Significance
Fertilizers are of key importance to sustain modern agriculture, but the long-term fate of fertilizer-derived nitrogen in the plant–soil–water system is not fully understood. This long-term tracer study revealed that three decades after application of isotopically labeled fertilizer N to agricultural soils in 1982, 12–15% of the fertilizer-derived N was still residing in the soil organic matter, while 8–12% of the fertilizer N had already leaked toward the groundwater. Part of the remaining fertilizer N still residing in the soil is predicted to continue to be taken up by crops and to leak toward the groundwater in the form of nitrate for at least another five decades, much longer than previously thought.
Keywords: nitrogen cycle, nitrate leaching, isotopic biogeochemistry
Abstract
Increasing diffuse nitrate loading of surface waters and groundwater has emerged as a major problem in many agricultural areas of the world, resulting in contamination of drinking water resources in aquifers as well as eutrophication of freshwaters and coastal marine ecosystems. Although empirical correlations between application rates of N fertilizers to agricultural soils and nitrate contamination of adjacent hydrological systems have been demonstrated, the transit times of fertilizer N in the pedosphere–hydrosphere system are poorly understood. We investigated the fate of isotopically labeled nitrogen fertilizers in a three–decade-long in situ tracer experiment that quantified not only fertilizer N uptake by plants and retention in soils, but also determined to which extent and over which time periods fertilizer N stored in soil organic matter is rereleased for either uptake in crops or export into the hydrosphere. We found that 61–65% of the applied fertilizers N were taken up by plants, whereas 12–15% of the labeled fertilizer N were still residing in the soil organic matter more than a quarter century after tracer application. Between 8–12% of the applied fertilizer had leaked toward the hydrosphere during the 30-y observation period. We predict that additional exports of 15N-labeled nitrate from the tracer application in 1982 toward the hydrosphere will continue for at least another five decades. Therefore, attempts to reduce agricultural nitrate contamination of aquatic systems must consider the long-term legacy of past applications of synthetic fertilizers in agricultural systems and the nitrogen retention capacity of agricultural soils.
Increasing anthropogenic nitrogen inputs have recently been identified as one of the two major issues potentially compromising a safe operating space for humanity (1). In many regions, the amount of human-activated reactive nitrogen, primarily via application of synthetic fertilizers and cultivation of leguminous crops, exceeds now the amount of natural nitrogen as a result of population growth and the associated need for food production (2, 3). These anthropogenic nitrogen inputs have significantly impacted the nitrogen cycle in terrestrial and aquatic ecosystems (4, 5).
Increasing diffuse nitrate loading of surface waters and groundwaters has emerged as a major problem in many agricultural areas of the world resulting in contamination of drinking water resources abstracted from aquifers and eutrophication of freshwaters (6–8) and coastal marine ecosystems (9) despite the implementation of several diffuse pollution control directives (10, 11) and best management practices (12). Empirical correlations relating increased use of synthetic fertilizers, their application rates, land use change, and nitrate leaching suggest that the increased application of synthetic fertilizers is strongly connected with the increase of nitrate concentrations in groundwater and surface waters (13, 14), but quantitative data on transfer rates of fertilizer N into the hydrosphere are elusive. There is also considerable uncertainty regarding the transit time of anthropogenic nitrogen applied to agricultural soils between the topsoil and groundwater due to a poor mechanistic understanding of the timelines governing nitrogen cycling and nitrate transfer through soils (3, 15–17).
Previous studies on the fate of synthetic fertilizers and other nitrogen amendments in agricultural soils have been carried out at various long-term agricultural research sites (18–26). In several cases, fertilizer compounds artificially enriched in 15N have been used to successfully follow the uptake of fertilizer N by crops and retention of fertilizer N in soil organic matter. These tracer studies with labeled 15N compounds demonstrated that 40–60% of the fertilizer N is rapidly taken up by crops and is removed via harvest, whereas the remainder of the fertilizer N is incorporated into the soil organic matter pool and soil microbial biomass. From this fertilizer-derived soil N pool, nitrate may be formed and leached out of the soil zone especially outside of the growing season (27–29). To our best knowledge, no in situ studies have investigated the long-term fate of this fertilizer-derived N in soil organic matter and quantified to which extent and over which time periods fertilizer N stored in soil organic matter is rereleased for either uptake in crops or is exported toward the hydrosphere.
