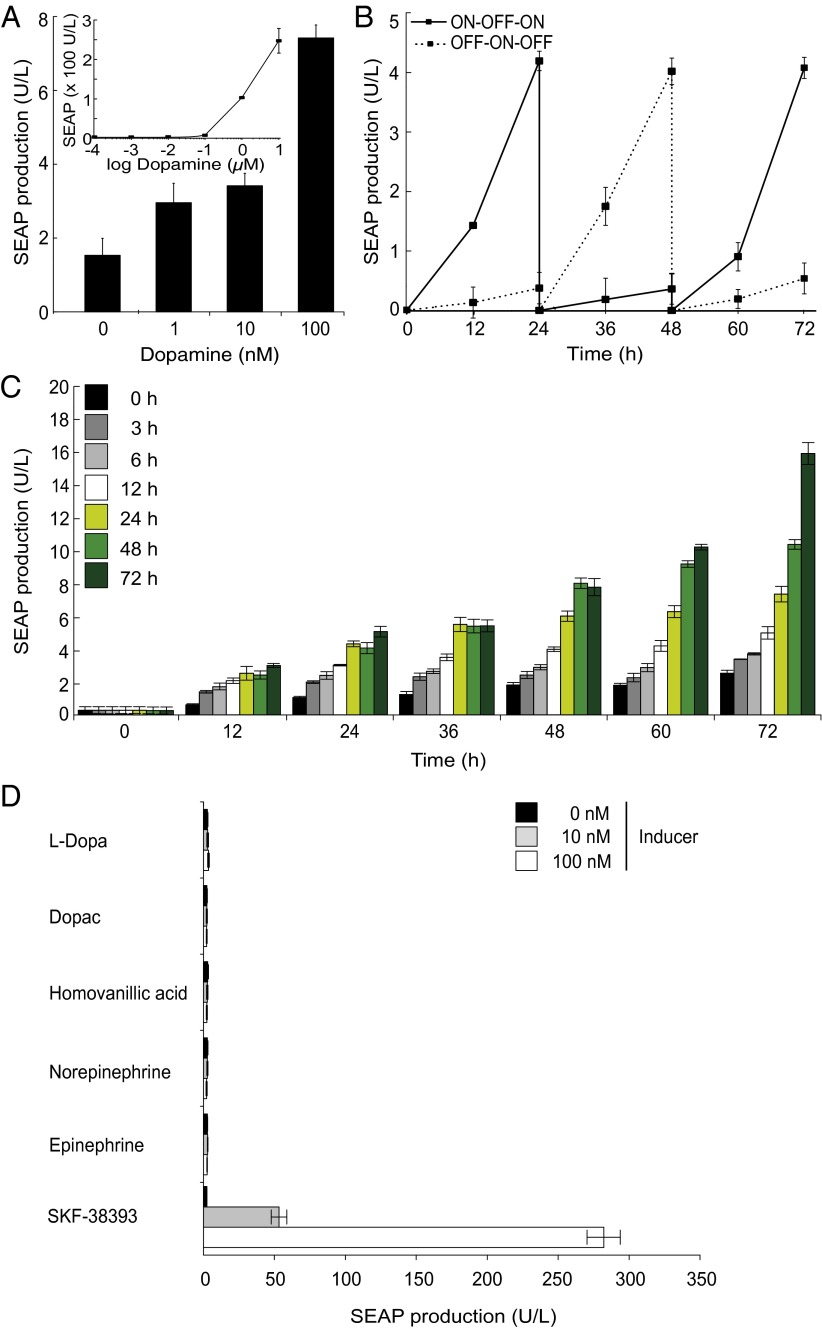
Sequential stable transfection and clonal selection of HEK-293 with pDRD1 (HEKDRD1) and pCK53 resulted in the double-transgenic cell line HEKREWARD, which ectopically expresses DRD1 and SEAP in a dopamine-inducible manner. With a sensitivity as low as 1 nM dopamine and maximum dopamine-triggered SEAP production up to 150-fold above basal expression, the HEKREWARD exhibited unprecedented dopamine responsiveness in the human physiologic range [1 ng/mL = 6.5 nM (19)] (Fig. 2A), while showing excellent neurotransmitter dose-dependent adjustability (Fig. 2A), reversibility (Fig. 2B), and time-programmable induction kinetics (Fig. 2C). To confirm the specificity of the dopamine-sensor device, we have exposed HEKREWARD to compounds that are metabolically related to dopamine such as its precursor l-Dopa (Levodopa), its breakdown products Dopac and homovanillic acid, as well as its hormone derivatives norepinephrine and epinephrine. Unlike dopamine and the DRD1-agonist SKF-38393, none of the metabolically related small molecules were able to trigger DRD1-driven SEAP expression, confirming the specificity of the dopamine-sensor device (Fig. 2D). Also, HEK-293 cells express the human β-2-adrenergic (25) and adenosine A2B receptors (26), both of which activate the same Gs-protein–coupled cAMP-signaling cascade as the synthetic dopamine sensor device when triggered by (nor-)epinephrine and adenosine, respectively. However, neither of the unrelated GPCRs were able to activate PCRE-driven SEAP expression at physiologic inducer concentrations (epinephrine/norepinephrine, 0–100 nM; adenosine, 0–2 µM; Fig. S2).
