Significance
The paper follows up the suggestion that pupillometry may reveal the presence of residual visual function of which subjects are unaware (blindsight) in the field defects caused by visual cortex lesions. In a group of 19 subjects with hemi-field cortical blindness, the pupil responses in the majority matched the psychophysical findings for the sensitive spatial frequencies, indicating that it is useful to carry out pupillometry in cortical blind fields as a predictor of intact visual psychophysical function for further rehabilitation procedures.
Keywords: spatial vision, incidence, screening
Abstract
Significantly above-chance detection of stimuli presented within the field defect of patients with postgeniculate lesions is termed “blindsight.” It has been proposed that those with blindsight are more likely to benefit from visual rehabilitation by repeated stimulation, leading to increased visual sensitivity within their field defect. Establishing the incidence of blindsight and developing an objective and reliable method for its detection are of great interest. Sudden onsets of a grating pattern in the absence of any change in light flux result in a transient constriction of the pupil, termed “pupil grating response.” The existence of pupil grating responses for stimuli presented within the blindfield has previously been reported in a hemianopic patient and two monkeys with removal of the primary visual cortex unilaterally. Here, we have systematically investigated the presence of a spatial channel of processing at a range of spatial frequencies using a psychophysical forced-choice technique and obtained the corresponding pupil responses in the blindfield of 19 hemianopic patients. In addition, in 13 cases we determined the pupil responses in a sighted field location that matched the blindfield eccentricities. Our findings demonstrate that blindfield pupil responses are similar to those for the sighted field, but attenuated in amplitude. Pupillometry correctly characterized the presence or absence of a significant psychophysical response and thus is worth measuring in the cortically blindfields as a predictor of intact psychophysical capacity. The incidence of blindsight where detection performance had been investigated psychophysically over a range of spatial frequencies was 70%.
Pupil size is determined by antagonistic interactions of two sets of iris muscles: the parasympathically controlled sphincter muscle and the radial muscle controlled by the sympathetic nervous system (1). Under free viewing conditions, the pupil diameter fluctuates within a few micrometers to the millimeter range. Of pupillary reflexes, the pupil light reflex is the oldest [first documented by Al Razi (2)] and is largely mediated by the midbrain, specifically the Edinger–Westfal nuclei of the tectum (3), the retinal input to which can remain intact after visual cortex lesions. Therefore, deficit in pupil light reflex is to be expected in lesions of the optic tract, manifesting in “relative afferent pupillary defects” (4). However, postgeniculate lesions can also influence the light reflex, demonstrating the cortical influence on the control of the pupil size (5).
In addition to changes in light flux, pupil size is modulated by other stimulus features such as color, motion, and structure, as well as by emotional stimuli and states. A transient constriction of the pupil diameter is elicited by interchanges of monochromatic light (6) or the onset of chromatic information when the associated luminance contributions are minimized (7). In random dot patterns, a brief episode of coherent dot movement can also lead to a transient pupil response (8). Both color and movement pupil responses remain for stimuli presented within the field defect of a patient with blindness due to postgeniculate lesions (9). The pupil response amplitude after the onset of a checkerboard pattern is systematically modulated by the square size in the checkerboard, with no responses present at below the limits of visual acuity (10). In healthy adults, the sudden onset of grating patterns also leads to a transient constriction of the pupil, and the profile of response amplitudes varies with spatial frequency similar to that of the contrast sensitivity function (11).
Based on the above findings, the pupil response has been assumed to provide an objective measure of neuronal processing and has been used to study visual processing in the absence of subjective reports, e.g., in infants (12) and in nonhuman primates (13). The range of pupil responses elicited to the onset of spatial patterns, chromatic signals, and moving objects indicate that they are a by-product of cortical activity and therefore may be useful in assessing the extent of processing after brain injury.
Hemianopia refers to homonymous blindness in both eyes following postchiasmic lesions. Some patients with postgeniculate brain injuries may retain the ability to detect or discriminate visual stimuli presented within their field defect. This capacity is termed “blindsight” (14). One blindsight patient (GY) also showed blindfield pupil responses to the onset of gratings with an overall response profile closely matching his psychophysically determined residual spatial channel (15). Interestingly, the pupillometric and psychophysical findings in GY also matched those in two macaque monkeys with unilateral removal of visual cortex. These findings have led to a prediction that the noninvasive technique of pupillometry may be used as a rapid and objective screening method for the presence of residual visual processing in hemianopic patients (16). Here we report on a study in which we systematically mapped the psychophysical channel of processing in a group of hemianopic patients. In addition, at the same location and spatial frequency range, we obtained pupil responses. We have also repeated the pupillometric measurements for a location at the same eccentricity, but within the sighted field.
