Significance
Viruses exploit their host’s microtubule (MT) transport system to move within infected cells at various stages of their life cycle. The formation and stability of MT networks is controlled by specialized host proteins, which track MT ends. These proteins, called “+TIPs” (plus-end tracking proteins), stabilize subsets of MTs in response to various environmental signals. How viruses influence the organization of these critical intracellular transport networks to facilitate infection remains poorly understood. In this study, we demonstrate that herpes simplex virus type 1 (HSV-1), which infects an estimated 60–90% of the world’s population, encodes a kinase that targets specific +TIPs to stabilize MTs formed at the trans-Golgi network, an alternate MT organizing center and organelle central to viral envelopment, to facilitate HSV-1 spread.
Abstract
Although microtubules (MTs) frequently form highly dynamic networks, subsets of MTs become stabilized in response to environmental cues and function as specialized tracks for vesicle and macromolecular trafficking. MT stabilization is controlled by specialized plus-end tracking proteins (+TIPs) whose accumulation at the MT ends is facilitated by the end-binding protein, EB1, and regulated by various signaling pathways. As cargoes themselves, viruses are dependent on MTs for their intracellular movement. Although many viruses affect MT organization, the potential contribution of MT stabilization by +TIPs to infection remains unknown. Here we show that early in infection of primary human fibroblasts, herpes simplex virus type 1 (HSV-1) disrupts the centrosome, the primary MT organizing center in many cell types. As infection progresses HSV-1 induces the formation of stable MT subsets through inactivation of glycogen synthase kinase 3beta by the viral Ser/Thr kinase, Us3. Stable MT formation is reduced in cells infected with Us3 mutants and those stable MTs that form cluster around the trans-Golgi network. Downstream of glycogen synthase kinase 3beta, cytoplasmic linker-associated proteins (CLASPs), specialized host +TIPs that control MT formation at the trans-Golgi network and cortical capture, are specifically required for virus-induced MT stabilization and HSV-1 spread. Our findings demonstrate the biological importance of +TIPs to viral infection and suggest that HSV-1 has evolved to exploit the trans-Golgi network as an alternate MT organizing center to facilitate virus spread.
Microtubules (MTs) function in a variety of processes, including directed intracellular trafficking of cargos and changes in cell shape, polarity, and motility (1, 2). Consisting of polarized heteropolymers of α/β-tubulin, in most cells MT minus-ends are anchored at the perinuclear MT organizing center (MTOC), whereas their plus-ends radiate toward the cell periphery. In proliferating cells, the majority of MTs are highly dynamic, growing and shrinking through the addition or loss of tubulin subunits primarily at the plus-end. Dynamic instability facilitates intracellular sensing through “search-and-capture.” On encountering targets such as the cell cortex and in response to specific signals, subsets of MTs become stabilized and acquire posttranslational modifications such as acetylation and detyrosination (3). Stable MTs are recognized by specific motor proteins and act as specialized tracks for vesicle transport (1, 3). MT stabilization is mediated by proteins that track dynamic MT plus-ends and influence rates of MT growth, pause, and collapse, known as plus-end tracking proteins (+TIPs) (4). Central to plus-end tracking is EB1, a member of the end-binding family of proteins that recognizes growing MT ends. Although many +TIPs are capable of associating with MTs, it is their interaction with EB1 that mediates their specific accumulation at MT plus-ends (4). +TIP activity and interaction with EB1 responds to signals including Rho-Dia activation and PI3K-Akt-mediated inactivation of glycogen synthase kinase 3beta (GSK3β) to induce localized MT stabilization at specific sites (1, 3, 4). +TIPs also interact with components of organelles and cortical actin, playing important roles in actin–MT linkage at the cell periphery.
At least eight viruses, including six DNA viruses, induce tubulin acetylation to varying extents, and in some cases, evidence suggests that stable MTs may be important for infection (5–14). However, the underlying mechanisms by which many viruses remodel host MT networks and potential roles for +TIPs remain poorly understood. This includes herpes simplex virus type 1 (HSV-1), a large DNA virus that infects an estimated 60–90% of humans worldwide (15). HSV-1 replicates in the nucleus and then buds into the cytoplasm, where organelles of the trans-Golgi network (TGN) and endocytic pathways function in viral glycoprotein sorting, secondary envelopment, and trafficking of new viral particles to the cell surface (15, 16). Although HSV-1 is known to induce MT rearrangements (17, 18), the precise nature of these events, their regulation, and their role in infection have remained unclear. Here we show that on infection of primary human cells, HSV-1 disrupts the centrosome yet induces MT stabilization through the kinase activity of Us3, a viral regulator of GSK3β. Downstream of GSK3β, MT stabilization and virus spread are regulated by cytoplasmic linker-associated proteins (CLASPs), specialized +TIPs that control MT formation at the TGN and cortical capture of MTs. Our findings suggest that HSV-1 has evolved to exploit the TGN as an alternate MTOC and induces stable MTs to facilitate virus spread.