Fig. 2.
Characterization of the clonal HEKREWARD cell line stably transgenic for dopamine-responsive SEAP expression. (A) Dose-dependent SEAP production profile of HEKREWARD cultivated for 48 h in the presence of increasing dopamine concentrations. (B) Reversibility of dopamine-responsive SEAP expression was assessed by cultivating 2.5 × 106 HEKREWARD for 72 h while alternating the dopamine status of the culture (100 nM, ON; 0 nM, OFF) every 24 h. (C) SEAP expression kinetics of HEKREWARD exposed for different periods of time (color code) to 100 nM dopamine. (D) Specificity of the dopamine-sensor circuit. HEKREWARD cells were exposed for 48 h to various concentrations of different dopamine-related compounds, and corresponding SEAP production was profiled in the culture supernatant after 48 h. Data are mean ± SD; n = 4 independent experiments.
Dopamine- and Reward-Based Transgene Expression in Wild-Type Mice.
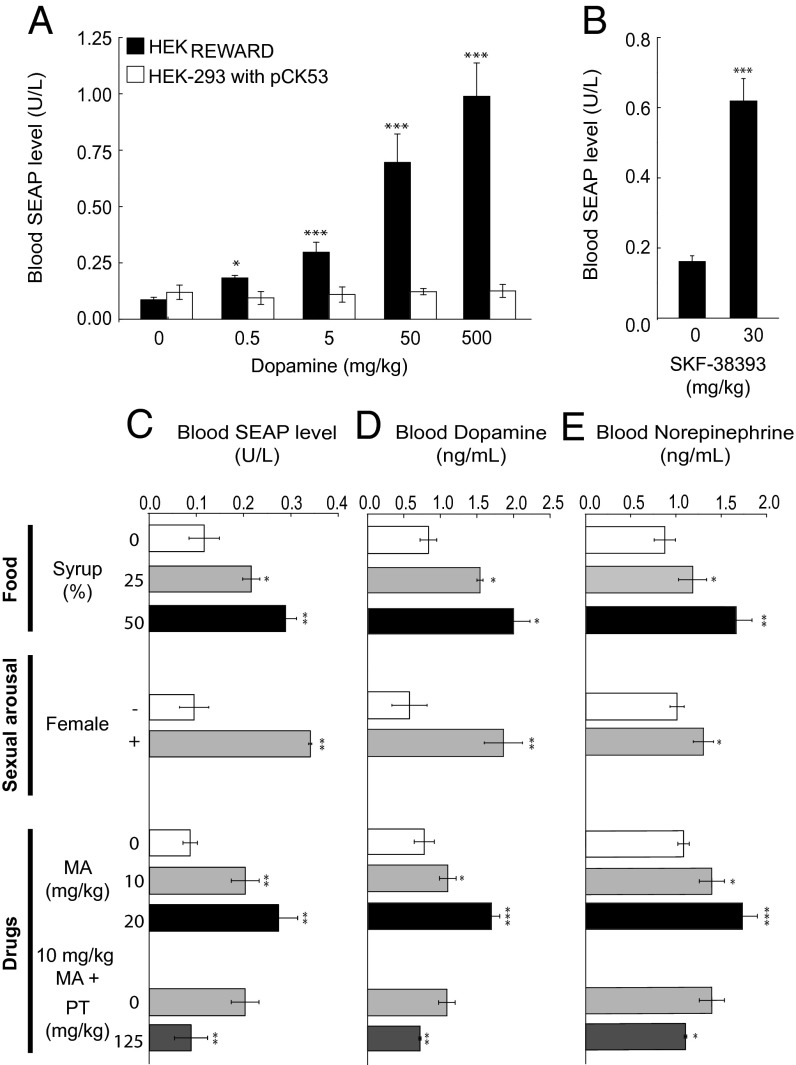
Covering a wide range of dopamine concentrations with high sensitivity, the dopamine-sensor device provides a way to precisely score peripheral physiologic dopamine levels. To validate dopamine-triggered transgene expression in vivo, we implanted wild-type mice with HEKREWARD, microencapsulated into clinically licensed alginate-poly-l-lysine-alginate capsules with a molecular cutoff of 72 kDa (27) to insulate the sensor device from the mouse physiology and its immune system and treated the animals with injections of diverse concentrations of dopamine or the synthetic DRD1 agonist SKF-38393. The SEAP levels profiled in the bloodstream of treated animals confirmed trigger-dose–dependent transgene expression in their peripheral circulation (Fig. 3 A and B). Control treatment groups implanted with pCK53-engineered HEK-293 showed no variations of basal SEAP levels in the blood, corroborating that HEK-293’s endogenous DRD1 expression was insufficient and that ectopic DRD1 production was required to mediate dopamine responsiveness in vivo (Fig. 3 A and B).