We aimed to address three questions. First, in a sample of clinical population, how many patients have above-chance detection (“blindsight”) within their blindfield measured using forced-choice guessing? Second, are there significant blindfield pupil grating responses, and if so, how are their amplitudes affected compared with the sighted field stimulation? Third, what is the sensitivity and the specificity of pupillometry in predicting blindsight performance?
Results
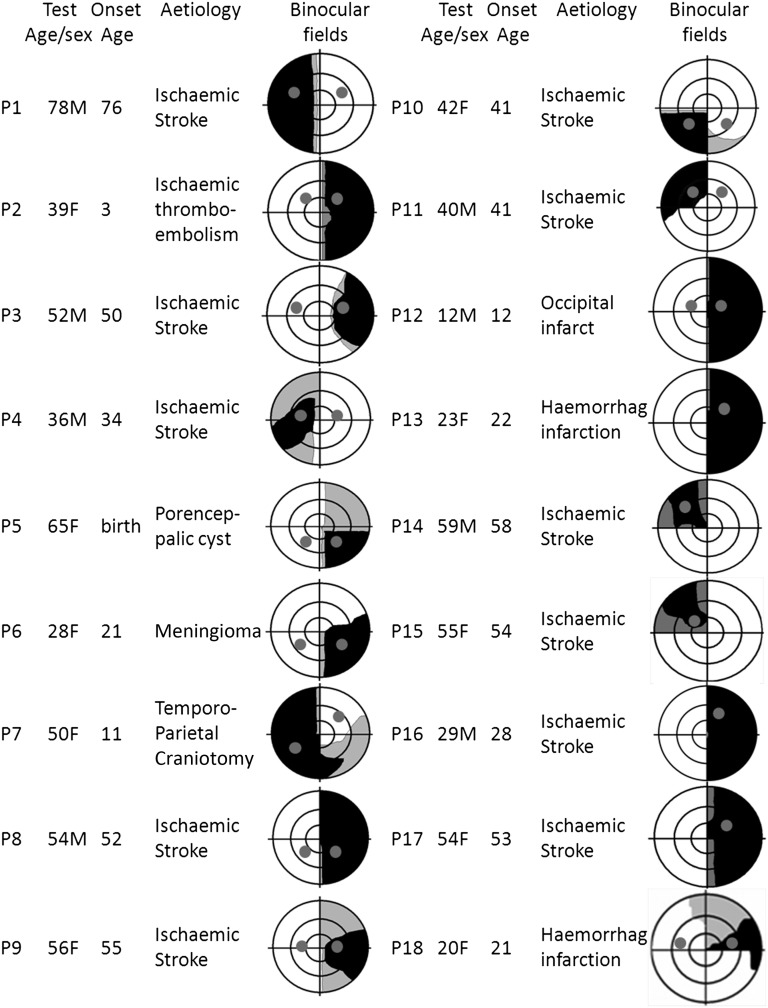
Eighteen patients with postgeniculate lesions were recruited for this study. A summary for each patient of the age when tested, sex, age at onset of injury, lesion etiology, and a schematic representation of their binocular central 30° visual field based on their monocular threshold perimetry is shown in Fig. 1. The visual field locations where testing within the field defect were conducted are also indicated by a gray disk on the binocular fields. For comparison, in 13 cases the pupillary responses were also obtained at a location within the sighted field and at the same eccentricity as the blindfield location.
Fig. 1.
Brief summary of details on patients participating in this study. Details include age at the onset of injury, when tested in the laboratory, sex, and the type of brain injury as well as a schematic representation of the binocular visual fields based on monocular results. Areas of reduced sensitivity in one or both eyes are shown in gray. Black areas are those where sensitivity of 0 db is measured in both eyes. The target locations for blind and sighted stimulus presentations are also depicted.
In addition to the 18 cases investigated in detail, we have obtained psychophysical and pupillometric data for a limited range of frequencies in the well-studied patient DB [patient 19 (P19)], an early reported case of blindsight (14, 17). The only other previously published finding was related to a well-studied patient GY (P20) (15). These data are included in sensitivity and specificity analysis only.