Results
HSV-1 Induces MT Reorganization and Stabilization in Primary Human Cells.
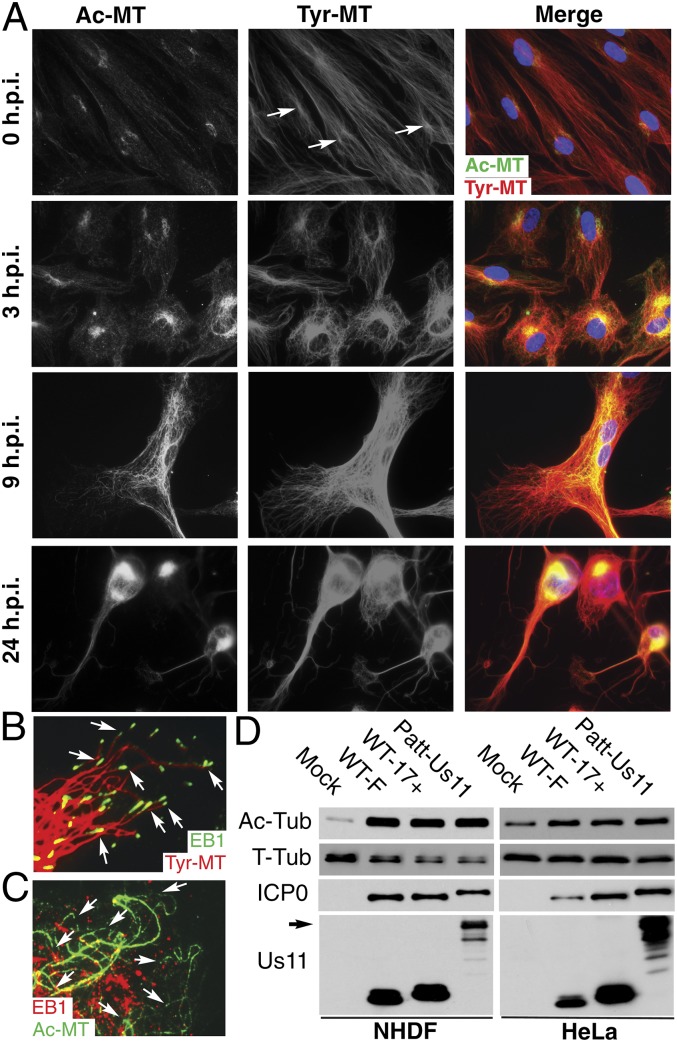
MT networks are rearranged in HSV-1-infected cells in a two-step process whereby, early in infection, MTs lose centrosomal focus and have been described as “disorganized.” Then, as infection progresses, MTs rearrange to form rings around the nucleus from which filaments radiate in a “criss-cross fashion” (17). HSV-1 also induces tubulin acetylation and detyrosination, although this has only been shown by Western blot (WB) analysis without visualization of filament organization (10, 11), whereas an independent immunofluorescence (IF) study has questioned whether HSV-1 affects stable MTs (19). As such, the potential induction and role of stable MTs during infection remains unclear. We characterized the effects of HSV-1 on MT organization in primary normal human dermal fibroblasts (NHDFs) at various times in hours postinfection (h.p.i.). Mock-infected or infected samples were fixed and stained for tyrosinated (Tyr) or acetylated (Ac) tubulin to detect dynamic or stable MTs, respectively. By 3 h.p.i., Tyr-MTs became less extended and accumulated at perinuclear regions compared with uninfected NHDFs (Fig. 1A). A low level of MT acetylation was also observed at 3 h.p.i., largely clustered near the nucleus. After early cell rounding, ∼70% cells became elongated, and cellular projections were formed between 7 and 16 h.p.i. (Fig. 1A). Samples at 9 h illustrated the extent to which Tyr-MT networks radiated toward the cell periphery and lacked a clear focus near the nucleus (Fig. 1A). Strong Ac-MT staining was observed at this point and continued until late in infection, when cells rounded up, typical of HSV-1-induced cytopathic effect. The organization of Tyr- and Ac-MTs suggested that MT networks in infected cells consisted of both dynamic and stabilized subsets (Fig. 1A). To confirm this, samples were stained for EB1, which forms a comet-like pattern on growing MT plus-ends (4). EB1 comets decorated dynamic Tyr-MT tips, where ends were distinguishable (Fig. 1B), but did not form comets on the ends of Ac-MTs (Fig. 1C), in line with their stable nondynamic nature. WB analysis confirmed that MT acetylation was induced by different HSV-1 strains in distinct human cell types, even at lower multiplicity of infection (m.o.i.) (Fig. 1D). This included HSV-1 Patton expressing a GFP-tagged late protein, Us11 (HSV-1-GFP-Us11) (20), used in the following assays.