Fig. 3.
Dopamine and reward-controlled transgene expression in mice. (A and B) Dopamine-responsive SEAP expression fine-tuning in mice. Wild-type mice implanted with microencapsulated HEKREWARD, HEK-293 transfected with pCK53 (PCRE-SEAP-pA) or nonengineered parental HEK-293 cells (2 × 106 cells/mouse) were injected with different doses of dopamine (A) or the synthetic DRD1 agonist SKF-38393 (B), and corresponding blood SEAP levels were profiled after 48 h. (C–E) Reward-controlled SEAP expression in mice. Wild-type mice implanted with microencapsulated HEKREWARD (2 × 106 cells/mouse) were exposed to different reward conditions including food (syrup/water mixtures), sexual arousal [treated male mice with (+) and without (−) female mouse company], and the addiction drug methamphetamine (MA) that was optionally coadministered with the ganglionic blocker pentolinium tartate (PT). SEAP (C), dopamine (D), and norepinephrine (E) levels were profiled in the blood of treated animals after 48 h. Data are mean ± SEM; statistics by two-tailed t test; n = 8 mice. *P < 0.05, **P < 0.005, ***P < 0.0001.
In the absence of a transfer across the blood–brain barrier and liver-based removal of catecholamines delivered from the gut via the portal vein, plasma dopamine was found to originate from the brain and be coreleased as a precursor of norepinephrine via sympathetic nerves enmeshing blood vessels throughout the body (17, 20). To test the potential direct correlation between brain activities and peripheral dopamine levels, we subjected wild-type mice containing HEKREWARD implants to different conditions that are known to stimulate the mammalian reward system by triggering the release of the neurotransmitter dopamine and profiled the resulting SEAP levels in the peripheral circulation. Sex, drugs, and food, the three major mammalian reward stimuli, all activated the implanted dopamine-sensor device and resulted in increased SEAP levels in the peripheral circulation (Fig. 3C). Sexual arousal was stimulated by transferring a wild-type female mouse to a group of male mice containing a dopamine-sensor implant. The blood SEAP levels of the male mice were significantly higher compared with the same male mice kept without female company, suggesting that SEAP expression was indeed triggered by sexual arousal controlling the reward system rather than by male rivalry. Likewise, when female mice with dopamine sensor implants were offered water-diluted sugar syrup under an all-you-can-drink condition, their blood SEAP levels were significantly higher compared with an identical treatment group kept on a standard water-drinking scheme. Interestingly, the higher the syrup content of the drinking water, more SEAP that could be detected in the blood of the mice, indicating that the animal’s reward system could be dose-dependently stimulated by the quality of the food (Fig. 3C). Because drug addiction is linked with the stimulation of the mammalian reward system, we tested the impact of methamphetamine, a major drug of abuse that has a long clinical history and a similar physiologic effect as today’s lifestyle drug cocaine (28, 29). Administration of methamphetamine to mice containing dopamine-sensor implants resulted in dose-dependent increase in blood SEAP levels. In all situations, by triggering the reward system—sex, drugs, and food—the dopamine-sensor device was able to capture changes in peripheral dopamine levels and coordinate a dose-dependent response that precisely adjusted SEAP expression to reach desired levels in the bloodstream of the animals. In all reward situations, the stimulated SEAP levels correlated with blood dopamine (Fig. 3D) and blood norepinephrine (Fig. 3E) levels, suggesting that reward-relevant dopamine levels seem to replicate in the peripheral circulation and that this neuronal-peripheral dopamine balance is mediated via sympathetic neurons. Indeed, when blocking the activity of sympathetic nerves by the ganglionic blocker pentolinium tartrate in mice implanted with the dopamine-sensor device and treated with methamphetamine, SEAP, dopamine, and norepinephrine were equally decreased to basal levels reached in the absence of the drug treatment (Fig. 3 C–E).