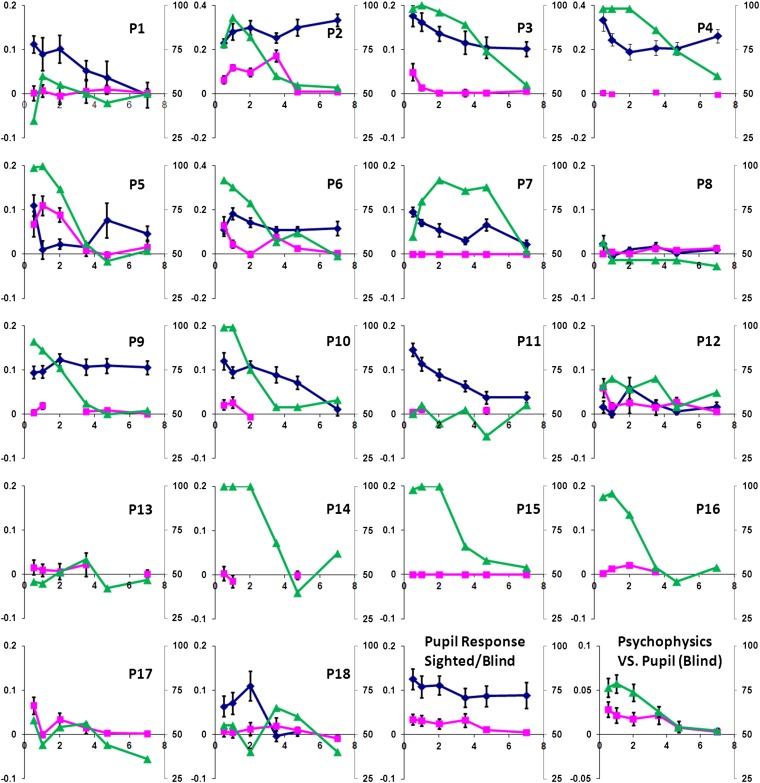
Fig. 2 summarizes the findings for each patient. For the psychophysical determinations, a Gabor patch at a range of spatial frequencies was presented at a blindfield location in one of two temporal intervals denoted by auditory beeps. The psychophysical data obtained using this temporal, two-alternative, forced-choice detection task are shown by green lines and symbols (triangles). A Gabor patch at a range of spatial frequencies was presented at a blindfield location in one of the two temporal intervals denoted by auditory beeps. Significantly above-chance detection was recorded in 12 of 18 cases. The average response profile of the spatial channel of processing obtained psychophysically in all 18 cases is shown in green in Fig. 2, Lower Right. This response profile shows peak sensitivity at 1 c/° (cycles per degree) and matches that reported previously for GY (15, 18). As DB also has above-chance detection at 1 c/°, the total number of blindsight cases is 14 of the total of 20. This finding would place the incidence of blindsight at 70% of patients with postgeniculate lesions whose spatial vision has been systematically investigated so far.
Fig. 2.
The response profile for the psychophysically determined spatial channel of processing is shown in green lines and symbols (triangles). Pupil responses in the blind and sighted fields are plotted in pink (squares) and blue (diamonds). The last two panels (Bottom Right) show the average responses. In all panels, the spatial frequency in cycles per degree is plotted along the horizontal axis; the left vertical axis indicates the pupil response amplitudes in mm; the right vertical axis indicates the percentage of correct responses in a two-alternative forced-choice psychophysical detection task.
We obtained blindfield pupil responses to a range of spatial frequencies in 18 patients and to 1 c/° stimuli in P19. In addition, in 13 cases we obtained the pupil responses at a location in the sighted field corresponding to the same eccentricity of that of the blindfield. In Fig. 2, the blind and sighted field data are shown in pink (squares) and blue (diamonds), respectively. As a group, significant blindfield pupil responses were obtained for all spatial frequencies for sighted and blindfield presentations (all P < 0.05, one sample t test, one-tail; for two-tail analysis, blindfield data for 2 and 3.5 c/° were not significant). A two-field (blind and sighted) × 7 (spatial frequencies) repeated-measure ANOVA revealed the main effect of field (df = 1, F = 15.47, P = 0.002, η2 = 0.563) and spatial frequency (df = 5, F = 8.33, P < 0.001, η2 = 0.410) and no significant field × spatial frequency interaction (df = 5, F = 1.01, P = 0.419, η2 = 0.078). This indicates that, overall, the pupil responses in the blindfield were similar in profile to those in the sighted field but of smaller amplitudes. This finding is consistent with those relating to detection of other features in the blindfield.