Fig. 1.
HSV-1 induces MT stabilization in primary human fibroblasts. (A–C) NHDFs were mock-infected or infected with HSV-1 strain F at m.o.i. 20. (A) Samples were fixed at the indicated times and stained for Tyr-MTs (red) and Ac-MTs (green). Arrows highlight representative perinuclear dots of Tyr-tubulin at the centrosome. (B and C) Infected samples fixed at 9 h.p.i. were stained for (B) Tyr-MTs (red) and EB1 (green) or (C) Ac-MTs (green) and EB1 (red). Magnification 100×. Arrows point to Tyr-MT ends with EB1 comets (B) or Ac-MTs lacking EB1 (C). (D) NHDFs or HeLa cells were infected at m.o.i. 2 for 16 h with the indicated HSV-1 strains, and lysates were analyzed by WB using the indicated antibodies. Ac-Tub, acetylated tubulin; T-Tub, total tubulin. The arrow points to GFP-tagged Us11 in Patton strain HSV-1-GFP-Us11 (Patt-Us11).
A Virus-Encoded Regulator of GSK3β Controls Stable MT Formation.
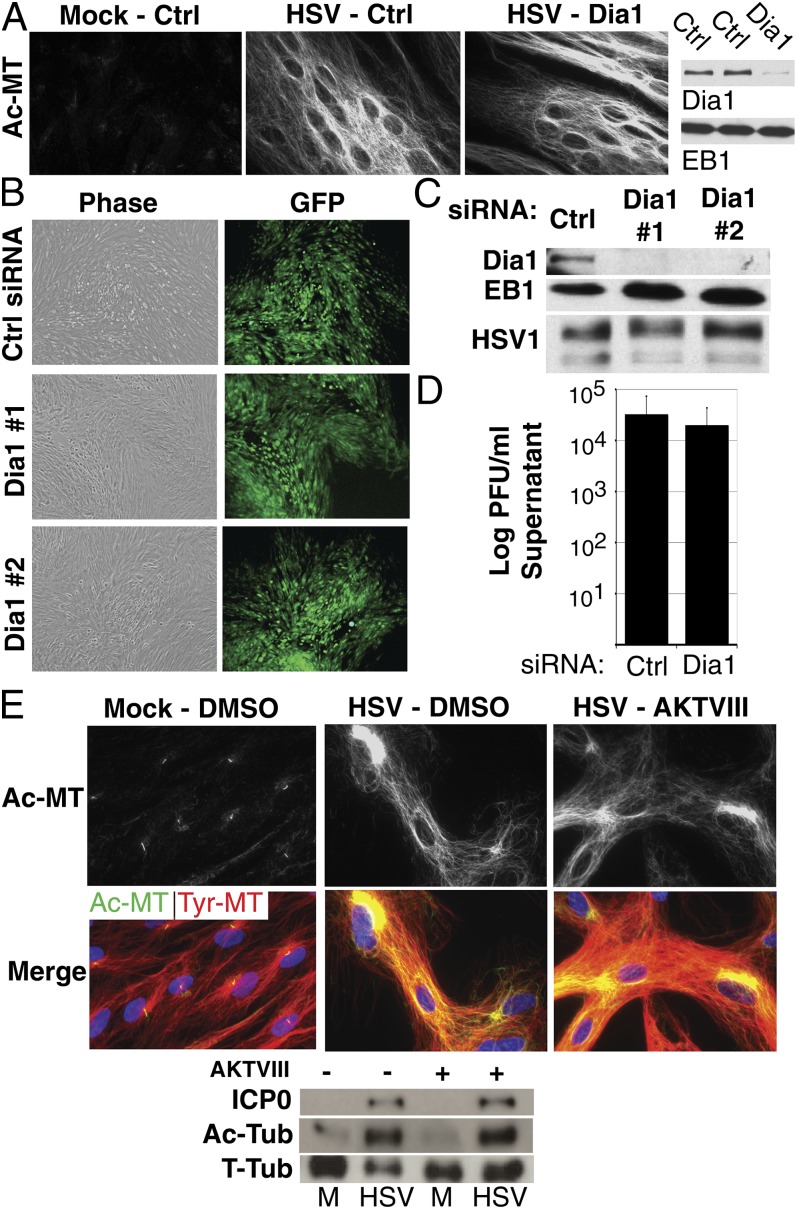
Two primary host signal pathways, namely, Rho-Dia and PI3K-Akt, effect changes in MT dynamics by other DNA viruses (7, 9, 14). To determine whether either pathway mediated MT stabilization by HSV-1, the effects of Dia1 depletion were first examined. After infection, no differences in Ac-MT intensity or organization were evident between control or Dia1 siRNA-treated cultures (Fig. 2A). To determine whether Dia1 affected HSV-1 replication, multicycle growth experiments were performed in which cultures were infected with one infectious particle of HSV-1-GFP-Us11 per 2,000 cells for 3 d. Neither of two Dia1 siRNAs detectably influenced virus spread compared with control siRNA, as determined by GFP signal (Fig. 2B) or WB analysis of viral antigen accumulation (Fig. 2C). Furthermore, Dia1 depletion had minimal effects on infectious virus release into culture supernatants in these assays (Fig. 2D). This suggested that Dia1 was not critical for virus-induced MT stabilization or HSV-1 spread.
