Significance
It has long been proposed that rules stemming from the mechanisms used during development can constrain the range of evolvable variations in a given form, but few empirical examples are known. We have focused on developmental processes determining proportions of phalanx size along individual digits (fingers/toes) of vertebrates. We find that phalangeal variation seen in nature is indeed constrained by an ancestral developmental program, limiting morphologies to a continuum from nearly equal-sized phalanges to a large-to-small gradient of relative sizes. Nonetheless, later innovations in distal regulation expanded variational possibilities for groups that needed greater grasping ability. These data provide a better understanding of how properties of developmental systems work in combination with natural selection to guide evolution of skeletal proportions.
Keywords: developmental module, developmental constraint, phalanx
Abstract
Evolutionary theory has long argued that the entrenched rules of development constrain the range of variations in a given form, but few empirical examples are known. Here we provide evidence for a very deeply conserved skeletal module constraining the morphology of the phalanges within a digit. We measured the sizes of phalanges within populations of two bird species and found that successive phalanges within a digit exhibit predictable relative proportions, whether those phalanges are nearly equal in size or exhibit a more striking gradient in size from large to small. Experimental perturbations during early stages of digit formation demonstrate that the sizes of the phalanges within a digit are regulated as a system rather than individually. However, the sizes of the phalanges are independent of the metatarsals. Temporal studies indicate that the relative sizes of the phalanges are established at the time of initial cell condensation. Measurements of phalanges across species from six major taxonomic lineages showed that the same predictable range of variants is conserved across vast taxonomic diversity and evolutionary time, starting with the very origins of tetrapods. Although in general phalangeal variations fall within a range of nearly equal-sized phalanges to those following a steep large-to-small gradient, a novel derived condition of excessive elongation of the distal-most phalanges has evolved convergently in multiple lineages, for example under selection for grasping rather than walking or swimming. Even in the context of this exception, phalangeal variations observed in nature are a small subset of potential morphospace.
The impacts of modularity and developmental constraint on trait evolvability are enduring themes in evolutionary developmental biology (1–6). Morphological modules are identified as strongly covarying structures; if the source of this covariance is developmental integration, we expect that development will produce a particular subset of phenotypic variations within populations rather than varying in all possible directions (4). This biased set of phenotypes then will influence evolutionary patterns by limiting options for natural selection. Theory suggests that evolution of stable integrated developmental units plays a major role in why we see the set of morphological forms that exist in nature today, in which some integrated developmental units become reinforced over generations and others break apart or reorganize when such innovations are selectively advantageous (4, 5). As a tool to assess modularity and constraint, morphospace, in which measurements are plotted and compared to analyze factors that affect size and shape, is commonly used as a means to compare differences in morphological form, (7).
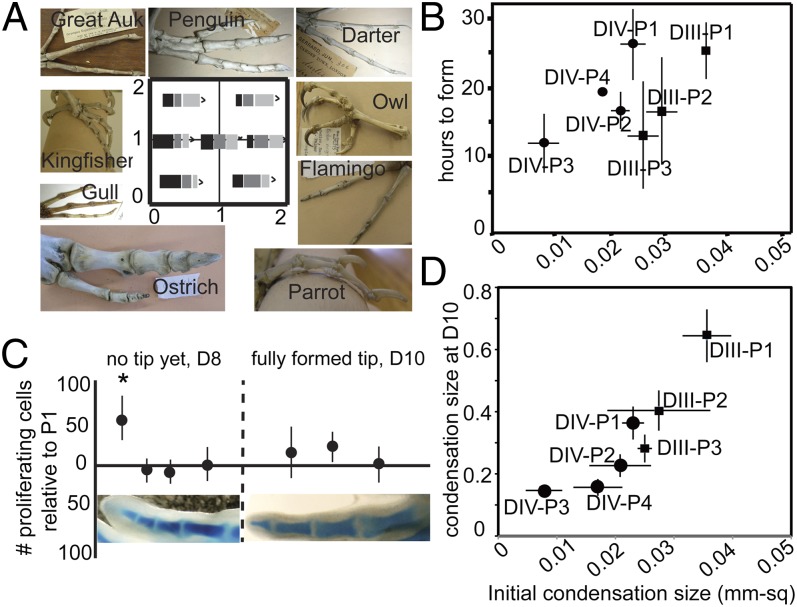
Modularity in the limb has been widely cited as the basis for the conservation of the basic three-part structure of the limb: the stylopod (upper arm or leg), zeugopod (lower arm or leg), and autopod (hand and wrist or foot and ankle). This structural conservation contrasts with the large range of adaptive variations in size and shape of limb segments among species. How this modular structure and variation apply to the most distal limb parts, the fingers and toes, has not been explored. Digits evolved much later than the antecedents of the more proximal limb structure and are thought to be neomorphic, arising with the invasion of land by amphibians in the Devonian era (8, 9). Clearly, fingers and toes are used for different functions in different species, and the number, size, and shape of phalanges varies tremendously across tetrapod taxa. Bird toes, for example, allow species to grasp prey, perch on branches, run, paddle, or dig. These different functions correlate with different skeletal proportions in the series of phalanges bones. Different positions of the toe joints, which determine proportions, thus are likely to be selectively advantageous for particular lifestyles (Fig. 1A).
Fig. 1.
(A) Morphospace potential for bird toe proportions. The x-axis shows the ratio of P2/P1; the y-axis is the ratio of P3/P1. Black rectangles indicate P1, dark gray rectangles indicate P2, and light gray rectangles indicate P3. The variations in the toe proportions of a variety of skeletal preparations of birds are shown. (B) In chick digits the number of hours required for a phalanx to develop from the proximal to the distal joint is related to the size of the initial (distal) condensation. (C) Density of proliferating cells (BrdU-incorporated cells) is relatively high in the distal-forming tip of digits, but once the distal joint is formed on a phalanx, proliferation is reduced. No significant differences in proliferation rate are found among formed phalanges within a digit. (D) The size of the initial condensation is correlated with size of the condensation when the final pattern is achieved (day 10) in digits III and IV in chicken embryos.