We investigated the long-term fate of isotopically (15N) labeled fertilizer nitrate in the plant–soil–water system of two intact lysimeters under rotating sugar beet and winter wheat cultivation at a site in France over a period of three decades (1982–2012). The objectives were i) to determine the extent to which fertilizer nitrate was taken up by crops, ii) to assess the mean residence time of fertilizer nitrogen in soil organic matter, and iii) to measure the rates at which fertilizer-derived nitrogen was exported as nitrate to the hydrosphere in the three decades after application of isotopically labeled fertilizer. The goal was to establish a complete 30-y mass balance of the fate of fertilizer N in an agricultural system and to quantify to which extent and over which time periods fertilizer N stored in soil organic matter is rereleased for either uptake in crops or export toward the hydrosphere.
Details about the experimental design are provided in the SI Methods. Two large (2 × 2 × 2 m) soil monoliths containing agricultural topsoils underlain by mineral soil were converted into lysimeters. For both lysimeters, the annual crop rotation was sugar beet–winter wheat with annual N fertilization rates of 120 kg N·ha−1·y−1 except in 1982. In the year of the tracer application (1982), Lys S was cropped with sugar beet whereas winter wheat was grown on Lys W. In 1982, both crops received a one-time 15N-labeled tracer application (635.3 mg 15N·m−2 on March 11 for wheat, 633.8 mg 15N·m−2 on April 7 for sugar beet) equivalent to a typical fertilizer application rate of 120 and 150 kg N·ha−1·y−1 for wheat and sugar beet, respectively. Nitrogen exports occurred annually by harvesting of wheat and sugar beets and via seepage water outflow in 2-m depth. Soils, harvest products, and seepage waters were sampled repeatedly, and chemical and isotopic analyses were conducted. Mass and isotope balances were conducted to assess the fate of the fertilizer applied in 1982 in the agricultural soils and its export via harvest products and toward the underlying aquifers (see SI Methods for further details).
Results and Discussion
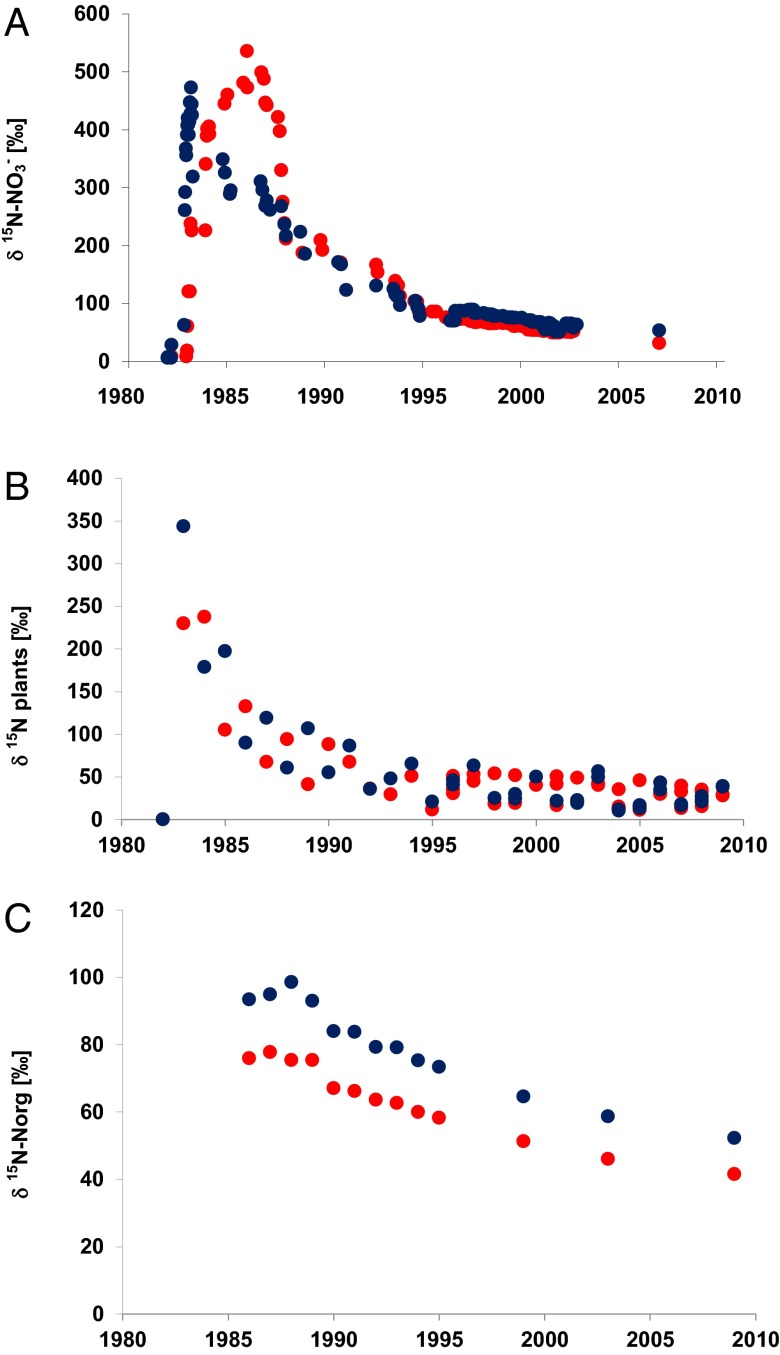
Before tracer application, δ15N values of nitrate in lysimeter outflow were on average 2.5‰. Following the application of the K15NO3− solution sprinkled uniformly on the surface of the two lysimeters in 1982, δ15N in seepage water nitrate steadily increased to peak values of 473‰ after 19 mo (577 d) in lysimeter W (Lys W) under wheat and 535‰ after 55 mo (1,653 d) in lysimeter S (Lys S) under sugar beet (Fig. 1A). Tritium measurements indicated that infiltration rates for precipitation water vary from 35 to 55 cm/y consistent with expected tracer migration times calculated based on water infiltration rates. Thereafter, δ15N values in seepage water nitrate decreased steadily reaching values of circa +200‰ in 1990, and +100‰ by 1996. During the last 14 y, δ15N values of seepage water nitrate in 2-m depth decreased slowly to values of +32‰ (Lys S) and +53‰ (Lys W) in 2008, indicating that isotopically labeled tracer N is still