Fig. 2.
Host Dia1 and Akt are not required for MT stabilization by HSV-1. (A) NHDFs were treated with Ctrl or Dia1 siRNAs and then mock-infected or infected at m.o.i. 10 for 16 h. (Left) Fixed samples were stained for Ac-MTs. (Right) Parallel samples were lysed and analyzed by WB with the indicated antibodies. (B–D) Dia1 has minimal effects on HSV-1 spread. NHDFs were treated with Ctrl or either of two different Dia1 siRNAs and then infected at m.o.i. 0.0005 for 3 d. (B) Representative phase and fluorescence (GFP) images of plaques formed by HSV-1-GFP-Us11. (C) WB analysis of lysates from B, using the indicated antibodies. HSV-1, antibody against HSV-1 virions. (D) Levels of infectious virus present in culture supernatants after infection with HSV-1 strain F, determined by titration on Vero cells. (E) NHDFs were treated with DMSO (−) or AKTVIII (+) and then infected at m.o.i. 10 for 16 h. (E, Top) Fixed samples were stained for Tyr-MTs (red) and Ac-MTs (green). (E, Bottom) WB analysis of lysates, using the indicated antibodies.
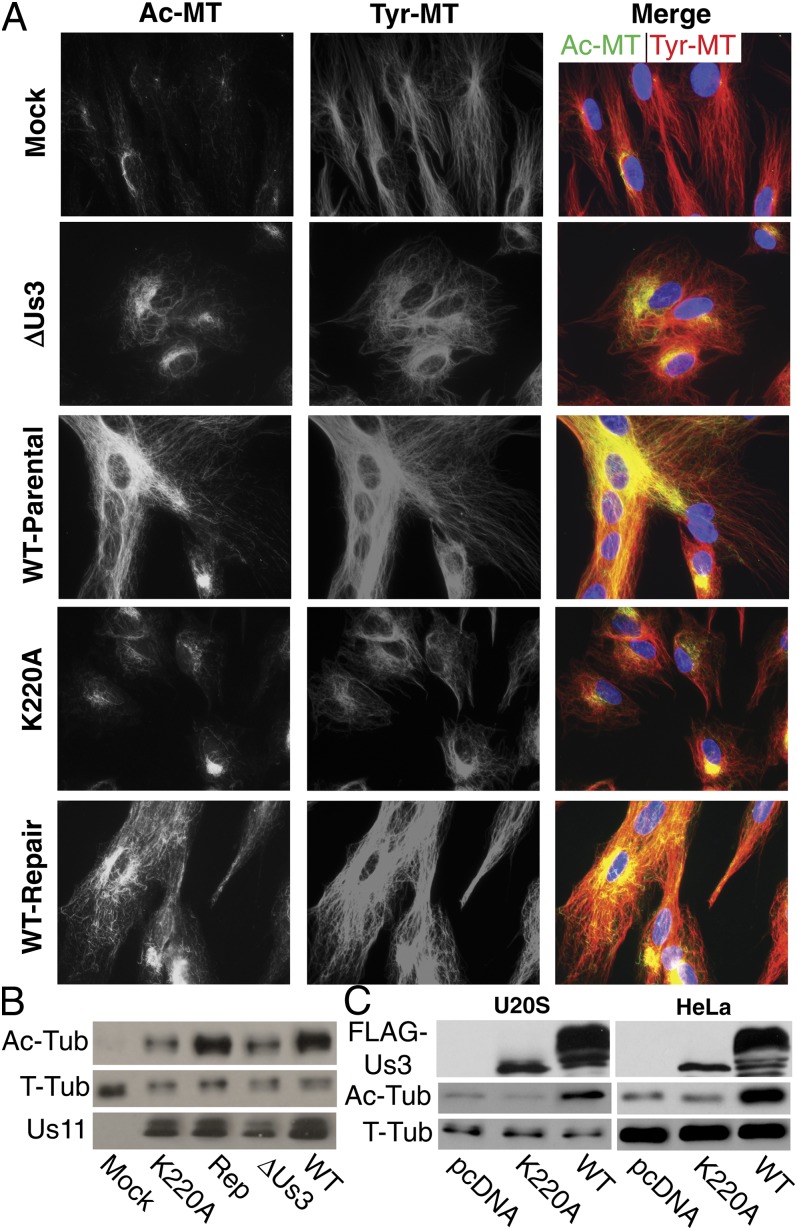
To determine whether host Akt signaling was involved, NHDFs were pretreated with the Akt inhibitor, AKTVIII, or DMSO control and then infected. AKTVIII did not detectably affect Tyr-MT reorganization or Ac-MT induction by HSV-1 (Fig. 2E). WB analysis confirmed that both viral protein production and Ac-MT induction by HSV-1 were insensitive to AKTVIII (Fig. 2E). However, interpretation of these findings is complicated by the fact that HSV-1 encodes a kinase that phosphorylates several Akt substrates yet does not share sequence homology with Akt and is AKTVIII-insensitive (21). Indeed, increased phosphorylation of the Akt substrate GSK3β was observed on infection with a virus encoding Us3, whereas no increase above mock-infected levels was detected with a virus expressing catalytically inactive Us3 (K220A) (22) (Fig. S1A). Furthermore, phosphorylation of GSK3β was sensitive to AKTVIII in both mock- and K220A-infected cultures, whereas active Us3 conferred insensitivity to this inhibitor (Fig. S1A). To determine whether Us3 contributed to MT stabilization NHDFs were infected with either of two independently generated Us3 mutants: a deletion mutant (ΔUs3) compared with its wild-type (WT) parental virus (23) or a virus encoding kinase-inactive Us3 (K220A) compared with its repair virus expressing WT Us3 (22). At 9 h.p.i., ∼70% cells infected with either WT or repair viruses appeared elongated with extensive Tyr- and Ac-MT networks radiating toward the cell periphery (Fig. 3A). In contrast, >95% cells infected with either of the Us3 mutants remained rounded throughout infection and contained Ac-MTs that stained less intensely and largely clustered at perinuclear sites (Fig. 3A). WB analysis of Ac-tubulin confirmed defects in MT stabilization in cells infected with Us3 mutants compared with either WT or repair viruses (Fig. 3B). GSK3β acts as a negative regulator of +TIP activity and is inactivated by phosphorylation, relieving its repressive effects to promote MT stabilization. As such, phosphorylation of GSK3β by Us3 likely induces MT stabilization. To test this, we examined the effects of siRNA-mediated GSK3β depletion. In uninfected cells, depletion increased MT acetylation, but in cells infected with WT HSV-1, which inactivates GSK3β, depletion had no effect on Ac-MT levels (Fig. S1B). In the absence of Us3, the viral function required to inactivate GSK3β, MT stabilization in infected cells became dependent on GSK3β depletion (Fig. S1 B and C). This demonstrated that inactivation of GSK3β by Us3 increased MT stability in infected cells. Finally, exogenous expression of Us3 in human cells induced MT acetylation, whereas a kinase inactive form (K220A) did not (Fig. 3C). These findings demonstrated that the kinase activity of Us3 was both necessary and sufficient to induce MT stabilization.
Fig. 3.
The kinase activity of Us3 induces stable MT formation. (A and B) NHDFs were mock-infected or infected at m.o.i. 10 for 9 h with the indicated viruses. (A) Fixed samples were stained for Tyr-MTs (red) and Ac-MTs (green). (B) Whole-cell extracts were analyzed by WB with the indicated antibodies. (C) U20S or HeLa cells were transfected with empty vector or plasmids encoding Flag-tagged Us3 or kinase inactive Us3 (K220A). Forty-eight hours later, lysates were analyzed by WB, using the indicated antibodies. WT Us3 autophosphorylates, resulting in altered migration compared with K220A. Ac-Tub, acetylated tubulin; T-Tub, total tubulin.