The extent to which the sizes of the skeletal elements of the limb are truly independent of one another has not been fully explored. Here we address this question from a developmental perspective, focusing on the most distal limb elements, the phalanges.
Results and Discussion
Restriction in the Variation of the Relative Proportions of Phalanges.
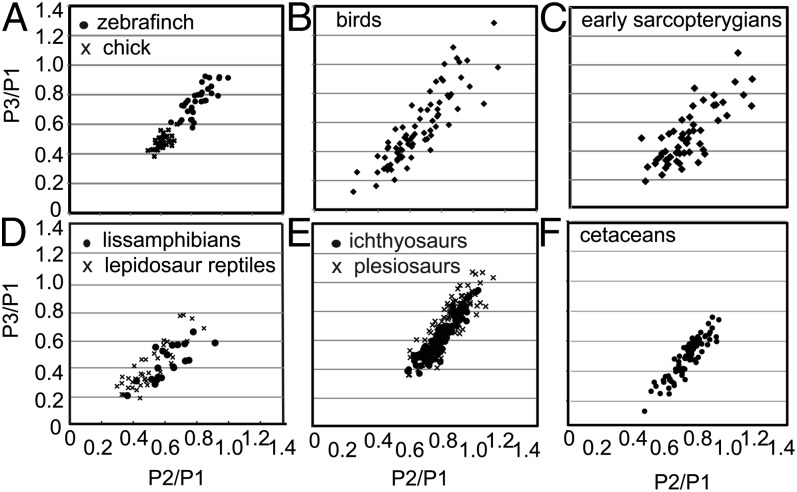
The phalanges form by a process of sequential segmentation. Cells are added continuously to the distal end of each digit ray from the so-called “digit-forming region” (DFR) at the tip (10). When the newly formed cartilage reaches a critical length, a joint is initiated behind the growing tip, establishing a phalanx behind the new joint, and growth of the digit ray continues distal to the new joint. The skeletal elements of the limb generally are considered to have unique individual developmental identities specified within the three major limb segments. However, whether this modularity applies to the phalanges has been controversial. Some have ascribed a uniquely specified identity to each phalanx (11), whereas others have suggested that the digits are specified as a whole with the phalanges being generated through a reiterative segmentation mechanism in the context of the digit identity (12). To differentiate between these hypotheses, we examined the variation in phalanx size within a single species. We reasoned that if phalanges are specified independently of one another, then the sizes of different homologous phalanges should vary independently among individuals. In contrast, if phalanges are established as part of a developmental module, then their proportions should covary. We measured the 2D area of the first, second, and third phalanges (P1–P3) of the fourth hind limb digit (digit IV) from radiographs of large collections of adult chicken and Zebra Finch skeletons. For each individual we plotted the ratio of P3/P1 versus the ratio of P2/P1, with each individual represented by a single point in morphospace. Strikingly, the plotted ratios for both species fall closely along a single line (Fig. 2A). With knowledge of this relationship, one can predict the size of P3 accurately by knowing the sizes of P1 and P2.
Fig. 2.
Variation in proportions of phalanges within and among vertebrate groups. All groups vary along a line from equal-sized to a proximodistal gradient. Ratios are taken from area measurements and are standardized against the size of the first phalanx. x-axis: P2/P1; y-axis: P3/P1. Plots of the proportions of individual phalanges are for digit IV, P1–3 in (A) individual Zebra Finch and chick populations, (B) birds, (D) lissamphibians, and reptiles. In hyperphalangic (C) early sarcopterygians, (F) cetaceans, (E) ichthyosaurs, and plesiosaurs, the plots are ratios of multiple sets of three sequential phalanges. Equations for the lines are Zebra Finch, y = 0.7528 + 0.1412, r2 = 0.43; chick, y = 0.63 × 0.03, r2 = 0.49; P < 0.0001; birds, y = 1.147 × −0.19, r2 = 0.77; early sarcopterygians, y = 0.944 × −0.17, r2 = 0.62; lissamphibians, y = 0.743 × −0.03, r2 = 0.605; reptiles, y = 0.949 × −0.08, r2 = 0.723; cetaceans, y = 1.21 × −0.39, r2 = 0.825; ichthyosaurs, y = 1.23 × −0.32; r2 = 0.71; plesiosaurs, y = 1.327 × −0.44; r2 = 0.854.
To verify that the apparent relationship between phalanges reflects a meaningful developmental integration in the formation of successive digits, we conducted simulations in which phalanx sizes were reassigned in series. The results substantiate that sizes of phalanges are highly unlikely to vary independently (P < 0.001) (SI Appendix).
Time and Size of Formation of Individual Phalanges.
The lack of independence among phalanges suggests that there might be a developmental linkage between the formation of successive phalanges in a growing digit. Previous studies have noted that there is a cyclic oscillation in the expression of the Hairy2 gene in the developing chick autopod, resembling the activity of the gene segmentation clock during somitogenesis (13). Moreover it was noted that the period of this oscillation, 6 h, is precisely half that of the formation of a phalanx, in particular the second phalanx (P2) of wing digit II, suggesting that phalanges could be generated on a periodic basis every two cycles of a clock (13). If true, this observation might suggest that segments would form at a constant rate and on a constant scale, with differences in phalanx size being attributable to subsequent differential growth. However, this previous study determined the timing of the formation of only a single phalanx.