Reward-Based Attenuation of High Blood Pressure in Hypertensive Mice.
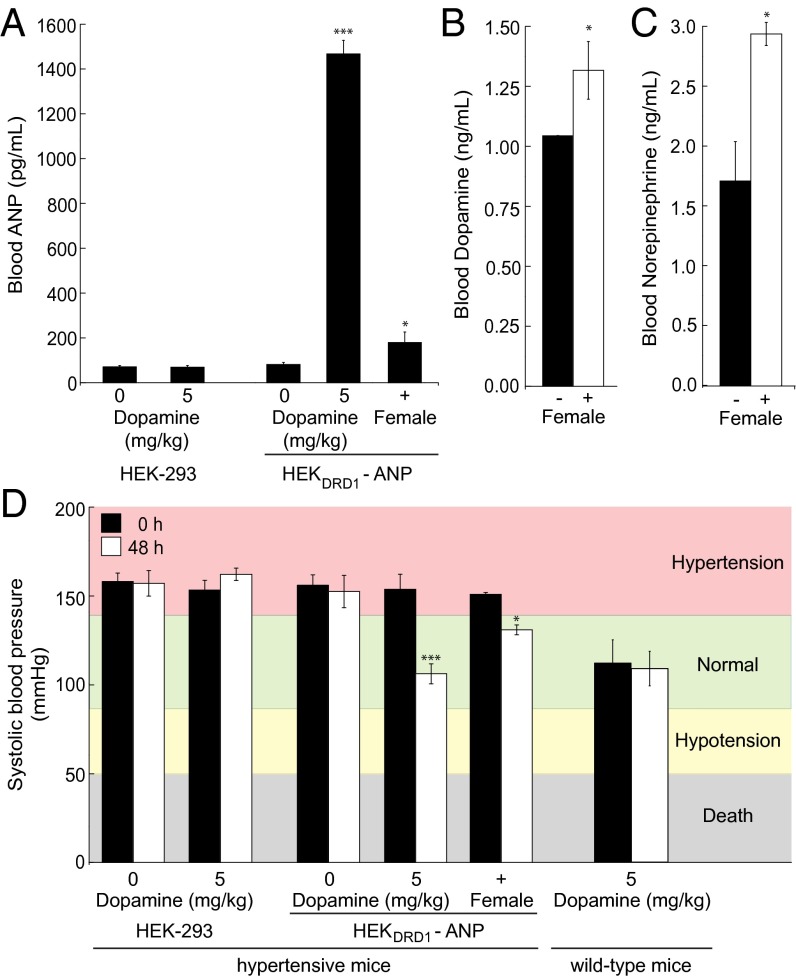
To confirm dopamine-based reward-triggered transgene expression in a prototypical therapeutic context, we chose to link the synthetic dopamine-sensor device to expression of the half-life–optimized (30) atrial natriuretic peptide (ANP; pKR147, PCRE-ANP-pA), a powerful vasodilator attenuating high blood pressure (31–33). After validation of dopamine-triggered ANP expression in vitro (Fig. S3), we implanted microencapsulated pKR147-transgenic HEKDRD1 into male mice serving as an animal model for hypertension. When hypertensive male animals containing an implanted dopamine sensor-controlled ANP production device had their reward system triggered by sexual arousal mediated by female company, they showed significant increases in serum ANP (Fig. 4A), dopamine (Fig. 4B), and norepinephrine (Fig. 4C) levels compared with an equally treated male-only community. As a consequence of sexual arousal-triggered ANP expression, the hypertonic systolic blood pressure of treated animals reversed to normal levels matching those observed for dopamine treatment (positive control), whereas the blood pressure of isolated male groups and the treatment groups implanted with nonengineered parental cells (negative control) continued to show severe hypertonia (Fig. 4D).
Fig. 4.
