Acute inflammation is an innate host-defense mechanism against invading pathogens and tissue injury. Neutralization of the offending insult ideally prompts resolution of inflammation and restoration of tissue homeostasis. Resolution of inflammation is an active process governed by resolution programs (1), involving a novel group of lipid and protein mediators, which possess dual anti-inflammatory and proresolution properties (2). Excessive inflammation or failure of the initial inflammatory response to resolve in a timely manner results in nonresolving inflammation and persisting tissue damage, which are now considered as critical components of many chronic human diseases (3). Intriguingly, many pro- and anti-inflammatory signals and proresolving circuits converge on select receptors, which integrate contrasting cues to determine the course of inflammation. Among these receptors is ALX/FPR2 (lipoxin A4 receptor or formyl peptide receptor 2; hereafter referred to as ALX), which binds both protein and lipid ligands that evoke opposing biological responses (4, 5). At present, little is known about the molecular basis for such diverse actions. In PNAS, Cooray et al. (6) present another advance in understanding signaling through the ALX receptor. Using coimmunoprecipitation and bioluminescence resonance energy transfer (BRET) assays in transfected cells combined with functional assays in leukocytes and in vivo, the authors provide important insights into how ligand-biased activation and conformational change in ALX shape opposing biological functions.
FPRs are a group of G-protein–coupled receptors (GPCRs) that are expressed predominantly by phagocytes and play a critical role in host defense. The three human FPRs, FPR1, ALX, and FPR3 share significant sequence homology (5). FPRs were identified as receptors for N-formylated peptides released from bacteria and mitochondria upon cell damage. ALX binds an unusually large number of structurally diverse ligands and conveys contrasting biological signals. For example, ALX mediates the proinflammatory actions, including leukocyte trafficking, activation, and delaying neutrophil apoptosis of the acute-phase protein serum amyloid A (SAA) and the antimicrobial peptide LL-37 (7, 8). Annexin A1 (AnxA1), AnxA1-derived peptide Ac2-26, and lipoxin A4 (LXA4) also signal through ALX to inhibit leukocyte recruitment, enhance neutrophil apoptosis, and macrophage efferocytosis (2, 7, 9–11), key events in inflammatory resolution. Lipid and peptide ligands act with different affinities and bind to distinct pockets on the receptor, thus making a direct competition unlikely (4). Monomer FPRs have traditionally been perceived as receptors that can recognize N-formylated peptides as well as LXA4. In this model, the tightly packed structure of FPRs would assure ligand specificity with docking of G proteins to the intracellular surface of the activated receptor as a transduction mechanism. Consistently, peptide Ac2-26 was found to signal through both ALX and FPR1 monomers (6).
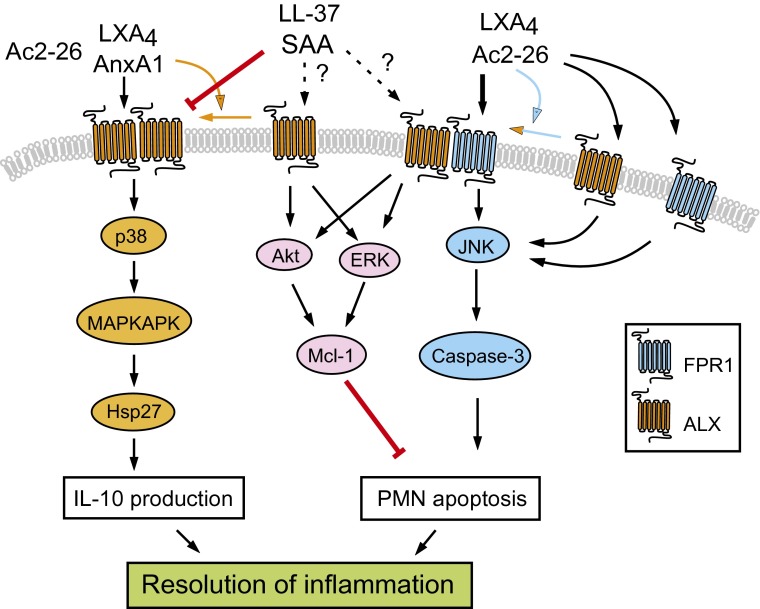
GPCRs can form dimers that affect receptor mobility, assembly, and activation (12). Although dimerization of GPCRs may not generally be required for ligand recognition, emerging evidence indicates that GPCR dimers may affect signaling functions and that ligand selectivity may be directly related to different GPCR conformation (12, 13). Cooray et al. document that ALX/ALX homodimers and ALX/FPR1 heterodimers constitutively occur in transfected HEK293 cells and human leukocytes (6). The authors also show that dimer formation and change in receptor conformation (assessed by BRET signal) can be induced in a ligand-specific fashion, and this does not appear to be simply a consequence of ligand–receptor interaction. Thus, AnxA1 stimulated ALX homodimerization and activated the p38 MAPK/MAPKAPK/Hsp27 signaling cascade to augment production of the anti-inflammatory cytokine IL-10 (Fig. 1). Peptide Ac2-26 facilitated ALX/FPR1 dimer formation and activated the JNK-caspase-3 pathway to induce neutrophil apoptosis, a critical control point in the resolution of inflammation (14). Peptide Ac2-26 effectively counteracted the potent apoptosis-suppressing signals from SAA. Although not shown in this study, it is likely that peptide Ac2-26 would induce degradation of the antiapoptotic protein Mcl-1 (Fig. 1), a master regulator of neutrophil life span. Cooray et al. (6) identify similarities in peptide Ac2-26 signaling through ALX and FPR1 monomers, but also differences that suggest a distinct function for the ALX/FPR1 dimer. Intriguingly, SAA does not seem to affect assembly or conformation of the ALX/ALX and ALX/FPR1 dimers.