To address the timing of phalanx formation more generally, we used two approaches. First, we examined specimens from a fine-scale (2-h) time series through phalangeogenesis in chicks. In addition, we removed one foot of a chicken embryo in ovo, let the embryo grow a variable (4–48) number of hours, harvested the second foot, and then counted the differences in the number of joints formed in the first and second feet to determine the maximum number of hours between the formation of the proximal and distal joints of a given phalanx (SI Appendix). Both sets of observations showed that there is clear variation in the number of hours required to form different phalanges, with the phalanges that ultimately will be the largest having the longest period of formation (∼25 h), and the smaller phalanges having significantly shorter periods (8–15 h) (Fig. 1B).
The difference in the timing of formation of the different phalanges could, in principle, reflect distinct rates of the condensation process in different elements; alternatively, the difference could be attributable to distinctions in the size of the condensations when they first form. To address these possibilities, we next measured condensation sizes in 161 chicken embryos and 168 Zebra Finch embryos that had been fixed during the period of phalangeogenesis and then stained with Alcian Blue cartilage stain and cleared with KOH. The smallest condensation size was determined by measuring the area of condensation just after the proximal joint of the phalanx was initiated. Down-regulation of cartilage matrix precedes formation of the joint interzone (i.e., the Alcian Blue staining becomes clear), and thus the earliest stages of joint position can be seen easily in Alcian Blue-stained hind limbs. In evaluating whether our sample size was sufficient to detect the initial condensations, we considered that five samples within 5% of the smallest measurement indicated a sampling level at which the smallest initial condensation size was known with confidence (SI Appendix). We found that initial condensation sizes differed among phalanges and were significantly correlated with final phalanx size (P < 0.0001, ANOVA) (Fig. 1D).
Although different phalanges form on a scale proportional to their final size, differences in the lengths also could be influenced by differential rates of growth. Therefore, we next used BrdU incorporation to evaluate the proliferation rates of the newly formed phalanges to evaluate the alternative hypothesis, i.e., that differing proliferation rates determine differing phalanx sizes. Although there is a higher rate of proliferation in the distal tip, where the DFR contributes cells to the condensation distal to the most recently formed joint, we saw no significant differences in the percentage of proliferating cells within the different phalanges (i.e., segments that have both proximal and distal joints; Fig. 1C). Thus, although proliferation may amplify differences in the relative size of the phalanges (larger phalanges having more total proliferating cells), the essential differences in relative phalanx size appear to be determined by the length of time that has elapsed and hence the number of additional cells added to the growing segment before a new joint forms.
Testing Developmental Modularity Within a Digit.
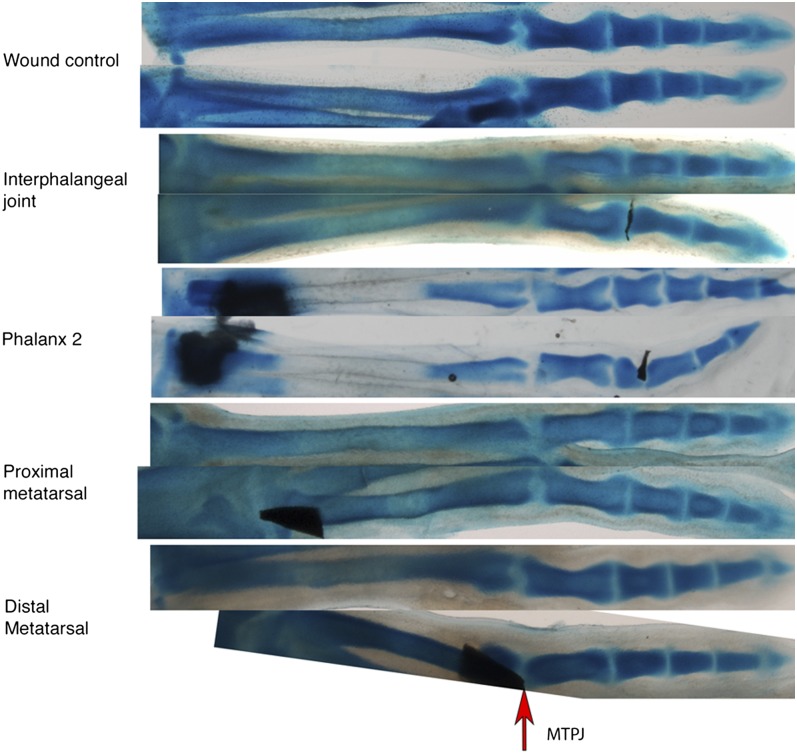
Within a given digit, the high degree of correlation in the variation of phalanx size suggested that phalanges are likely to develop as a modular unit. However, the different phalanges within a module are not all the same size but rather decrease successively in size. A parsimonious explanation would be that each phalanx negatively influences the size of the next, to an extent characteristic of each digit. Because we have found that the proportions of the various phalanges are established at the time of prechondrogenic condensation, such a negative influence, if it exists, must act at the time when the cartilage anlagen is first being set aside (14). To test this notion, we inserted a thin foil barrier into the distal phalanx-forming region, sometimes ending up in the forming joint interzone between the first and second phalanx at day 6.5 in the chick. The hope was that such a barrier would effectively block the influence of the proximal differentiating phalanx on the formation of the next. Indeed, such manipulations consistently resulted in the next forming phalanx (P2) being significantly longer than seen in the unoperated contralateral digit (n = 28/36) (Fig. 3). This phenotypic effect was rarely seen in wound controls (n = 2/27), indicating that the operation itself was not disruptive of phalanx length. Importantly, the impermeable barriers not only affected the next forming phalanx but also led to subsequent phalanges being of extended length, even though they were formed at a distance from the barrier and well after the digit had healed from the manipulation (Fig. 3). In essence, after insertion of the barrier, the still-to-be-formed phalanges behaved as a modular set, seemingly starting a decreasing sequence anew, distal to the barrier. In most cases the overall length of the digit with the barrier insertion was very close to that seen on the contralateral side, indicating that only the modular segmentation process, and not the overall growth parameters, had been affected. In addition, as expected, the terminal phalanx, which is known to be formed by a process different from that forming the proximal phalanges (15), always was unaffected.