Reward-controlled expression of the atrial natriuretic peptide (ANP) in hypertensive mice. (A–C) Hypertensive mice were implanted with pKR147 (PCRE-ANP-pA)-transgenic HEKDRD1 (HEKDRD1-ANP) or nonengineered parental HEK-293 and either treated with dopamine injections or exposed to sexual arousal [male with (+) female company]. ANP (A), dopamine (B), and norepinephrine (C) levels were profiled in the blood of treated animals, and their systolic blood pressure was scored (D) after 48 h and compared with the blood pressure of wild-type mice. Data are mean ± SEM; statistics by two-tailed t test; n = 8 mice. *P < 0.05, ***P < 0.0001.
Discussion
Natural rewards, such as food and sexual arousal that can also be simulated by addictive drugs, trigger a dopamine release in the brain (1, 3), which increases the central sympathetic outflow and results in accumulation of the neurotransmitter in peripheral circulation (21). Capitalizing on this correlation between reward-related brain activities and dopamine levels in the blood, we have designed a synthetic dopamine sensor-effector device that interfaces with peripheral dopamine, remotely wires into reward-associated behavior, and converts transient reward stimuli into a dose-dependent systemic expression response. When operating in implanted designer cells self-connecting to the peripheral circulation (34–39) and insulated from the host immune system inside semipermeable microcapsules (27), the synthetic dopamine sensor-effector device functionally interfaced with reward-associated brain activities and successfully managed antihypertensive treatment in an animal model by coordinating expression of the clinically licensed atrial natriuretic protein. Synthetic interfaces between brain activities and peripheral circulation may enable therapeutic interventions to be programmed by the autonomic nervous system and its quick-response sympathetic branch to deliver instantaneous therapeutic impact in acute situations. Also, functional interconnection of recurrent behavioral activities with production and systemic delivery of protein therapeutics will likely foster gene- and cell-based treatment strategies that require seamless and self-sufficient drug dosing at regular intervals. With the advent of personalized medicine, it may now be foreseen that autologous designer cells that are implanted into humans inside vascularizing semipermeable microcontainers could be remote-controlled by brain activities to produce and systemically deliver protein therapeutics at the right time and dose (40–43). Provided that such designer cell implants are scalable to the patients’ needs and could be replaced by ambulant interventions at patient-compliant intervals when becoming fibrotic, such brain-instructed peripheral designer cell implants could make the taking of pills in specified amounts and in precise doses a thing of the past (43–46).
Materials and Methods
Dopamine Sensor-Effector Components.
Comprehensive design and construction details for all expression vectors are provided in Table S1. Key plasmids include pDRD1, which encodes constitutive expression of human DRD1 (PhCMV-DRD1-pA); pCK53, which harbors a SEAP expression unit driven by a synthetic promoter (PCRE) containing a CREB-dependent CRE [(PCRE-SEAP-pA (23)]; and pKR147, which manages PCRE-driven expression of a modified mouse atrial natriuretic peptide (PCRE-ANP-pA).
Cell Culture and Transfection.
Human embryonic kidney cells [HEK-293, American Type Culture Collection (ATCC): CRL-11268], baby hamster kidney cells (BHK-21, ATCC: CCL-10), African green monkey kidney cells (COS-7, ATCC: CRL-1651), and human fibrosarcoma cells (HT-1080, ATCC: CCL-121) were cultured in DMEM (Invitrogen) supplemented with 10% (vol/vol) FCS (PAN Biotech lot no. P251110) and 1% (vol/vol) penicillin/streptomycin solution (PAN Biotech). Wild-type Chinese hamster ovary cells (CHO-K1, ATCC: CCL-61) were cultured in ChoMaster HTS (Cell Culture Technologies) supplemented with 5% (vol/vol) FCS and 1% penicillin/streptomycin solution. All cell types were cultivated at 37 °C in a humidified atmosphere containing 5% CO2. For cotransfection of HEK-293 and CHO-K1, 60,000 cells seeded per well of a 24-well plate 12 h before transfection were incubated for 4 h with a DNA-Ca3(PO4)2 precipitate (0.6 µg total DNA; cotransfection, 0.5 µg of receptor-encoding vector and 0.1 µg of reporter plasmid), subjected to a glycerol shock (CHO-K1 only; 30 s in ChoMaster HTS containing 15% glycerol) and cultivated for indicated periods of time in 0.4 mL DMEM or ChoMaster HTS containing different concentrations of trigger compounds (37). Likewise, BHK-21, COS-7, and HT-1080 were cotransfected overnight using FuGENE 6 (Roche Diagnostics AG).
Design and Characterization of Stable Transgenic Cell Lines.