exported from the lysimeters almost three decades after tracer application. The elevated δ15N values and their sluggish decrease in seepage water nitrate are indicative of significant tracer retention in the soil–plant system, because the pore-space of the lysimeters had been flushed more than 10 times during the observation period. Nitrate collected in outflow from both lysimeters between 2001 and 2009 was also analyzed for oxygen isotope ratios yielding an average δ18Onitrate value of −0.5 ± 2.8‰ (n = 16).
Fig. 1.
The δ15N values for seepage water nitrate (A), plants (B), and soil organic matter (C) for the two types of lysimeters under sugar beet (Lys S in red) and under wheat (Lys W in blue).
Before application of the 15N tracer, the δ15N value of total nitrogen in plants was 0‰. The δ15N values of total N in the harvest products increased to +230‰ (Lys S) and +340‰ (Lys W) after the first growing season (Fig.1B), indicating that a considerable portion of the labeled 15N was taken up by the crops in the first growing season. The δ15N values of total N in the harvested crops decreased markedly in the following years to +67‰ (Lys S) and +119‰ (Lys W) in 1987 and to +28‰ (Lys S) and +38‰ (Lys W) in 2009. Even 27 y after tracer application, the δ15N values of the crops were still significantly higher than natural abundance nitrogen isotope ratios observed before tracer application suggesting continued availability of isotopically labeled N applied in 1982.
Before application of the 15N tracer (1976–1981), δ15N values of total N in soils ranged between 4.4 and 5.4‰. Three years after tracer application (1985), δ15N of total nitrogen in soil organic matter had maximum values of +98‰ (Lys S) and +105‰ (Lys W) (Fig. 1C). Thereafter, δ15Ntotal values of soil organic matter decreased exponentially to +52.2‰ (Lys W) and +41.5‰ (Lys S) in 2009. This indicates significant retention of isotopically labeled fertilizer N more than a quarter century after application, with slightly higher tracer contents in the lysimeters cropped with sugar beets (Lys S) compared with those planted with wheat (Lys W).
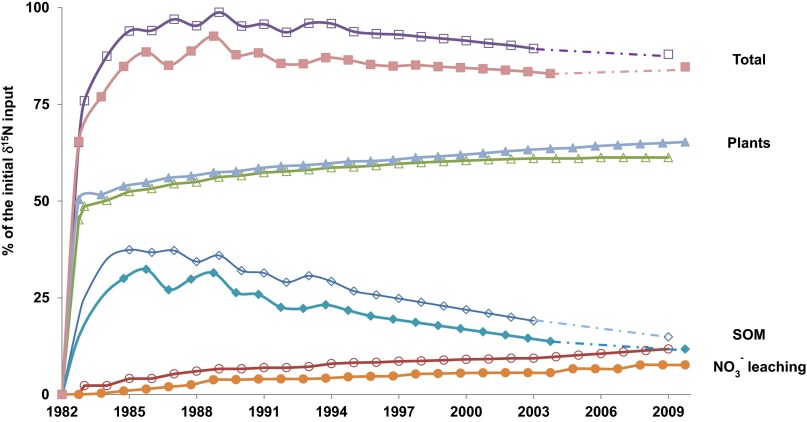
Isotope and mass balances were used to determine the extent to which fertilizer nitrate was taken up by crops, to assess the mean residence time of fertilizer nitrogen in soil organic matter, and to measure the rates at which fertilizer-derived nitrogen was exported to the hydrosphere over an observation period of almost three decades. In the first year of the experiment, between 45.2% (Lys W) and 50.4% (Lys S) of the 15N-labeled fertilizer nitrate-N was taken up by the winter wheat and sugar beet crops, respectively (Fig. 2). In subsequent years, additional crop uptake of 15N-labeled fertilizer N was observed at average annual rates between 0.3% (lysimeter S) and 0.5% (lysimeter W) of the labeled fertilizer N applied in 1982. Twenty-seven years after tracer application, between 65.3% (Lys S) and 61.3% (Lys W) of the applied tracer had been cumulatively taken up by the crops and was exported from the soil–plant system via harvest (Fig. 2).