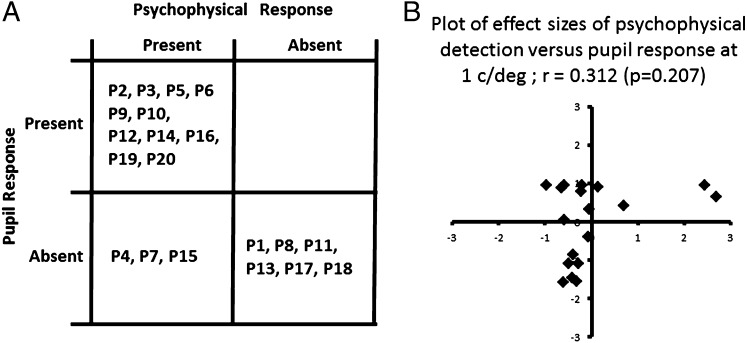
When the suitability of pupil responses as a predictor of significant psychophysical processing is to be investigated, it is necessary to consider the data on an individual basis. If the gold standard as far as detection of blindsight is concerned—i.e., the significantly above-chance detection using a two-alternative forced-choice task—then significant detection can be determined using a binomial distribution at a given probability range (i.e., P < 0.05, 0.01, or 0.001). Similarly, the criteria for a significant pupil response can be set by comparing the response amplitude against zero (one-sample t test) for each individual at the range of frequencies measured at a similar probability range (see Materials and Methods for summary). An alternative approach would be to choose the conditions and the significance level to be set a priori. Given that all of the evidence indicates that the psychophysically determined spatial channel has peak sensitivity at 1 c/°, one may measure the pupil response also at this same spatial frequency to develop a rapid and objective test for the presence of blindsight. Fig. 3A summarizes the findings for each case. Of the 14 patients with significant psychophysical detection, 11 also had significant pupil responses at 1 c/°. However, all 6 cases without blindsight also did not have significant pupil response. This distribution indicates that pupillometry can detect the presence or absence of blindsight with 85% accuracy. Crucially, for specificity of 1.0, the technique has a sensitivity of 0.79.
Fig. 3.
(A) Response matrix summarizing the presence or the absence of significant (P < 0.05) psychophysical and pupil responses in all 20 cases. (B) The relationship between the amplitude of psychophysical and pupil responses is explored by converting the response amplitudes to z-scores and plotting them against each other. The plot shows no significant correlation between these measures of effect size.
At the same spatial frequency it is of interest to establish if there is relationship between the effect sizes for both psychophysical and pupillometric findings. That is, does high percentage accuracy in detection necessarily lead to a larger pupil response and vice versa, or is it the presence and/or the absence of significant responses that is the crucial factor, and the response amplitudes are not interrelated? To investigate this point, for each patient, we have transformed the deviation from the mean score of all patients to a z-score that is a direct indicator of the effect size for both psychophysical and pupillometric data. These z-scores are plotted against each other in Fig. 3B. There is no significant correlation between the two parameters (P = 0.207), indicating that, although the presence or absence of a significant pupil response can predict the presence/absence of blindsight, their signal strengths are not necessarily related.
Discussion
Establishing the presence or absence of residual visual processing within the clinically blind area of patients with lesions along the visual pathways is of interest. Recent reports on animal models (19) and a human patient (20) with lesions extending to the lateral geniculate nucleus showed the absence of any residual visual processing within the field defect. However, in cases of postgeniculate lesions where some visual capacities are maintained, there is more likelihood of benefiting from rehabilitation techniques (21). Therefore, fast and effective screening for the presence of blindsight may be a useful and effective way of determining the probability of a successful rehabilitation outcome. Psychophysical determination of the full range of residual processing in a patient relies on two prerequisites: (i) the patient must be willing to play a guessing game, choosing between temporally or spatially separated alternatives in a seemingly random manner and (ii) the patient must cope with concentrated and lengthy testing sessions. Pupillometric studies, on the other hand, are less demanding; are independent of any subjective responses regarding the stimuli (although background emotional states may be important); and can be automated. Current investigation shows that the presence of a significant pupil response always accompanies the presence of a psychophysical channel of processing. Therefore, we propose that this method is useful in flagging the presence of residual vision within the blind visual field. If the pupil response is absent, then there is a one in three probability of the existence of a residual processing that can then be investigated using psychophysical techniques alone.