HEKDRD1, the stable transgenic HEK-293–derived cell line constitutively expressing human DRD1 was designed by cotransfection of pDRD1 (PhCMV-DRD1-pA; 1.5µg) and pZeoSV2 (0.1 µg), followed by selection of a polyclonal population for 10 d in DMEM containing 200 µg/mL (wt/vol) zeocin (Invitrogen), FACS-mediated single-cell cloning, and clonal expansion. Stable transgenic cell clones were transfected with pCK53 (PCRE-SEAP-pA) and profiled for dopamine responsiveness. The best-in-class cell line HEKDRD1 (cell clone no. 18 of five randomly picked clones) was chosen for further experiments. The stable double-transgenic cell line HEKREWARD (cell clone no. 12 of five randomly picked clones) was constructed by cotransfecting HEKDRD1 with pCK53 (PCRE-SEAP; 0.5 µg) and pPUR (2.5 µg), followed by selection of a polyclonal population for 10 d in DMEM containing 200 µg/mL (wt/vol) zeocin and 0.5 µg/mL (wt/vol) puromycin (InvivoGen), FACS-mediated single-cell cloning, and clonal expansion. Dopamine responsiveness of individual clones was profiled, and HEKREWARD was chosen for further experiments. For characterization of transgene induction kinetics and adjustability, HEKREWARD cells (120,000 cells/mL) were cultivated for up to 72 h in DMEM containing different dopamine concentrations while continuously scoring SEAP expression levels. The reversibility of the dopamine control switch was validated by cultivating HEKREWARD cells (120,000 cells/mL) for 72 h while alternating the dopamine status of the culture (100 nM, ON; 0 nM, OFF) every 24 h.
Analytical Assays.
SEAP.
Human placental SEAP levels were profiled (i) in cell culture supernatants using a standard p-nitrophenylphosphate–based light absorbance time course (47) and (ii) in blood samples using a chemiluminescence-based assay (Roche Applied Science).
Norepinephrine and Dopamine.
Blood levels of these catecholamines were quantified using a TriCAT ELISA (IBL International).
ANP.
Atrial natriuretic peptide was quantified (i) in cell culture supernatants via its fragment crystallizable (FC) fusion tag using a mouse IgG ELISA (Immunology Consultant Laboratory, Inc., cat. no. E90-G) and (ii) in blood samples using an ANP ELISA kit (Abcam).
Semiquantitative RT-PCR.
One microgram of total cellular RNA, isolated from HEK-293 and HEKDRD1 using the ZR RNA MicroPrep kit (Zymo Research), was reverse-transcribed using SuperScript II Reverse Transcriptase (Life Technologies). Specific RNA levels were quantified with the Mastercycler ep gradient/S system (Vaudaux-Eppendorf) and primers specific for human dopamine receptor D1 (OKR75, 5′-CCAGCCTCAGCAGGACATATG-3′; OKR76, 5′-CTTTCCGGTTGAGAACATTCG-3′; target size: 211 bp) and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) (OKR500, 5′-CCATCACCATCTTCCAGGAG-3′; OKR501, 5′-CCTGCTTCACCACCTTCTTG-3′).
Fluorescence Microscopy.
EGFP expression of pKR43-transfected HEK-293 was visualized using a Leica TCS-SP5 confocal microscope (Leica, Microsystems) equipped with a HCX PL APO 40.0 × 0.85 dry objective, a 488-nm/509-nm laser for excitation, a 500-nm/600-nm emission filter set, and Leica Application Suite software (version 2.6.0.7266).
Viability Assay.
Individually treated nontransfected and pDRD1-transfected HEK-293 cells were washed and resuspended in PBS before mixing with Trypan Blue solution to determine viable cells using a hemacytometer.
Chemical Compounds.
Pentolinium tartrate (12.5 g/L stock solution in water; Thermo Fisher Scientific Inc.). Adenosine, l-DOPA, DOPAC, the antipsychotic drug Fluphenazine, the PKA inhibitor H-89, homovanillic acid, epinephrine, norepinephrine (all 10 mM stock solution in water; all Sigma-Aldrich Chemie), dopamine (in vitro, 10 mM; in vivo, 8.3 g/L stock solution in water), SKF-38393 (in vitro, 10 mM; in vivo: 3 g/L stock solution in water), and metamphetamine (in vivo, 2 g/L stock solution in water) were purchased from Sigma-Aldrich Chemie.
Animal Experiments.