Fig. 2.
Cumulative budget of 15N-labeled fertilizer nitrogen based on mass and isotope balances for plants, soil organic matter (SOM), and nitrate in lysimeter outflows for Lys S (full symbols) and Lys W (empty symbols).
Three years after tracer application, between 32.3% (Lys S) and 37.4% (Lys W) of the 15N-labeled fertilizer were detected in the soil organic matter (Fig. 2). Subsequently, the amount of tracer 15N recovered in the soils decreased by circa 0.9% per annum. At the end of the observation period in 2009, between 11.8% (Lys S) and 14.9% (Lys W) of the 15N-labeled N still resided in the soil organic matter (Fig. 2). The observed decrease of the 15N tracer in soil organic matter between 1985 and 2009 is partially explained by plant uptake (4.9 and 5.5% in 27 y after the 1982 growing season) and nitrate leaching as seepage water outflow from the lysimeters, as described below.
Three years after tracer application, i.e., in 1985, between 1.4% (Lys S) and 4.1% (Lys W) of the applied 15N-labeled nitrate had been exported with the seepage water outflow in 2-m depth. During the following 24 y an average of 0.4% of the applied tracer was exported annually with the seepage water nitrate flux from the plant–soil system with comparatively little variability of hydrological 15N exports between wet and dry years. The cumulative nitrate exports toward the hydrosphere accounted for 7.6% (Lys S initially cropped with sugar beets) and 11.8% (Lys W, initially wheat) of the 15N-labeled fertilizer N applied in 1982 throughout the 27-y observation period (Fig. 2). δ18O–NO3− values of lysimeter outflow nitrate collected for both lysimeters in 2001, 2003, 2005 (only Lys W), 2008, and 2009 (only Lys W) averaged −0.5 ± 2.8‰ (n = 16). Nitrate-containing fertilizers (i.e., +22–25‰) and atmospheric nitrate deposition (>50‰) have δ18O values typically >20‰ (30, 31). The observed low δ18O–NO3− values indicate that the exported nitrate was not directly derived from the applied fertilizer, but from nitrification of soil organic matter (32, 33). During ammonification of soil organic matter followed by nitrification, three new atoms of oxygen are incorporated into the newly formed nitrate molecule, two of which are derived from water resulting in low δ18O values of nitrate typically around 0‰ (34). Therefore, the combination of δ15N and δ18O measurements indicates that a significant portion of the 15N-labeled fertilizer nitrate was first incorporated into the soil organic matter either directly by uptake in the soil microbial community or via plant root decomposition after harvest. Subsequently, the 15N-labeled organic N was remineralized and some of this newly formed nitrate is continuously exported toward the hydrosphere.
In summary, between 61 and 65% of the applied fertilizer N was taken up by plants during this three-decade experiment (Fig. 2). A significant part of the applied nitrate that was not taken up by the crops after 15N-labeled fertilizer application was rapidly incorporated into the soil organic matter pool (initially between 32 and 37%), and between 12 and 15% of the tracer remained in the soil organic matter pool 28 y after fertilizer application (Fig. 2). Oxygen isotope measurements on seepage water nitrate collected at 2-m depth below the root zone confirmed that 15N enriched nitrate was derived from mineralization of soil organic matter. These soil-internal processes resulted in a continuous leaching of circa 0.4% of the applied fertilizer N per year as labeled nitrate toward the groundwater for more than a quarter of a century after fertilizer application. Throughout the observation period, between 8 and 12% of the labeled fertilizer N was exported toward the hydrosphere (Fig. 2).