The TGN Acts as an Alternate MTOC in Infected Cells.
Stable MT formation involves nucleation of MTs from an MTOC followed by growth and stabilization of filaments. Previous studies have suggested that HSV-1 disrupts the MTOC, based on observations that MTs are not clearly focused on a single perinuclear site (the centrosome) (17, 18), which is also evident in Tyr-MT staining patterns in infected NHDFs by 3 h.p.i. (Fig. 1A). As the centrosome could be simply obscured by dense MT networks in infected cells, we directly tested for its disruption by staining for the centrosomal marker γ-tubulin. Although discrete centrosomal puncta were readily detected in uninfected NHDFs, these were lost in infected NHDFs (Fig. S2A). A similar finding was reported while this manuscript was in preparation (24), directly demonstrating that HSV-1 disrupts the centrosome.
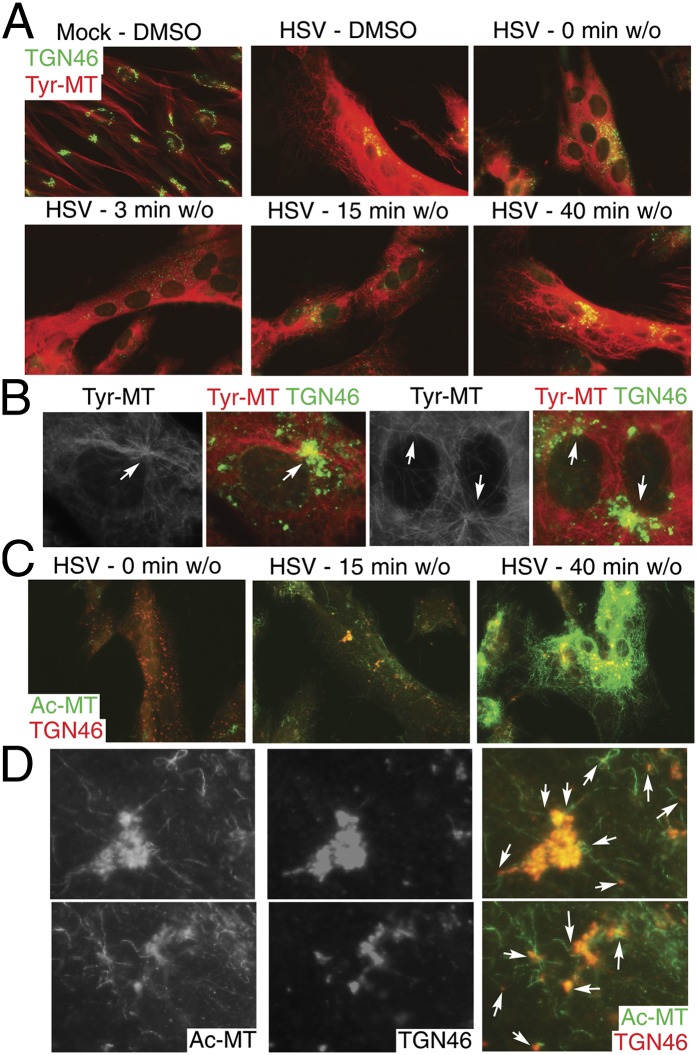
The TGN has been shown to act as an alternate MTOC for noncentrosomal MT arrays (25, 26). To test whether HSV-1 might exploit the TGN as an MTOC, we examined MT regrowth and acetylation using nocodazole washout assays. NHDFs were infected for 14 h and then treated with 10 μM nocodazole for 3 h to disrupt both dynamic and stable MTs. IF analysis showed that new Tyr-MTs began forming in infected cells within 3 min of nocodazole washout and appeared to originate from multiple sites, many of which stained for the TGN marker, TGN46 (Fig. 4 A and B). At this later point in infection, the TGN was largely dispersed, in line with previous reports (17, 27, 28). By 15–40 min after washout, extensive Tyr-MT networks had reformed. In washout samples, acetylation of newly formed MTs was detected within 15 min and became extensive after 40 min (Fig. 4C). Higher-magnification images illustrated how these Ac-MTs originated from TGN-positive sites (Fig. 4D). Furthermore, although Ac-MTs formed inefficiently in cells infected with Us3 mutant virus (Fig. 3), those that did form were found clustered around the TGN (Fig. S2B), suggesting defects in the stabilization of MTs originating from the TGN in the absence of Us3. In contrast, Ac-MTs in WT-infected cells formed extensive arrays that appeared to emanate from the TGN, which was more intact at this earlier 9-h point. Overall, these findings demonstrated that MTs were capable of dynamic growth late in infection and that HSV-1 rapidly induced acetylation of MT subsets that originated from the TGN.