Intraperitoneal implants were produced by encapsulating HEKREWARD, pKR147-transgenic HEKDRD1, pCK53-engineered HEK-293, as well as native HEK-293 into coherent alginate-poly-(l-lysine)-alginate beads (400 µm; 200 cells/capsule) using an Inotech Encapsulator Research Unit IE-50R (Buchi Labortechnik AG) adjusted to the following settings: 200-µm nozzle with a vibration frequency of 1,023 Hz and 900 V for bead dispersion, 20-mL syringe operated at a flow rate of 403 units. Serum-free DMEM (400 µL) containing 2 × 106 microencapsulated transgenic cells (200 cells/capsule) were injected intraperitoneally into wild-type female or male oncins France souche 1 (Charles River Laboratories) or male hypertensive mice (BPH/2J, The Jackson Laboratory). One hour after capsule implantation, the animals were treated intraperitoneally with chemicals [dopamine (0, 0.5, 5, 50, 500 mg/kg), SKF-38393 (0, 30 mg/kg), methamphetamine (0, 10, 20 mg/kg), methamphetamine (10 mg/kg) plus pentolinium tartrate (125 mg/kg)] or rewarded by either dopamine-free syrup [25, 50% (vol/vol in H2O); Goldsaft Zuckerruebensirup] or sexual arousal (one female mouse in a group of six male mice). Control animals were intraperitoneally implanted with nonengineered parental cells, received no chemicals, or were not rewarded (water instead of syrup; same-sex groups). Blood levels of SEAP and ANP (serum isolation using microtainer serum separator tubes; Becton Dickinson) as well as systolic blood pressure (tail-cuff plethysmography; NIBP LE-5002; Harvard Apparatus) were profiled 48 h after treatment or reward. All experiments involving animals were performed according to the European Parliament and European Union Directive 2010/63/EU, approved by the French Republic (no. 69266309), and carried out by Ghislaine Charpin-El Hamri at the Institut Universitaire de Technology, F-69622 Villeurbanne Cedex, France.
Supplementary Material
Acknowledgments
We thank Tomonori Katsuyama for assistance with microscopy and Pratik Saxena, Barbara Geering, Nadezda Masloboeva, and William Bacchus for critical comments on the manuscript. This work was supported by a European Research Council advanced grant (ProNet 321381).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312414110/-/DCSupplemental.
References
- 1.Bello EP, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14(8):1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song in male European starlings. Physiol Behav. 2006;88(4–5):575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Hull EM. Sex, drugs and gluttony: How the brain controls motivated behaviors. Physiol Behav. 2011;104(1):173–177. doi: 10.1016/j.physbeh.2011.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamford NS, et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42(4):653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- 5.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42(6):939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 8.Blum K, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): A commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stice E, Yokum S, Zald D, Dagher A. Dopamine-based reward circuitry responsivity, genetics, and overeating. Curr Top Behav Neurosci. 2011;6:81–93. doi: 10.1007/7854_2010_89. [DOI] [PubMed] [Google Scholar]
- 10.Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Brain Res Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10(3):211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 12.Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24(3):165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 14.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 15.Pardal R, Ortega-Sáenz P, Durán R, López-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131(2):364–377. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 16.Biaggioni I, Hollister AS, Robertson D. Dopamine in dopamine-beta-hydroxylase deficiency. N Engl J Med. 1987;317(22):1415–1416. doi: 10.1056/NEJM198711263172212. [DOI] [PubMed] [Google Scholar]
- 17.Rubí B, Maechler P. Minireview: New roles for peripheral dopamine on metabolic control and tumor growth: Let’s seek the balance. Endocrinology. 2010;151(12):5570–5581. doi: 10.1210/en.2010-0745. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang G-J, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein DS, et al. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab. 1999;84(7):2523–2531. doi: 10.1210/jcem.84.7.5864. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein DS, Holmes C. Neuronal source of plasma dopamine. Clin Chem. 2008;54(11):1864–1871. doi: 10.1373/clinchem.2008.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Loon GR. Plasma dopamine: Regulation and significance. Fed Proc. 1983;42(13):3012–3018. [PubMed] [Google Scholar]