There are two further points that are worth mentioning. One is related to the response amplitudes and a second to the incidence of blindsight. Comparison of pupil responses in the blind and sighted fields to a range of spatial frequencies shows that, on average, the pupil response amplitudes are reduced by one log unit in the blindfield compared with the sighted field. The fact that postgeniculate lesions result in smaller amplitude pupil grating responses reflects the cortical (i.e., top–down) influences on the midbrain mechanisms controlling the pupil response. A similar reduction in sensitivity in the blindfield compared with the visual processing at a comparable eccentricity within the sighted field has been reported previously for orientation discrimination (14), chromatic processing (22), and contrast sensitivity (23). In all cases, the response profiles within the field defect are similar to those of the sighted field albeit with overall reduced sensitivity. The findings reported here are also in agreement with the previously reported pupillary responses in cases of achromatopsia (cortical color blindness) where the pupil response is absent when color changes are ineffective (24). Both sets of findings further emphasize the cortical origins of the pupil response.
The incidence of blindsight has been the topic of debate (see ref. 25 for review). Variation in the reported incidence is a direct consequence of the definition of this phenomenon. Once the existence of a residual capacity within the field defect of a clinical cohort is investigated, by definition, those with above-chance performance are identified as positive cases. Therefore, the incidence is a function of the methods applied in the detection task. A patient may show evidence of effective processing for a set of features and no above-chance detection or discrimination of a different set of features. In addition, some features may be more likely to be preserved than others. Using the redundant target paradigm, Marzi and colleagues (26) found reliable and consistent evidence for redundancy gain in only 1 of 20 cases investigated whereas all patients investigated by Huxlin showed evidence for reliable motion detection (27). We have previously proposed that methods examining the activation of a wide range of spatial and temporal channels leading to residual vision are more likely to show the presence of blindsight where it exists. Here we have found evidence for blindsight in 70% of cases. However, it remains to be investigated whether those without blindsight as defined by detection of spatial pattern would be capable of detecting other stimulus categories such as facial expressions or chromatic targets. Such abilities, should they exist, would mean that it is the lack of blindsight that is a rare phenomenon and not its presence.
A brief discussion of data reported in Fig. 3A is noteworthy. In the majority of cases (17 of 20), the presence or absence of significant detection abilities determined psychophysically matched the presence or absence of a significant pupil response. Nevertheless, we found three cases where no significant pupil responses were present when there was positive psychophysical evidence for above-chance detection. This is indeed intriguing. Significant psychophysically determined detection capability may rely on visual processing within the intact extrastriate areas in the ipsi-lesioned hemisphere. Alternative pathways may also include processing in cortical and/or subcortical areas in the contralesioned hemisphere activated via colossal connections, and it might be that pupillary responses are masked by autonomically mediated emotional changes. An intriguing possibility stems from the fact that the pupil responses are normally consensual and their presence for sighted-field targets were confirmed in at least two cases (P4 and P7). This finding indicates that activation of the contralesioned hemisphere results in normal pupil responses. Nevertheless, activity in the contralesioned hemisphere may not be sufficient to induce a pupil response for blindfield target presentation. It may be the case that the behavioral findings relate to processing in either or both hemispheres, whereas pupil responses rely mainly on activity in the ipsi-lesioned hemisphere. Further research is needed to investigate this possibility.
Materials and Methods
Participants.
Patients were selected from those attending the Acute Stroke Unit, Ophthalmology, or Neurology Departments of the Aberdeen Royal Infirmary. The main inclusion criterion was a homonymous visual-field defect after damage to the occipital cortex or optic radiation as determined by computer tomography scans during admission. All those with significant mobility problems, previous ocular surgery, or any retinopathy or cataract were excluded. None of the patients demonstrated evidence of significant cognitive impairment. Fig. 1 shows a summary of information regarding the patients, extent of binocular field defects, as well as field locations examined. Ethical approval was granted for this study by the Grampian Research Ethics Board, National Health Service, UK, and the study was carried out in accordance with the Helsinki convention. Data were collected over multiple recording sessions, the number of visits varying between patients depending on their availability and their ability to concentrate.
Experimental Setup.
Stimuli were programmed on an IBM compatible PC and were generated using a SVGA graphics card (VSG 2/5, Cambridge Research Systems). Stimuli were presented on a 21-inch monitor (Sony Multiscan G520) at a 100-Hz refresh rate. The monitor was enclosed in a cubicle with a chin headrest mounted at the edge of the cubicle at a viewing distance of 76 cm. The inside of the cubicle and all exposed surfaces were covered in nonreflective matt black felt to minimize scattered light from the areas surrounding the monitor. The monitor gamma corrections were carried out using a luminance meter (LumCal, Cambridge Research System) at 256 linear steps, and individual stimuli were subsequently checked using a separate luminance meter (Minolta LS100, Konica Minolta). The screen background luminance was 37 cd/m2 at the xy chromaticity of (0.309, 0.353) measured using a colorimeter (Tektronix J17 photometer with J1820 chromaticity head, Tektronix Inc.) and subtended 26.6° × 20.6°. The patient’s eye fixation was continually monitored using an ASL 5000 Pupillometer (Applied Science Laboratories), and any occasional trials containing eye movements were discarded.