Fig. 4.
The TGN acts as an alternate MTOC in HSV-1-infected cells. NHDFs were mock-infected or infected with HSV-1 at m.o.i. 5 for 14 h. Cultures were treated for 3 h with DMSO or 10 μM nocodazole. Nocodazole was then washed out (w/o) for the indicated times. (A) Fixed samples were stained for Tyr-MTs (red) and TGN46 (green). (B) Higher-magnification images of samples from A illustrate Tyr-MT growth from TGN structures, highlighted by arrows. (C) Fixed samples were stained for TGN46 (red) and Ac-MTs (green). (D) Higher magnification of samples 15 min after w/o in B. Arrows point to Ac-MTs radiating from TGN46-positive structures.
CLASPs Are Required for HSV-1-Induced MT Stabilization and Virus Spread.
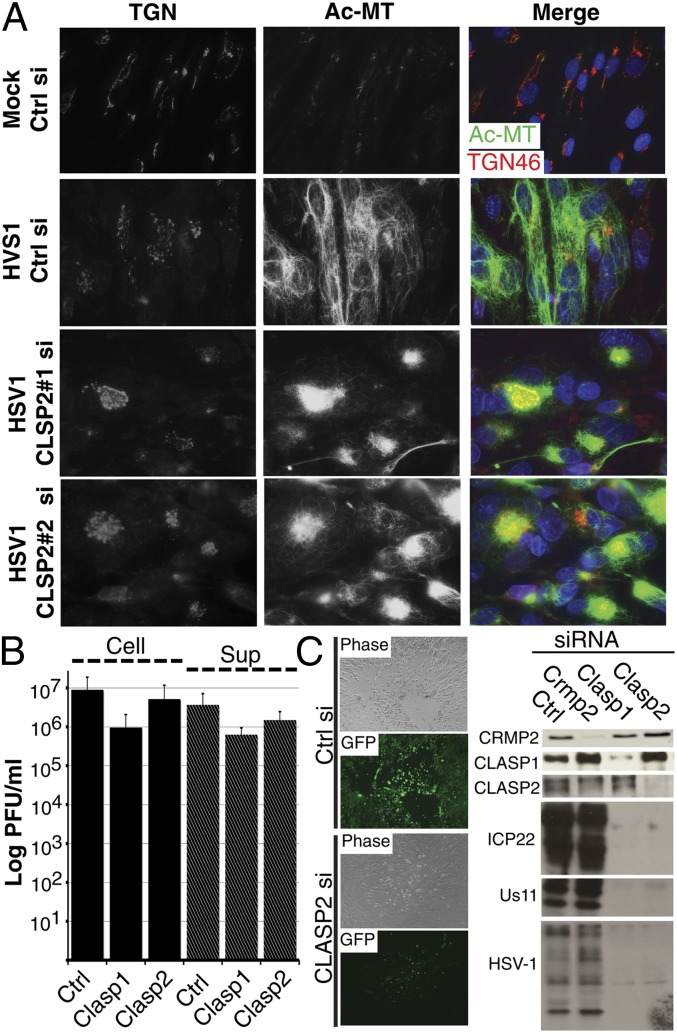
Among GSK3β-regulated +TIPs, CLASP2 controls MT nucleation at the TGN and stabilization at the cell periphery (26, 29). To test whether CLASP2 was required for HSV-1-induced MT stabilization, NHDFs were treated with control or CLASP2 siRNAs and then either mock-infected or infected. IF analysis demonstrated that compared with control siRNA-treated cells, CLASP2 depletion resulted in reduced Ac-MT staining and clustering around the TGN in infected cells (Fig. 5A and Fig. S3A). Similar effects were observed when the functionally related family member, CLASP1, was depleted, whereas depletion of a distinct GSK3β-regulated +TIP, collapsin response-mediating protein 2 (CRMP2) (30) had no effect (Fig. S3A). Compared with control siRNA-treated samples depletion of CLASP1, CLASP2, or CRMP2 did not affect early or late viral protein production (Fig. S3B). However, when infectious virus levels were measured, CLASP depletion was found to result in ∼two- to sevenfold reductions in both cell-associated virus and infectious virus in culture supernatants (Fig. 5B). This suggested a role for CLASPs in viral particle formation. In addition, Ac-MTs are also likely to play important roles in the cell–cell spread of HSV-1. To test this, CLASP1, CLASP2, or CRMP2 were depleted, and then cultures were infected at low m.o.i. with HSV-1-GFP-Us11 for 3 d. Although large plaques and a strong GFP signal were evident in control siRNA-treated or CRMP2-depleted cultures, depletion of CLASP1 or CLASP2 resulted in reduced plaque size and GFP signal resulting from impaired virus spread from the site of initial infection (Fig. 5C and Fig. S4). WB analysis confirmed these spreading defects, which resulted in reduced accumulation of early, late, and structural viral proteins in CLASP-depleted cultures (Fig. 5C). Overall, these findings demonstrate that CLASPs were not required for HSV-1 to enter cells and synthesize viral proteins but were required for both maximal infectious virus production and spread to neighboring cells.