- 22.LeBlanc J, Ducharme MB. Plasma dopamine and noradrenaline variations in response to stress. Physiol Behav. 2007;91(2–3):208–211. doi: 10.1016/j.physbeh.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Kemmer C, et al. A designer network coordinating bovine artificial insemination by ovulation-triggered release of implanted sperms. J Control Release. 2011;150(1):23–29. doi: 10.1016/j.jconrel.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Yu P, et al. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66(6):2167–2180. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- 25.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390(6655):88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 26.Cooper J, Hill SJ, Alexander SP. An endogenous A2B adenosine receptor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells. Br J Pharmacol. 1997;122(3):546–550. doi: 10.1038/sj.bjp.0701401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, He H, Yen C, Ho W, Lee LJ. A biodegradable, immunoprotective, dual nanoporous capsule for cell-based therapies. Biomaterials. 2008;29(31):4253–4259. doi: 10.1016/j.biomaterials.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Tominaga GT, Garcia G, Dzierba A, Wong J. Toll of methamphetamine on the trauma system. Arch Surg. 2004;139(8):844–847. doi: 10.1001/archsurg.139.8.844. [DOI] [PubMed] [Google Scholar]
- 29.Shaner JW. Caries associated with methamphetamine abuse. J Mich Dent Assoc. 2002;84(9):42–47. [PubMed] [Google Scholar]
- 30.Mezo AR, et al. Atrial natriuretic peptide-Fc, ANP-Fc, fusion proteins: Semisynthesis, in vitro activity and pharmacokinetics in rats. Bioconjug Chem. 2012;23(3):518–526. doi: 10.1021/bc200592c. [DOI] [PubMed] [Google Scholar]
- 31.Schillinger KJ, et al. Regulatable atrial natriuretic peptide gene therapy for hypertension. Proc Natl Acad Sci USA. 2005;102(39):13789–13794. doi: 10.1073/pnas.0506807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Therrien J-P, et al. A gene therapy approach for long-term normalization of blood pressure in hypertensive mice by ANP-secreting human skin grafts. Proc Natl Acad Sci USA. 2010;107(3):1178–1183. doi: 10.1073/pnas.0908882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin KF, Chao J, Chao L. Human atrial natriuretic peptide gene delivery reduces blood pressure in hypertensive rats. Hypertension. 1995;26(6 Pt 1):847–853. doi: 10.1161/01.hyp.26.6.847. [DOI] [PubMed] [Google Scholar]
- 34.Ye H, Daoud-El Baba M, Peng R-W, Fussenegger M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science. 2011;332(6037):1565–1568. doi: 10.1126/science.1203535. [DOI] [PubMed] [Google Scholar]
- 35.Ye H, et al. Pharmaceutically controlled designer circuit for the treatment of the metabolic syndrome. Proc Natl Acad Sci USA. 2013;110(1):141–146. doi: 10.1073/pnas.1216801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber W, et al. Macrolide-based transgene control in mammalian cells and mice. Nat Biotechnol. 2002;20(9):901–907. doi: 10.1038/nbt731. [DOI] [PubMed] [Google Scholar]
- 37.Fussenegger M, Schlatter S, Dätwyler D, Mazur X, Bailey JE. Controlled proliferation by multigene metabolic engineering enhances the productivity of Chinese hamster ovary cells. Nat Biotechnol. 1998;16(5):468–472. doi: 10.1038/nbt0598-468. [DOI] [PubMed] [Google Scholar]
- 38.Ruder WC, Lu T, Collins JJ. Synthetic biology moving into the clinic. Science. 2011;333(6047):1248–1252. doi: 10.1126/science.1206843. [DOI] [PubMed] [Google Scholar]
- 39.Khalil AS, Collins JJ. Synthetic biology: Applications come of age. Nat Rev Genet. 2010;11(5):367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orive G, et al. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004;22(2):87–92. doi: 10.1016/j.tibtech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Catena R, et al. Improvement of the monitoring and biosafety of encapsulated cells using the SFGNESTGL triple reporter system. J Control Release. 2010;146(1):93–98. doi: 10.1016/j.jconrel.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Chang TMS. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005;4(3):221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs-Tulleneers-Thevissen D, et al. Beta Cell Therapy Consortium EU-FP7 Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56(7):1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- 44.Ausländer S, Wieland M, Fussenegger M. Smart medication through combination of synthetic biology and cell microencapsulation. Metab Eng. 2012;14(3):252–260. doi: 10.1016/j.ymben.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: The next pillar of medicine. Sci Transl Med. 2013;5(179):179ps177. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fussenegger M. Synthetic biology: Synchronized bacterial clocks. Nature. 2010;463(7279):301–302. doi: 10.1038/463301a. [DOI] [PubMed] [Google Scholar]
- 47.Schlatter S, Rimann M, Kelm J, Fussenegger M. SAMY, a novel mammalian reporter gene derived from Bacillus stearothermophilus alpha-amylase. Gene. 2002;282(1–2):19–31. doi: 10.1016/s0378-1119(01)00824-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.