Psychophysical investigations.
For psychophysical investigations, the stimuli were Gabor patches spatially limited in diameter to 10° (±2 × spatial SD of 2.5°). The Gabor patch included vertical sine waves with both the onset and the offset of the stimuli temporally smoothed using a temporal Gaussian function. The stimulus duration was limited to 2 s (4 × temporal SD of 500 ms). In addition, the Gabor patches were temporally modulated at 10 Hz. The fixation point was a high-contrast black crosshair subtending 0.5°. The spatial Gabor patches were presented within the field defect of the patients, ensuring a minimum of a 3° margin between the edge of the stimulus and the edge of the perimetrically determined blindfield. The spatial frequencies tested were 0.5, 1.0, 2.0, 3.5, 4.7, and 7 c/°. The space-averaged luminance of the stimuli was always the same as the background luminance (37 cd/m2) to avoid artifacts as a result of changes in total light flux. Measurements were conducted using a temporal two-alternative forced-choice technique (18) when only one interval contained a spatial pattern. In all but four cases (P1, P8, P11, and P18) a minimum of five blocks of trials were recorded, each block containing 60 trials (10 repetitions at each spatial frequency). Significant psychophysical detection at each spatial frequency refers to probability of detection calculated using binomial distribution (two-tailed).
Pupillometric investigations.
Pupil diameter was recorded using a pupillometer (ASL5000, Applied Sciences Laboratories) with modified optics to allow high precision (> 0.01 mm) measurements. Similar to previous research (9), responses were measured to the onset of high-contrast vertical sinewave grating (98%). Circular stimuli (10° diameter) were placed at the same retinal locations and spatial frequencies as those for psychophysical measurements conducted in each patient. Measurements were obtained in blocks of 60 trials (10 at each frequency), and on average four to five blocks of recordings were obtained within the blindfield and three to four blocks within the sighted field of the patients with presentation orders being arranged by field and counterbalanced between sighted and blindfields where possible.
Analysis of pupil data.
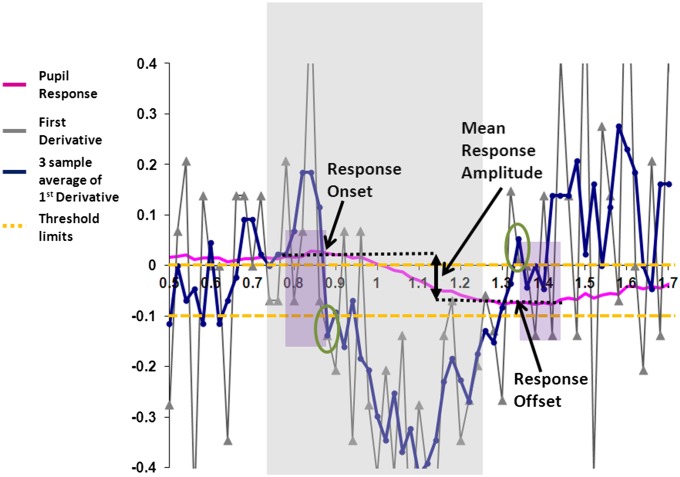
Individual pupil traces recorded are inherently noisy, so to increase the signal-to-noise ratio, a number of traces (30–60) are often averaged. The pink line in Fig. 4 shows a typical average pupil trace. To quantify the response amplitude, previous studies have marked the points of deflections indicating the start of pupil and points of maximum constrictions by visual inspection. The calculated difference in pupil diameter between the two points is taken as the response amplitude (8, 11, 15). Although the technique has high interexperimenter reliability (28), it is nevertheless subjective and open to bias. We have developed an objective technique for identifying the two points of deflection denoting the start and end of pupil responses to automate the process and identify the response objectively.
Fig. 4.