Fig. 3.
Barrier experiments. Tantalum foil barriers inserted into developing phalanges affect the size of the distal phalanges. Barriers inserted into developing metatarsals affect metatarsal length but not the patterning of the phalanges. The top digit in each pair is the contralateral control. Proximal is to the left. Images are aligned by metatarsal–phalangeal joint (MTPJ).
Many influential evolutionary studies treat the metapodials as separate from the phalanges, likely because they are observed to vary independently of the loss of the digits (16–18). In contrast to their obvious functional and variational independence from the phalanges within a digit, the morphogenesis of metapodials appears to be exactly the same as that of the phalanges. Thus, the metacarpal and metatarsal bones often are viewed as forming from the same “digital rays” as the phalanges distal to them. No histological or structural differences or differences in gene expression have been reported (19–21). Here, we used an experimental approach to test whether metatarsals are part of the same developmental module as the phalanges. We inserted foil barriers in joint-forming regions between the metatarsal and the presumptive proximal phalanx on day 5.5 of chick development, and embryos were fixed at day 10 when the tip had formed. We found that, although the metatarsals were consistently and variably shortened (25/25 of affected digits were shortened), the phalanges developed normally 100% of the time (Fig. 3). Thus, the metatarsals do not appear to influence the segmentation process forming the phalanges and accordingly appear to be a distinct developmental module.
The Impact of Modular Development on the Evolution of Phalanges.
We have found that the phalanges of a given digit are formed as a module. Therefore, although the absolute sizes of homologous phalanges vary within a population, the set of phalanges in any digit maintains predictable size ratios. One consequence of this limit on the range of variation within a population would be a limited range of phenotypes upon which selective forces can act. Thus, in theory, the modular development of the phalanges within a digit would be expected to result in a consequent bias in the evolution of phalangeal patterns. To look at this possibility, we examined the ratios of phalangeal sizes within representative individuals from all available vertebrate taxa that have three or more phalanges in a series (excluding the clawed tip). Area (2D) measurements were taken from digital images of articulated skeletons or fossils or from radiographs of museum specimens. Size proportions were plotted separately for each major group. In some species the sequential phalanges in a given digit are almost the same size, whereas in others there is a dramatic decrease in size from phalanx to phalanx. However, the ratios of the sizes of the phalanges within these digits form a set of proportion variations that is consistent in all taxa (Fig. 2). For example, in birds, when proportions are plotted with P2/P1 vs. P3/P1, the variational distribution of proportions of digit IV, P1–3 in all birds appears to be an extension of the variation found among individuals in chick and Zebra Finch populations (Fig. 2). Statistical analysis shows a high likelihood that both chick and Zebra Finch populations fit the same regression line, which is not detectably different from the regression line for all bird species (P < 0.0001) (SI Appendix). These results demonstrate that the influence of modular development on variation within bird populations has indeed influenced evolution within class Aves as a whole. The same restriction in phalangeal variation is seen in other taxonomic groups, including land reptiles, plesiosaurs, ichthyosaurs, amphibians, and cetaceans. The variation is limited so that, by knowing the sizes of two phalanges, one can reliably predict the size of a third.
To see if the developmental integration in the formation of the digital skeleton and the consequent predictability of relative phalanx sizes is a basal feature of tetrapods, we measured the phalanges present in the earliest autopods known from the fossil record, including several extinct amphibian species (22, 23), and from lungfish embryos, because they are a living representative of a basal sarcopterygian, the group from which tetrapod vertebrates evolved (24). The ratios of phalanx sizes in these early autopods are similar to those seen in modern tetrapods, suggesting that the digit skeletal elements have been formed as a developmental module with biased variations since the origination of the autopod, with metapodials later evolving into a separate module. Indeed when each major modern taxon was plotted against basal groups as a whole, no significant differences in slope were observed (Fig. 2 and SI Appendix).
Innovation in the Evolution of the Distal Phalanges in Derived Groups.
There is one major and very informative exception to this overall pattern. In the hindlimbs of some birds and in the forelimbs of some bipedal dinosaurs and climbing or digging reptiles, the penultimate phalanx (the last phalanx before the tip) is elongated. All these exceptions apparently are coincident with the lack of primary dependence on the limb for propulsion. For example, after examining digital images of a diverse sample of 76 species of birds, P4 of digit IV (and sometimes P3 of digit III) was seen to be significantly longer than anticipated, based on the foregoing analysis, in a number of different species (Fig. 4 and SI Appendix). Differences in the relative length of the penultimate phalanx of digits III and IV has been noted previously as a key variable that has been interpreted functionally relative to the lifestyle of birds along a simple spectrum from arboreal to cursorial (25, 26). However, these prior studies did not consider the penultimate phalanges in the context of the overall proportions of the proximal phalanges.
Fig. 4.