Overall mass balances for 15N detected in crops, soils, and seepage water accounted in the first years of the experiment for between ∼88% (Lys S) and ∼95% (Lys W) of the labeled fertilizer N. Throughout the experiment, the mass balance calculations revealed a slightly increasing deficit of 15N of up to 15.3% for Lys S and 12.1% for Lys W in 2009 (Fig. 2). This discrepancy is not thought to be due to unaccounted losses to the hydrosphere, because all of the seepage water exported from the lysimeters was quantitatively recovered and regularly analyzed. Therefore, we hypothesize that the mass balance deficit for 15N was caused by gaseous losses of N via volatilization (NH3) and/or denitrification (e.g., N2, N2O) of either fertilizer N after tracer application or labeled N released from the soil organic matter pool. The observed percentage of gaseous loss of fertilizer nitrogen is in good agreement with values reported in the literature (35).
These results provide evidence that a significant portion of fertilizer N is incorporated in the soil organic matter pool, which constitutes a temporary nitrogen reservoir for the fertilizer N. In 2003, 21 y after 15N application, between 13.7% (Lys S) and 19.0% (Lys W) of the 15N-labeled N was still residing in the soil organic matter pool. Remineralization of fertilizer-derived N incorporated into the soil organic matter pool gradually releases 15N-labeled N that is then taken up by plants, is lost to the atmosphere via volatilization or denitrification, or is leached toward aquifers in low doses over more than 25 y after application of the 15N-labeled fertilizer.
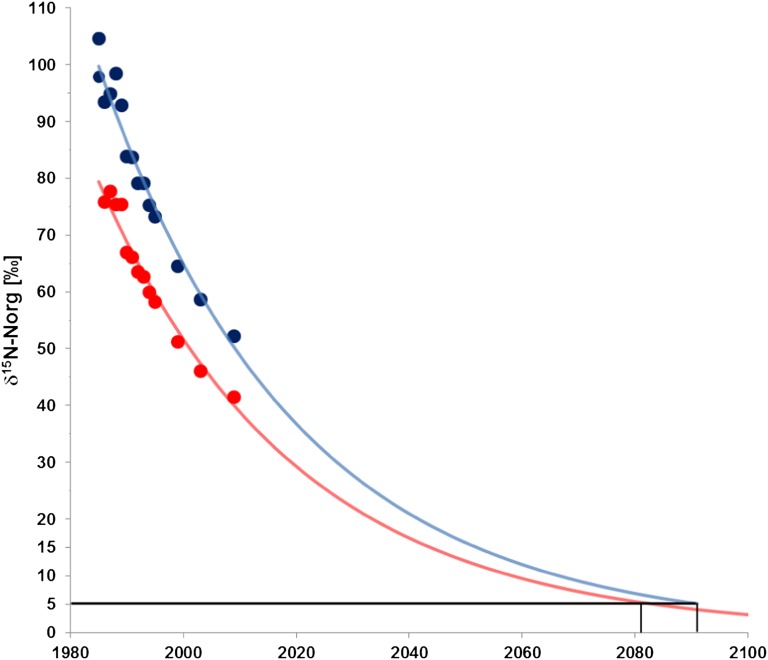
Using a simple decay function fitted to the isotope data for soil nitrogen shown in Figs. 1C and 2 it is predicted that it will take circa 100 y to reach the background δ15N values of +5‰ measured for soil N before tracer application (Fig. 3). Hence, the model suggests that it will take at least another five decades until the remaining tracer N is removed from the soil system. Assuming similar proportions of N transformation in the plant–soil–water system as in the last three decades, the remaining fertilizer-derived 15N in the soil organic matter (12–15%) will be subject in approximately equal proportions to plant uptake (4–5%), seepage water export as nitrate (4–5%), and removal via soil-internal processes such as volatilization and denitrification (2–7%). It is estimated that seepage water export of labeled 15N applied with a nitrate fertilizer in 1982 will continue for at least another five decades. This suggests that between 12 and 17% of the initially applied 15N-labeled fertilizer are subject to low-dose continuous release with seepage water nitrate toward the hydrosphere over a time period of more than eight decades.
Fig. 3.
Decay functions fitted to observed δ15N values of soil organic matter from Lys S (red) and Lys W (blue). The model suggests that it will take circa 100 y to reach the background δ15N values of circa +5‰ observed before tracer application.