A graphical representation of a method for detection of pupil response. A typical average pupil response is shown in pink. The time interval of interest is between 250 and 750 ms poststimulus onset. The first derivative (blue line) and three-sample average (gray) lines are also shown together with threshold limits (yellow lines). The response onset is when the first derivative is negative and the three-sample average is lower than the threshold value (0.1 mm/s). Response plateaus when both the first derivative and the three-sample average are zero or positive. The difference between the pupil diameters averaged over six samples immediately before the response onset and after the response offsets reflects the mean response amplitude.
Based on previous research, the pupil constriction is expected to have response latencies between 250 and 750 ms poststimulus onset (8). The pupil average diameter trace was first differentiated with respect to time. The first differential is inherently noisy due to minor fluctuations between two consecutive samples. A smoothing procedure was carried out by obtaining a three-sample window average (averaging each sample and its immediately preceding and following sample) of the first differential. The response onset was defined at a point in time when both the first differential was negative (indicating a reduction in diameter) and the three-sample average value exceeded a set criterion (0.1 mms−1). The response offset was determined when both the first differential and its three-sample average were zero or positive (indicating that the response had reached its maximum and started to plateau or head back toward the steady-state level). The pupil response amplitude was defined as the difference between the average of six samples (120 ms at a 50-Hz sampling rate) before and including response onset diameter, and the average of six samples post-response offset, including the diameter at offset. To validate this approach, we obtained pupil grating responses in normal observers (n = 6) under 24 different experimental conditions (spatial frequencies, contrasts, and eccentricities). Fifty responses under each condition were averaged for each participant, the response onsets were detected, and amplitudes were determined using both visual inspection and the automated techniques. The response amplitudes between the two techniques were highly correlated (Spearman’s rho correlation coefficient = 0.994, P < 0.001). Crucially, the inspection technique showed a lack of pupil response in 3 of 144 conditions. The same three conditions were also identified as those without pupil responses using the automated technique. Once the average response amplitude is determined, the variance is calculated by measuring the diameter change between the same two time intervals identified using the automated algorithm for each individual trace obtained in every trial and testing of the dataset for significant deviation from zero using a one sample t test. Pupil response amplitudes plotted in Fig. 2 for sighted and blindfield stimulations were determined using the automated technique described above.
Acknowledgments
The equipment for the study was funded by a Royal Society (London) project grant to A.S. The study was funded by Chief Scientist’s Office Grant CZB/4/30 from the Scottish Executive.
Footnotes
The authors declare no conflict of interest.
References
- 1.Loewenfeld IE. The Pupil: Anatomy, Physiology, and Clinical Applications. Oxford: Butterworth-Heinemann; 1999. [Google Scholar]
- 2. Al Razi (903) translated as Liber ad Ammansorem (1187), referenced at US National Library of Medicine. Available at www.nlm.nih.gov/exhibition/islamic_medical/islamic_06.html. Accessed May 28, 2013.
- 3.Zinn KM. The Pupil. Springfield, IL: Charles C Thomas Publisher; 1972. [Google Scholar]
- 4.Bell RA, Thompson HS. Relative afferent pupillary defect in optic tract hemianopias. Am J Ophthalmol. 1978;85(4):538–540. doi: 10.1016/s0002-9394(14)75251-1. [DOI] [PubMed] [Google Scholar]
- 5.Brindley GS, Gautier-Smith PC, Lewin W. Cortical blindness and the functions of the non-geniculate fibres of the optic tracts. J Neurosurg Psychiat. 1969;32(4):259–264. doi: 10.1136/jnnp.32.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohn M, Clynes M. Color dynamics of the pupil. Ann N Y Acad Sci. 1969;156(2):931–950. doi: 10.1111/j.1749-6632.1969.tb14024.x. [DOI] [PubMed] [Google Scholar]
- 7. Barbur JL, Birch J, Harlow AJ (1993) Colour vision testing using spatiotemporal luminance masking. Psychophysical and Pupillometric Methods: Colour Vision Deficiencies XI, ed Drum B (Kluwer Academic Publishers, Dordrecht, The Netherlands), pp 417–426.