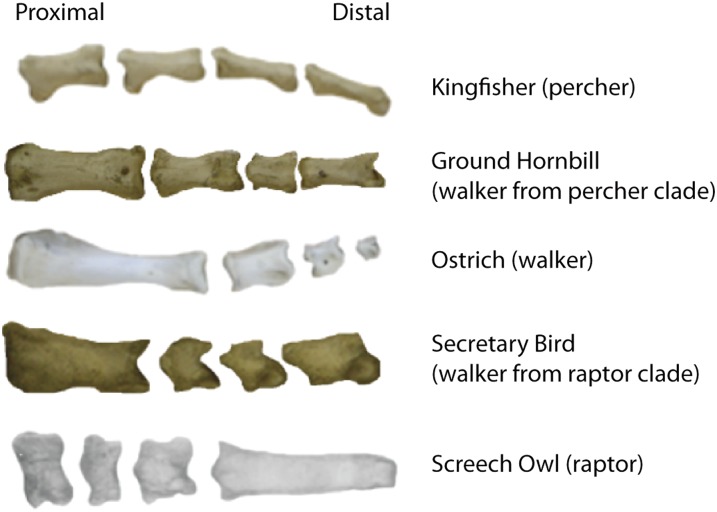
Examples of convergence in phalanges proportions. From left to right, P1–P4 are aligned; ungual phalanges (tips) have been removed. Phalanges proportions evolve along predicted lines, e.g., the Ground Hornbill, a walking species whose closest taxon is primarily perching, has evolved toward the walking variant by elongating P1 and developing a steeper proximodistal gradient.
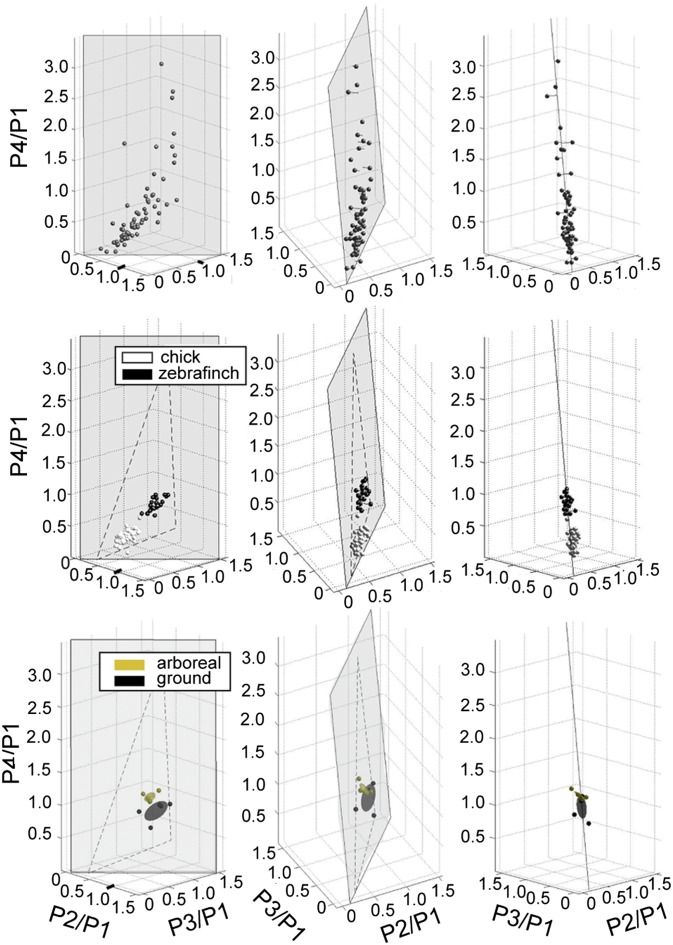
To gain a better understanding of the variation in digit IV, we plotted the data for digit IV in 3D morphospace (plotting the ratios of P2/P1, P3/P1, and P4/P1, along the three Cartesian axes) (Fig. 5 and SI Appendix). If digit IV, P4 were regulated independently, all points would be expected to be directly vertical in the z plane above the x–y line. In contrast, we found the observed proportions fell within a roughly triangular plane that was significantly off the vertical. This “tilted” position of the plane results from the observation that, in the species with the most elongated P4s, the P3 is also slightly larger than expected by the basal pattern (see screech owl digit IV in Fig. 4). One plausible developmental model for this pattern of distal elongation would be a response to a distal signal extending growth and delaying segmentation of the distal phalanges superimposed on the general developmental program leading to proportional decrease in sequential phalanx size. As is consistent with this model, the phalangeal elongation we observe is always in the distal-most phalanges. This pattern also is reflected in the triangular shape of the plane, which indicates that some conceivable variations in proportion on the plane are never found in nature (e.g., long-short-long-short). This restricted morphospace is highly improbable unless all phalanges covary as part of the same module (SI Appendix) (27). In other words, compared with the majority of vertebrate digits, the distal-most phalanges in birds can become relatively longer than expected from the basal pattern, creating an increased range of variability for these groups. Variation among individuals within populations also falls within this same morphospace (Fig. 5B).
Fig. 5.
(Top) Phalanges proportions for digit IV in birds fall roughly within a triangular plane in 3D morphospace. (Middle) Chick (white dots) and Zebra Finch (black dots) individuals fall within the same morphospace plane as proportion variations among species. (Bottom) Galapagos finch species that are more arboreal (yellow) have altered proportions as compared with more ground-dwelling species (black). Ellipses are centroid + 1 SD.