It is often assumed that most of the nitrate contained in fertilizers is used by plants for their growth or quickly leached out of the root zone (3, 4, 36, 37). Using 15N-labeled tracer techniques combined with the determination of oxygen isotope ratios of nitrate this long-term lysimeter study demonstrates that a significant portion of nitrate fertilizer applied in 1982 was incorporated (32–37% in 1985) and partly retained for more than a quarter century (14–19% in 2009) in the soil organic matter pool of an agricultural soil. Hence, a significant part of the applied nitrate fertilizer is incorporated in the soil organic matter entering the soil nitrogen cycle with an estimated mean residence time of circa three decades. Mineralization of this 15N-labeled soil organic matter pool continuously produced nitrate available for uptake by plants in the growing season and for export to the hydrosphere in approximately equal proportions. Our 30-y study demonstrates that a portion of the nitrogen applied as nitrate fertilizer is available for decades after application. This long-term retention and recycling of fertilizer N and release of nitrate has several implications. Soil organic matter management is crucially important for maximizing the long-term benefit of fertilizer applications for crop yields and for minimizing nitrate export to the hydrosphere. For example, bypassing the retention capacity of the soil organic matter pool by intensive tile drainage systems increases significantly the transfer of fertilizer-derived nitrate to rivers, aquifers, and estuaries (38–40). Also, due to the long mean residence time of fertilizer N in soils the effects of changes in soil management practices on nitrate loading of the hydrosphere may be considerably delayed. For instance, studies of the Mississippi River Basin have revealed a decrease in anthropogenic N inputs without any concurrent reductions in riverine nitrate loading (41–43).
Our findings reinforce the importance of soil organic matter management in agricultural soils as a buffer to mitigate diffuse nitrogen pollution of surface waters and groundwaters. They stress the need to take into account this long-term N-recycling component in soil N and catchment models to better understand and simulate nitrate-leaching lag times often observed between fertilizer N applications to soils and nitrate transfers in drainage basins. Our data also imply that the current trends of nitrate concentration increases observed in hydrological systems associated with many agricultural areas of the world are the result of both current and past activities throughout the last decades. Therefore, mitigation or restoration measures must take into account the delay resulting from legacies of past applications of synthetic fertilizers in agricultural systems.
Methods
The study was carried out over a 30-y period since 1981 using two lysimeters in the chalk area located under in situ environmental conditions near Châlons en Champagne, France (48°58’N, 4°19’E). Each lysimeter consisted of an intact unaltered soil monolith (2 × 2 × 2 m) surrounded by a lysimetric tank. Soil organic matter and harvest products of wheat and sugar beets were sampled annually, air dried, ground and sieved through a 1 mm mesh for soils and 80-µm for plants, and total N contents were determined using an elemental analyzer. Isotope abundance ratios of total nitrogen for plant materials and soil organic matter were determined by continuous flow isotope ratio mass spectrometry coupled to an elemental analyzer (EA-CF-IRMS). Nitrate concentrations in the lysimeter seepage water were determined by automated colorimetry (44). Nitrogen isotope ratios of nitrate in lysimeter seepage water were determined either with the Kjeldahl distillation procedure or with the ammonium diffusion technique using Devarda reagent (45, 46). Oxygen isotope ratios of seepage water nitrate were determined using an adaptation of the method described by Silva et al. (47). δ18O-NO3− values were determined after conversion of nitrate to pure silver nitrate, which was converted to CO via pyrolysis in a glassy carbon reactor (TC/EA) at 1350 °C followed by mass spectrometric measurements. δ18O values of nitrate are reported with respect to Standard Mean Ocean Water.
Supplementary Material
Acknowledgments
We thank the Institut National de Recherche Agronomique (INRA), Gonzague Alavoine, Jean-Louis Ballif, Danièle Denys, Bruno Mary, Christian Herre, Marie-Jeanne Herre, Francis Millon, and Sylvie Millon for providing the set up of the Fagnières Lysimetric experimental site, the field assistance, the collection and analysis of plant, soil, and water samples or the data collection, and Micheline Grably for isotopic measurements.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305372110/-/DCSupplemental.
References
- 1.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461(7263):472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 2.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of earth’s ecosystems. Science. 1997;277(5325):494–499. [Google Scholar]
- 3.Galloway JN, et al. The nitrogen cascade. Biosciences. 2003;53(4):341–356. [Google Scholar]
- 4.Galloway JN, et al. Nitrogen cycles: Past, present, and future. Biogeochemistry. 2004;70:153–226. [Google Scholar]
- 5.Burt TP, Howden NJK, Worrall F, Whelan MJ. Importance of long-term monitoring for detecting environmental change: Lessons from a lowland river in south east England. Biogeosciences. 2008;5:1529–1535. [Google Scholar]
- 6.Spalding RF, Exner ME. Occurrence of nitrate in groundwater- A review. J Environ Qual. 1993;22:392–402. [Google Scholar]
- 7.Altman SJ, Parizek RR. Dilution of non-point source nitrate in ground water. J Environ Qual. 1995;24:707–718. [Google Scholar]
- 8.Wassenaar LI. Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of 15N and 18O in NO3- Appl Geochem. 1995;10:391–405. [Google Scholar]
- 9.Zillen L, Conley DJ, Andren T, Andren E, Bjorck S. Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth Sci Rev. 2008;91(1–4):77–92. [Google Scholar]
- 10. Commission of the European Communities (1991) Directive EC 91/676/EEC (European Union, Brussels)
- 11. European Commission (2000) European Commission Directive 2000/60/EC. Official Journal of the European Community L327.