Fig. 1.
Biased ALX ligands and ALX dimers modulate resolution of inflammation. Annexin A1 (AnxA1) or the nonselective FPR ligand peptide Ac2-26 induces ALX homodimerization and conformational change, and activates the p38 MAPK/MAPKAPK/Hsp27 pathway to release IL-10. Peptide Ac2-26 (and LXA4) evokes ALX/FPR1 dimerization and signals through the ALX/FPR1 dimer and to a lesser efficacy through the ALX and FPR1 monomers to activate the JNK/caspase-3 pathway, leading to apoptosis in neutrophils (PMN, polymorphonuclear neutrophil leukocytes), one of the control points in resolution. Activation of this pathway overrides the apoptosis suppressing action of SAA and LL-37, although the receptors mediating this latter action remain to be identified. SAA counters AnxA1-induced conformational change in ALX homodimers and blocks anti-inflammatory circuits.
These unique findings allow us to comment on the complexity of ALX signaling. There is evidence that AnxA1 functions as one of the major effectors of LXA4 in the inflamed microvasculature (15). LXA4 did not mimic nor interfere with AnxA1-induced conformational change in ALX (6), providing additional insight into the molecular mechanisms of this endogenous anti-inflammatory network. LXA4 was shown to override the apoptosis-delaying actions of SAA in neutrophils (7), whereas SAA can overwhelm the protective signaling by LXA4 in patients with chronic obstructive pulmonary disease (16). Although the functional ALX isomer for SAA signaling remains to be identified, SAA reduction of AnxA1-evoked conformational change in the ALX/ALX dimer and the
Cooray et al. present another advance in understanding signaling through the ALX receptor.
resulting impaired activation of anti-inflammatory circuits may provide a molecular explanation for SAA-mediated defective resolution. It is noteworthy that LXA4 and resolvin E1 counterregulated the leukotriene B4 (LTB4)/LL-37 proinflammatory circuit, which is mediated by the LTB4 receptor BLT1 and ALX (8). Resolvin E1 can activate a proresolution circuit through BLT1 (17), highlighting the complex interplay between these lipid mediators and their receptors. Despite these fascinating findings, many intriguing questions about ALX remain. What is the minimal functional unit for ALX dimers? Studies on LTB4 receptors revealed that BLT1 forms a homodimer that binds to a single G protein, resulting in an asymmetric state where only one receptor protomer is active to transmit a signal (18). How do the protomers in ALX dimers affect each other to generate ligand-biased intracellular signals? Would ALX assemblies be transient and dynamic in the inflammatory microenvironment, perhaps representing an additional control mechanism for preventing excessive inflammation? These issues notwithstanding, the notion of ligand-biased control of ALX dimerization and activation of anti-inflammatory and proresolving circuits are an important advance in the understanding of the dynamic processes that orchestrate resolution of inflammation. These results also provide a basis for development of novel therapeutical approaches, such as cleavage-resistant AnxA1 (19) or stable LXA4 analogs (20), which could mimic endogenous pathways to dampen inflammation. If the rapid advancements in the resolution field in the past years are any indication, we may be much closer to achieving this than we now imagine.
Acknowledgments
This work was supported by Grant MOP-97742 from the Canadian Institutes of Health Research.
Footnotes
The author declares no conflict of interest.
See companion article on page 18232.
References
- 1.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 2.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Chiang N, et al. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58(3):463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 5.Ye RD, et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61(2):119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooray SN, et al. Ligand-specific conformational change of the G-protein–coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci USA. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Kebir D, et al. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: A novel mechanism for resolution of inflammation. J Immunol. 2007;179(1):616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 8.Wan M, Godson C, Guiry PJ, Agerberth B, Haeggström JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25(5):1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- 9.Solito E, et al. A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J. 2003;17(11):1544–1546. doi: 10.1096/fj.02-0941fje. [DOI] [PubMed] [Google Scholar]
- 10.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9(1):62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 11.Vago JP, et al. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J Leukoc Biol. 2012;92(2):249–258. doi: 10.1189/jlb.0112008. [DOI] [PubMed] [Google Scholar]
- 12.Lohse MJ. Dimerization in GPCR mobility and signaling. Curr Opin Pharmacol. 2010;10(1):53–58. doi: 10.1016/j.coph.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Mary S, et al. Ligands and signaling proteins govern the conformational landscape explored by a G protein-coupled receptor. Proc Natl Acad Sci USA. 2012;109(21):8304–8309. doi: 10.1073/pnas.1119881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi AG, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12(9):1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 15.Brancaleone V, et al. Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature. J Immunol. 2011;186(8):4905–4914. doi: 10.4049/jimmunol.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozinovski S, et al. Serum amyloid A opposes lipoxin A₄ to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2012;109(3):935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci USA. 2012;109(37):14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damian M, Martin A, Mesnier D, Pin JP, Banères JL. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 2006;25(24):5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalli J, et al. pro-resolving and tissue protective actions of annexin A1-based cleavage resistant peptides are mediated by FPR2/ALX. J Immunol. 2013;190:6478–6487. doi: 10.4049/jimmunol.1203000. [DOI] [PubMed] [Google Scholar]
- 20.Petasis NA, et al. Design and synthesis of benzo-lipoxin A4 analogs with enhanced stability and potent anti-inflammatory properties. Bioorg Med Chem Lett. 2008;18(4):1382–1387. doi: 10.1016/j.bmcl.2008.01.013. [DOI] [PubMed] [Google Scholar]