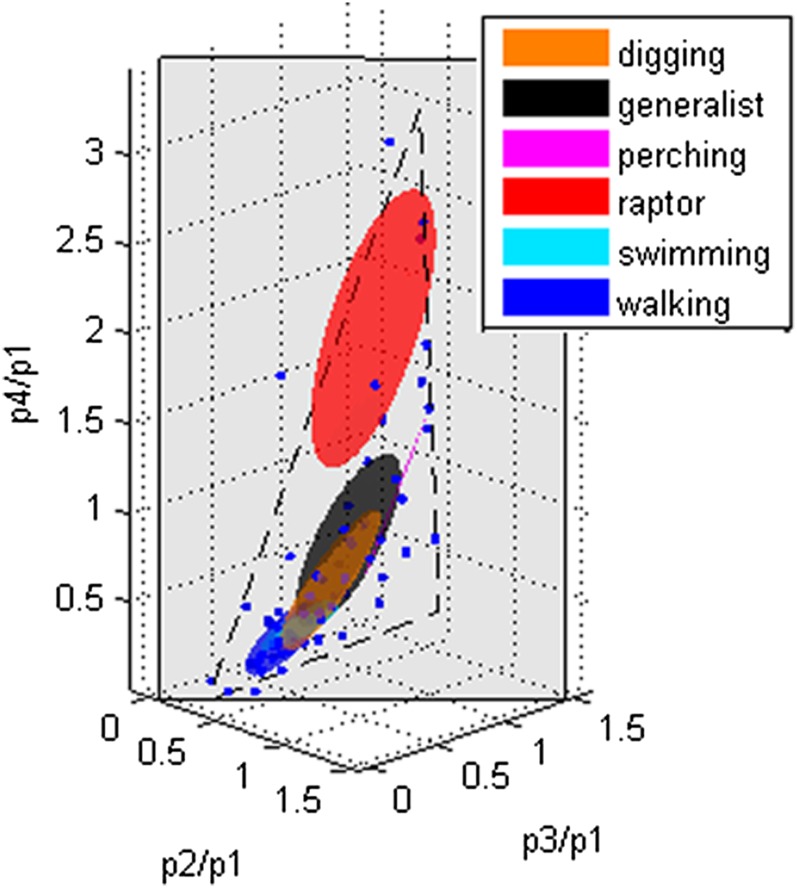
We next tested whether particular proportions in birds were correlated with function. Birds were categorized according to known lifestyles or behaviors involving toes of the particular species. The functional categories included raptors, perchers, diggers, walkers, swimmers, and generalists, with species categorized based on published literature. We found that particular proportions in 3D space were associated with particular functional categories (Fig. 6), with evolution occurring along lines in morphospace that suggest tradeoffs between competing functions (27).
Fig. 6.
Ellipses showing the patterns of the phalanges by functional groups established by known lifestyles of the birds. Ellipses are defined by the centroid + 1 SD in all directions for each functional group.
Indeed, by comparing variations found within and between related groups, we found evidence of convergence for function in several groups. For example, the secretary bird (Sagittarius serpentarius) is from a raptor clade, but its toes function not only for grasping prey on the ground but also for walking (28, 29). The proportions of the secretary bird’s phalanges are intermediate between those of raptors and flightless birds (walkers), with a P1 that is greatly elongated compared with that of any other raptor we measured (Fig. 4). In other words, given sufficient time and selective pressure, the proportions of phalanges in bird digits evolve as a module along predictable lines in morphospace (27), e.g., between optimized walking and grasping functions (Fig. 6).
Thus, despite the highly conserved relationships among phalangeal sizes attained in evolution, when dependence on walking is reduced for a given limb, the distal phalanges appear to be able to elongate dramatically, particularly in association with perching and grasping functions. Moreover, this evolution can occur relatively rapidly. We examined the range of variation in digit IV, P4 in a group of species that have undergone a relatively recent adaptive radiation, the Darwin's finches, which evolved in the Galapagos Islands within the last 3 million years (30). We found that in the more arboreal finches the distal phalanges are more elongated, consistent with the overall correlation between arboreality and P4 length among all birds. The variation in these finches again remains within the predicted plane in 3D morphospace (Fig. 5C).
Birds that are diggers, perchers, and raptors have eluded the constrained range of variation in phalangeal proportions through a developmental innovation that apparently has been layered on top of the basal modular phalanx program, allowing greater range of variation in the size of the distal phalanx than otherwise possible. In arboreal and digging mammals, e.g., in Grant’s Golden Mole (31) and in certain extinct taxa of sloths (32), this evolutionary challenge has been circumvented differently, by condensing phalangeal elements in normal proportions but then fusing phalanges with one another or with the metapodial to produce a long segment from shorter phalanges. Other arboreal mammals, such as the Two-Toed Sloth (33, 34), have paralleled the digging/perching/predatory birds in extending their distal phalanges to a greater extent than the proximal ones. An insight into at least a potential mechanism by which this extension might have occurred comes from a study (15) in which Fgf signaling was maintained experimentally at the distal tip of a growing digit ray. The result was an elongation of the penultimate phalanx, although the terminal claw was of normal proportions (as is the case in birds with a naturally extended penultimate P4 of digit IV). Thus, one might postulate that, in addition to the modular developmental program influencing the developing phalanges, the length of the phalanges also is positively influenced by signals such as Fgf produced at the tip of the DFR. One also could postulate that maintaining or increasing the level of this distal signal can superimpose an extension of the distal-most phalanges on the normal pattern of regularly decreasing phalanx size.
Conclusions
Although previous studies have reported that proportions of limbs and other skeletal elements are evolvable but are significantly constrained within and between segments (18, 35–37), the developmental basis for these observations has been unclear. Focusing on the distal elements of the autopod, we have found that the formation of the phalanges of each digit is specified as a developmental module rather than as a series of independently specified elements. As a consequence, there is a developmental bias, so that in independent vertebrate lineages the proportions of phalanges have evolved repeatedly within a limited subgroup of all possible variations in morphospace (Fig. 7). The variation arises developmentally during a short period of morphogenesis during which joint positioning is established. The scenario we propose involves an ancestral developmental system that allowed variations ranging from equal-sized to a large-to-small gradient, with later innovations in distal regulation that opened up new variational possibilities for groups that needed greater grasping ability. These data thus provide a better understanding of how the properties of developmental systems work in combination with natural selection to guide evolution of skeletal proportions in vertebrates.