- 12.Wassenaar LI, Hendry MJ, Harrington N. Decadal geochemical and isotopic trends for nitrate in a transboundary aquifer and implications for agricultural beneficial management practices. Environ Sci Technol. 2006;40(15):4626–4632. doi: 10.1021/es060724w. [DOI] [PubMed] [Google Scholar]
- 13.Howarth RW, et al. Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic ocean: Natural and human influences. Biogeochemistry. 1996;35:75–139. [Google Scholar]
- 14.Donoso G, Cancino J, Magri A. Effects of agricultural activities on water pollution with nitrates and pesticides in the Central Valley of Chile. Water Sci Technol. 1999;39(3):49–60. [Google Scholar]
- 15.Power JF, Schepers JS. Nitrate contamination of groundwater in North America. Agric Ecosyst Environ. 1989;26(3–4):165–187. [Google Scholar]
- 16.Strebel O, Duynisveld WHM, Böttcher J. Nitrate pollution of groundwater in western Europe. Agric Ecosyst Environ. 1989;26(3–4):189–214. [Google Scholar]
- 17.Hubbard RK, Sheridan JM. Nitrates in groundwater in the southeastern United States. In: Adriano DC, Iskandar AK, Murarka IP, editors. Advances in Environmental Science. Contamination of Groundwaters. Boca Raton, FL: CRC Press; 1994. pp. 303–345. [Google Scholar]
- 18.Shen SM, Pruden G, Jenkinson DS. Mineralization and immobilization of nitrogen in fumigated soil and the measurement of microbial biomass nitrogen. Soil Biol Biochem. 1984;16(5):437–444. [Google Scholar]
- 19.Wickramasinghe KN, Rodgers GA, Jenkinson DS. Transformations of nitrogen fertilizers in soil. Soil Biol Biochem. 1985;17(5):625–630. [Google Scholar]
- 20.Shen SM, Hart PBS, Powlson DS, Jenkinson DS. The nitrogen cycle in the Broadbalk wheat experiment: 15N-labelled fertilizer residues in the soil and the soil microbial biomass. Soil Biol Biochem. 1989;21(4):529–533. [Google Scholar]
- 21.Nicolardot B, Molina JAE. C and N fluxes between pools of soil organic matter: model calibration with long-term field experiment data. Soil Biol Biochem. 1994;26(2):245–251. [Google Scholar]
- 22.Jenkinson DS, Poulton PR, Johnston AE, Powlson DS. Turnover of nitrogen-15-labeled fertilizer in old grassland. Soil Sci Soc Am J. 2004;68(3):865–875. [Google Scholar]
- 23.Odell RT, Melsted SW, Walker WM. Changes in organic carbon and nitrogen of Morrow plot soils under different treatments, 1904–1973. Soil Sci. 1984;137(3):160–171. [Google Scholar]
- 24.Khan SA, Mulvaney RL, Ellsworth TR, Boast CW. The myth of nitrogen fertilization for soil carbon sequestration. J Environ Qual. 2007;36(6):1821–1832. doi: 10.2134/jeq2007.0099. [DOI] [PubMed] [Google Scholar]
- 25.Mulvaney RL, Khan SA, Ellsworth TR. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J Environ Qual. 2009;38(6):2295–2314. doi: 10.2134/jeq2008.0527. [DOI] [PubMed] [Google Scholar]
- 26.Nafizger ED, Dunker RE. Soil organic carbon trends over 100 years in the Morrow plots. Agron J. 2011;103:261–267. [Google Scholar]
- 27.Jenkinson DS, Parry LC. The nitrogen cycle in the Broadbalk wheat experiment: a model for the turnover of nitrogen through the soil microbial biomass. Soil Biol Biochem. 1989;21(4):535–541. [Google Scholar]
- 28.Recous S, Machet JM. Short-term immobilisation and crop uptake of fertilizer nitrogen applied to winter wheat: Effect of date of application in spring. Plant Soil. 1999;206:137–149. [Google Scholar]
- 29.Sieling K, Kage H. Efficient N management using winter oilseed rape. A review. Agron Sustain Dev. 2010;30:271–279. [Google Scholar]
- 30.Vitòria L, Otero N, Soler A, Canals A. Fertilizer characterization: Isotopic data (N, S, O, C, and Sr) Environ Sci Technol. 2004;38(12):3254–3262. doi: 10.1021/es0348187. [DOI] [PubMed] [Google Scholar]