- 8.Sahraie A, Barbur JL. Pupil response triggered by the onset of coherent motion. Graefes Arch Clin Exp Ophthalmol. 1997;235(8):494–500. doi: 10.1007/BF00947006. [DOI] [PubMed] [Google Scholar]
- 9.Barbur JL, Harlow AJ, Sahraie A. Pupillary responses to stimulus structure, colour and movement. Ophthalmic Physiol Opt. 1992;12(2):137–141. doi: 10.1111/j.1475-1313.1992.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 10.Slooter J, van Norren D. Visual acuity measured with pupil responses to checkerboard stimuli. Invest Ophthalmol Vis Sci. 1980;19(1):105–108. [PubMed] [Google Scholar]
- 11.Barbur JL, Thomson WD. Pupil response as an objective measure of visual acuity. Ophthalmic Physiol Opt. 1987;7(4):425–429. [PubMed] [Google Scholar]
- 12. Cocker KD, Moseley MJ, Stirling HF, Fielder AR. (1998) Delayed visual maturation: Pupillary responses implicate subcortical and cortical visual systems. Dev Med Child Neurol Mar 40(3):160–162. [DOI] [PubMed]
- 13.Gamlin PDR, Zhang H, Harlow AJ, Barbur JL. Pupil responses to stimulus color, structure and light flux increments in the rhesus monkey. Vision Res. 1998;38(21):3353–3358. doi: 10.1016/s0042-6989(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 14.Weiskrantz L. Blindsight: A Case Study and Implications. Oxford: Oxford University Press; 1986. [Google Scholar]
- 15.Weiskrantz L, Cowey A, Le Mare C. Learning from the pupil: a spatial visual channel in the absence of V1 in monkey and human. Brain. 1998;121(Pt 6):1065–1072. doi: 10.1093/brain/121.6.1065. [DOI] [PubMed] [Google Scholar]
- 16.Weiskrantz L. The Ferrier lecture, 1989. Outlooks for blindsight: Explicit methodologies for implicit processes. Proc R Soc Lond B Biol Sci. 1990;239(1296):247–278. doi: 10.1098/rspb.1990.0016. [DOI] [PubMed] [Google Scholar]
- 17.Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97(4):709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- 18.Barbur JL, Harlow AJ, Weiskrantz L. Spatial and temporal response properties of residual vision in a case of hemianopia. Philos Trans R Soc Lond B Biol Sci. 1994;343(1304):157–166. doi: 10.1098/rstb.1994.0018. [DOI] [PubMed] [Google Scholar]
- 19.Schmid MC, et al. Blindsight depends on the lateral geniculate nucleus. Nature. 2010;466(7304):373–377. doi: 10.1038/nature09179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahraie A, Trevethan CT, Macleod MJ, Weiskrantz L, Hunt AR. The continuum of detection and awareness of visual stimuli within the blindfield: From blindsight to the sighted-sight. Invest Ophthalmol Vis Sci. 2013;54(5):3579–3585. doi: 10.1167/iovs.12-11231. [DOI] [PubMed] [Google Scholar]
- 21.Sahraie A, et al. Improved detection following Neuro-Eye Therapy in patients with post-geniculate brain damage. Exp Brain Res. 2010;206(1):25–34. doi: 10.1007/s00221-010-2395-z. [DOI] [PubMed] [Google Scholar]
- 22.Stoerig P, Cowey A. Wavelength discrimination in blindsight. Brain. 1992;115(Pt 2):425–444. doi: 10.1093/brain/115.2.425. [DOI] [PubMed] [Google Scholar]
- 23.Sahraie A, Trevethan CT, MacLeod MJ. Temporal properties of spatial channel of processing in hemianopia. Neuropsychologia. 2008;46(3):879–885. doi: 10.1016/j.neuropsychologia.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Cowey A, Alexander I, Heywood C, Kentridge R. Pupillary responses to coloured and contourless displays in total cerebral achromatopsia. Brain. 2008;131(Pt 8):2153–2160. doi: 10.1093/brain/awn110. [DOI] [PubMed] [Google Scholar]
- 25.Sahraie A. Induced visual sensitivity changes in chronic hemianopia. Curr Opin Neurol. 2007;20(6):661–666. doi: 10.1097/WCO.0b013e3282f1c70f. [DOI] [PubMed] [Google Scholar]
- 26.Marzi CA, Tassinari G, Aglioti S, Lutzemberger L. Spatial summation across the vertical meridian in hemianopics: A test of blindsight. Neuropsychologia. 1986;24(6):749–758. doi: 10.1016/0028-3932(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 27.Huxlin KR, et al. Perceptual relearning of complex visual motion after V1 damage in humans. J Neurosci. 2009;29(13):3981–3991. doi: 10.1523/JNEUROSCI.4882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conway CA, Jones BC, DeBruine LM, Little AC, Sahraie A. Transient pupil constrictions to faces are sensitive to orientation and species. J Vis. 2008;8(3):1–11. doi: 10.1167/8.3.17. [DOI] [PubMed] [Google Scholar]