Fig. 7.
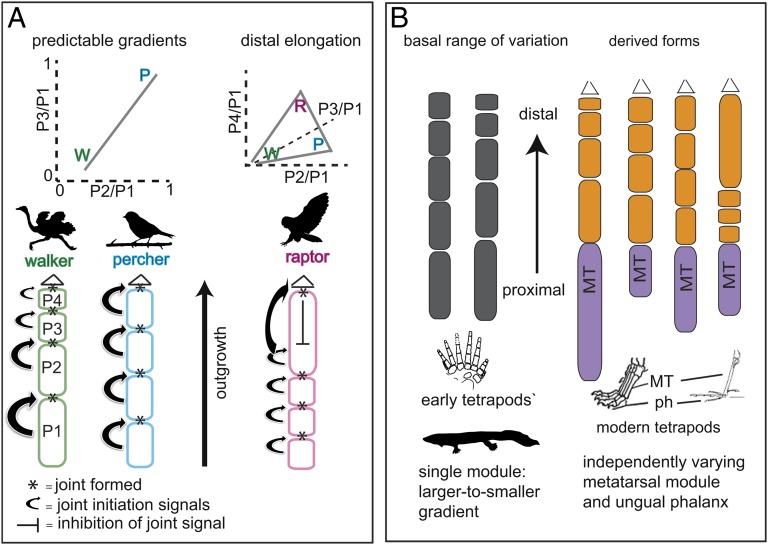
Summary of results. (A) Variants in phalanx proportions remain within a predictable range, from a large-to-small gradient in walkers to equal-sized in perchers to distal elongation of the penultimate phalanx in raptors. Walking and perching specialist phenotypes (W, P) are connected in morphospace by a line, where different phenotypes along the line show a trade off of functions (27). Three functions lead to a full triangle whose vertices are W, P, and the raptor specialist phenotype, R. Joint positions are determined in sequence as the digit grows. Distal elongation may be caused by the inhibition of the joint-initiation signals. (B) Hypothesis of origins of modules in the digit. Phalanges in early tetrapods vary as one module, whereas derived tetrapods have two independently varying modules. MT, metatarsal; ph, phalanx.
Methods
Analysis of Proportions and Morphospace.
Adult specimens of birds, reptiles, amphibians, cetaceans, plesiosaurs, ichthyosaurs, and Darwin’s finches were obtained from the American Museum of Natural History, the British Museum of Natural History, the Harvard Museum of Comparative Zoology, the Boston Museum of Science, and the Museum of the Rockies (see Dataset S1). Zebra Finch feet were obtained from university research animal colonies. The birds were excess breeders that were part of IACUC approved protocol BU11-026 in an AAALAC approved songbird breeding facility at Boston University; Chicken feet were obtained from Boston Chinese markets. From skeletal preparations and fossils, digital photographs were taken from the dorsal (top) side of the foot; for museum skins, digital X-ray was used to obtain images of phalanges. For some of the early sarcopterygian taxa, which are extremely rare and difficult to access, published photographs or illustrations were used to obtain measurements. Measurements of phalanges areas were made using ImageJ software by tracing around each phalanx. Proportions (P2/P1, P3/P1, and P4/P1) were plotted in 2D or 3D morphospace for visualization of the variation. Using MatLab, principal component analysis data were used to measure variance and to establish the relationships between various sets of proportions. A statistical protocol was used on generated randomized datasets to establish whether the observed relationships between phalanges proportions were statistically significantly different from random (described in detail in SI Appendix). Functional data on the use of toes by bird species were obtained from published sources (SI Appendix).
Developmental Analyses.
We used two methods to calculate the number of hours elapsed between the formation of the proximal joint and the formation of the distal joint in a phalanx. First, several large batches of chick eggs were incubated synchronously, and a 2-h time series was collected and stained with Alcian Blue. Condensation sizes for each phalanx were measured over each time period. The average elapsed time between proximal and distal joint formation was calculated to estimate the time of formation for a given phalanx. A second method involved removing one limb, allowing the embryo to grow for 4–48 h longer, then removing the other limb, performing Alcian staining, and counting the difference in the number of joints. The maximum number of hours before a new joint was observed in a digit was determined by estimating the number of hours required to form a given phalanx. Proliferation rates were calculated by labeling developing embryos with BrdU (Invitrogen EdU kits Alexa Fluor 555) and counting the numbers of labeled cells in 200-μm quadrants. Barrier experiments were conducted using tantalum foil implants into digit IV of chick hindlimbs either on day 5 (metatarsal barriers) or on day 6–7 (phalanges barriers). Embryos were collected at day 10, fixed, and Alcian stained. For wound controls foil barriers were inserted and then were removed about 1 min later. Alcian Blue-stained condensations were measured and compared among treatment groups.
Supplementary Material
Acknowledgments
We thank the members of the C.J.T. and K.D.K. laboratories for comments on the research and manuscript, L. Mahler and S. Mallick for assistance with data analysis, C. Koeppl for providing embryos, and the British Museum of Natural History and Museum of Comparative Zoology at Harvard University for access to specimens. This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant P01DK056246 (to C.J.T.) and funds from the University of Massachusetts Dartmouth (to K.D.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315213110/-/DCSupplemental.