- 31.Kendall C, Elliott EM, Wankel SD. Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener RH, Lajtha K, editors. Stable isotopes in ecology and environmental science. 3rd Ed. London: Blackwell Publishing; 2007. pp. 375–449. [Google Scholar]
- 32.Anderson KK, Hooper AB. O2 and H2O are each the source of one NO2- produced from NH3 by Nitrosomas. 15N-NMR evidence. FEBS Lett. 1983;65:236–240. [Google Scholar]
- 33.Hollocher TC. Source of the oxygen atoms of nitrate in the oxidation of nitrite by Nitrobacter agilis and evidence against a P-O-N anhydride mechanism in oxidative phosphorylation. Arch Biochem Biophys. 1984;233(2):721–727. doi: 10.1016/0003-9861(84)90499-5. [DOI] [PubMed] [Google Scholar]
- 34.Mayer B, Bollwerk SM, Mansfeldt T, Hütter B, Veizer J. The oxygen isotope composition of nitrate generated by nitrification in acid forest floors. Geochim Cosmochim Acta. 2001;65:2743–2756. [Google Scholar]
- 35.Groffman PM, et al. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol Appl. 2006;16(6):2091–2122. doi: 10.1890/1051-0761(2006)016[2091:mfmdda]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Green CT, Böhlke JK, Bekins BA, Phillips SP. Mixing effects on apparent reaction rates and isotope fractionation during denitrification in a heterogeneous aquifer. Water Resour Res. 2010;46:1–19. [Google Scholar]
- 37.Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451(7176):293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- 38.McIsaac GF, Hu X. Net N and riverine export from Illinois agricultural watersheds with and without extensive tile drainage. Biogeochemistry. 2004;70:251–271. [Google Scholar]
- 39.David MB, Drinkwater LE, McIsaac GF. Sources of nitrate yields in the Mississippi River Basin. J Environ Qual. 2010;39(5):1657–1667. doi: 10.2134/jeq2010.0115. [DOI] [PubMed] [Google Scholar]
- 40.Steinheimer TR, Scoggin KD, Kramer LA. Agricultural chemical movement trough a field-size watershed in Iowa: Surface hydrology and nitrate losses in discharge. Environ Sci Technol. 1998;32:1048–1052. [Google Scholar]
- 41.Goolsby DA, Battaglin WA, Aulenbach BT, Hooper RP. Nitrogen flux and sources in the Mississippi River Basin. Sci Total Environ. 2000;248(2–3):75–86. doi: 10.1016/s0048-9697(99)00532-x. [DOI] [PubMed] [Google Scholar]
- 42.McIsaac GF, David MB, Gertner GZ, Goolsby DA. Relating net nitrogen input in the Mississippi River basin to nitrate flux in the lower Mississippi River: A comparison of approaches. J Environ Qual. 2002;31(5):1610–1622. doi: 10.2134/jeq2002.1610. [DOI] [PubMed] [Google Scholar]
- 43.Hong B, Swaney DP, Howarth RW. Estimating net anthropogenic nitrogen inputs to U.S. watersheds: Comparison of methodologies. Environ Sci Technol. 2013;47(10):5199–5207. doi: 10.1021/es303437c. [DOI] [PubMed] [Google Scholar]
- 44.Dorich R, Nelson DW. Evaluation of manual cadmium reduction methods for determination of nitrate KCl extracts of soils. Soil Sci Soc Am J. 1984;48:72–75. [Google Scholar]
- 45.Bremner JM, Keeney DR. Steam distillation methods for determination of ammonium nitrate and nitrite. Anal Chim Acta. 1965;32:485–495. [Google Scholar]
- 46.Sebilo M, Mayer B, Grably M, Billiou D, Mariotti A. The use of the “Ammonium diffusion” method for δ15N-NH4+ and δ15N-NO3 - measurements: Comparison with other techniques. Environ Chem. 2004;1(2):99–103. [Google Scholar]
- 47.Silva SR, Kendall C, Wilkison DH, Chang CCYC, Avanzino RJ. A new method for collection of nitrate from fresh water and analysis for its nitrogen and oxygen isotopes ratios. J Hydrol. 2000;228(1-2):22–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.