References
- 1.Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev Camb Philos Soc. 1991;66(2):101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 2.Gould SJ. Ontogeny and Phylogeny. Cambridge, MA: Harvard Univ Press; 1977. [Google Scholar]
- 3.Kirschner M, Gerhart J. Evolvability. Proc Natl Acad Sci USA. 1998;95(15):8420–8427. doi: 10.1073/pnas.95.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klingenberg CP. Morphological integration and developmental modularity. Annu Rev Ecol Evol Syst. 2008;39:115–132. [Google Scholar]
- 5.Schlosser G, Wagner G. Modularity in Development and Evolution. Chicago: Univ of Chicago Press; 2004. [Google Scholar]
- 6.West Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003. [Google Scholar]
- 7.Eble GJ. Developmental and non-developmental morphospaces in evolutionary biology. In: Chapman RE, Rasskin-Gutman D, Wills M, editors. Morphospace Concepts and Applications. Cambridge, UK: Cambridge Univ Press; 1999. [Google Scholar]
- 8.Hall BK. Fins into Limbs: Evolution, Development, Transformation. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 9.Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388(6643):639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Hasso SM, Fallon JF. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc Natl Acad Sci USA. 2008;105(11):4185–4190. doi: 10.1073/pnas.0707899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolpert L (2002) Limb patterning: Reports of model’s death exaggerated. Current Biology 12(R):R628–R630. [DOI] [PubMed]
- 12.Richardson MK, Jeffery JE, Tabin CJ. Proximodistal patterning of the limb: Insights from evolutionary morphology. Evol Dev. 2004;6(1):1–5. doi: 10.1111/j.1525-142x.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- 13.Pascoal S, et al. A molecular clock operates during chick autopod proximal-distal outgrowth. J Mol Biol. 2007;368(2):303–309. doi: 10.1016/j.jmb.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 14. Hartmann C, Tabin C (2001) Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 104(3):341–351. [DOI] [PubMed]
- 15.Sanz-Ezquerro JJ, Tickle C. Fgf signaling controls the number of phalanges and tip formation in developing digits. Curr Biol. 2003;13(20):1830–1836. doi: 10.1016/j.cub.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Gatesy SM, Middleton KM. Bipedalism, flight and the evolution of theropod diversity. J Vertebr Paleontol. 1997;17:308–329. [Google Scholar]
- 17.Shapiro M, Hanken J, Rosenthal N. Developmental basis of digit loss in the Australian lizard Hemiurgis. J Exp Zool B Mol Dev Evol. 2003;297:48–56. doi: 10.1002/jez.b.19. [DOI] [PubMed] [Google Scholar]
- 18.Young NM, Wagner GP, Hallgrímsson B. Development and the evolvability of human limbs. Proc Natl Acad Sci USA. 2010;107(8):3400–3405. doi: 10.1073/pnas.0911856107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, et al. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18(19):2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Settle SH, Jr, et al. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254(1):116–130. doi: 10.1016/s0012-1606(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 21.Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999;209(1):11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- 22.Coates MI, Clack JA. Polydactyly in the earliest known tetrapod limbs. Nature. 1990;347:66–69. [Google Scholar]
- 23.Carroll R. The Rise of Amphibians: 365 Million Years of Evolution. Baltimore: Johns Hopkins Univ Press; 2009. [Google Scholar]
- 24. Ahlberg PE 2003. Fossils, developmental patterning, and the origin of tetrapods. The New Panorama of Animal Evolution, eds Legakis A, Sfenthourakis S, Polymeni R, Thessalou-Legaki M (Pensoft Publishers, Moscow) pp 44–54.
- 25. Hopson JA 2001. Ecomorphology of avian and non-avian theropod phalangeal proportions: Implications for the arboreal vs terrestrial origin of birds. New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom, eds, Gautheir J, Gall LF (Peabody Museum of Natural History, Yale University, New Haven, CT)
- 26. Kambic RE 2008. Multivariate Analysis of Avian and Non-Avian Theropod Pedal Phalanges. M.S. thesis (Montana State University, Bozeman, MT)
- 27.Shoval O, et al. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science. 2012;336(6085):1157–1160. doi: 10.1126/science.1217405. [DOI] [PubMed] [Google Scholar]
- 28.Steyn P. Birds of Prey of Southern Africa: Their Identification and Life Histories. Dover, NH: Tanager Books, Inc.; 1983. [Google Scholar]
- 29. Ferguson-Lees J Christie. D. 2001. Raptors of the World. (Houghton–Mifflin Company, New York)
- 30.Grant BR, Grant PR. Lack of premating isolation at the base of a phylogenetic tree. Am Nat. 2002;160(1):1–19. doi: 10.1086/339987. [DOI] [PubMed] [Google Scholar]
- 31.Kindahl M. The embryonic development of the hand and foot of Eremitalpa (Chrysochloris) grani (broom) Acta Zool. 1949;30:133–152. [Google Scholar]
- 32. Naish D (2005) Fossils explained 51(6): Sloths. Geology Today 21:232–238.
- 33.Mendel FC. Foot of two-toed sloths: Its anatomy and potential uses relative to size of support. J Morphol. 1981a;170:357–372. doi: 10.1002/jmor.1051700307. [DOI] [PubMed] [Google Scholar]
- 34.Mendel FC. Use of hands and feet of two-toed sloths (Choloepus hoffmanni) during climbing and terrestrial locomotion. J Mammal. 1981b;62:413–421. [Google Scholar]
- 35. Adams DC, Nistri A (2010) Onotogenetic convergence and evolution of foot morphology in European cave salamanders (Family: Plethodontidae). BMC Evol Biol Jul 16:10:216. [DOI] [PMC free article] [PubMed]
- 36. Kavanagh KD, Evans AR, Jernvall J (2007) Predicting evolutionary patterns of mammalian teeth from development. Nature 449:427–432. [DOI] [PubMed]
- 37. Young NM (2013) Macroevolutionary diversity of amniote limb proportions predicted by developmental interactions. J Exp Zool B Mol Dev Evol 320(7):420–427. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.