Fig. 5.
CLASPs are required for HSV-1-induced stable MT formation and virus spread. NHDFs were treated with Ctrl or CLASP2 siRNAs and then infected. (A) Samples infected at m.o.i. 10 for 16 h were fixed and stained for TGN46 (red) and Ac-MTs (green). (B) Samples were infected at m.o.i. 5 for 16 h, and then levels of cell-associated (Cell) and supernatant (Sup) infectious virus were determined by titration on Vero cells. (C) siRNA-treated NHDFs were infected with HSV-1-GFP-Us11 at m.o.i. 0.0005 for 3 d. (Left) Representative phase and fluorescence (GFP) images of plaques formed in control or CLASP2 siRNA-treated cultures. (Right) WB analysis of whole-cell extracts shows depletion of CLASP1, CLASP2, and CRMP2 and their effects on the accumulation of early (ICP22), late (Us11), and HSV-1 virion (HSV-1) proteins.
Discussion
Although studies using chemical agents that affect MT polymerization have long established their importance to infection, the mechanisms by which many viruses manipulate MT networks to facilitate their replication and spread remain unclear. Here, we show that HSV-1 induces MT stabilization in primary human cells and demonstrate that this involves GSK3β inactivation by the viral kinase Us3. Our findings demonstrate that downstream GSK3β-regulated +TIPs, CLASPs mediate stable MT formation and suggest that HSV-1 has evolved to exploit the TGN as an alternate MTOC to facilitate virus spread.
Us3 is a multifunctional Ser/Thr kinase that, although dispensable during high m.o.i. infection in many cell lines, is important for efficient anterograde movement and HSV-1 spread in vivo and in specific cell types, notably including NHDFs (21, 22, 31–34). In terms of cytoskeletal regulation, a number of its properties potentially position Us3 as a central regulator of host MTs that may underlie differences with other viral strategies. Kaposi’s sarcoma–associated herpesvirus activates ERK and PI3K through engagement of viral glycoprotein gB with host integrin receptors to stabilize MTs within minutes of infection (9). In contrast, HSV-1 Us3 suppresses both ERK and PI3K-Akt activity (33, 35, 36). Furthermore, specific downstream substrates such as GSK3β are regulated independent of host PI3K-Akt signaling and are, instead, directly controlled by Us3 (21, 33, 37, 38). Here, we find that Us3 induces MT acetylation in transfected cells and is required for efficient stable MT formation in infected cells. Low levels of Ac-MTs that do form in cells infected with Us3 mutants may reflect basal Akt activity, host responses to infection, or the activity of other viral factors. Exogenous expression of the HSV-1 protein VP22 increases MT acetylation although the underlying mechanism, and a role for VP22 in MT stabilization during infection has never been identified (10, 19). Although other factors may contribute to MT stabilization, our findings demonstrate that Us3 targets GSK3β and plays a central role in stable MT formation by HSV-1. Us3 also regulates actin polymerization and the formation of cellular projections (39, 40). Combined with our findings, this positions Us3 as a central regulator of cytoskeletal organization in infected cells. Vaccinia virus–encoded F11 also regulates both actin and MT dynamics by inactivating RhoA to facilitate MT-dependent trafficking of virions to the cell surface (14). Unlike Us3, F11 does not have kinase activity highlighting fundamental differences in the strategies used by these viruses to target the host cytoskeleton. Organization of actin and MTs is intricately linked and coordinately regulated (1, 2). Viral functions such as Us3 and F11 that target both cytoskeletal networks likely orchestrate widespread changes in cellular architecture, motility, and intracellular trafficking that contribute to efficient virus spread.
Stable MT formation by HSV-1 involved GSK3β inactivation by Us3 and required the downstream GSK3β-regulated +TIPs, CLASPs. Depletion of CRMP2 had no effect on MT stabilization or virus spread, demonstrating specificity in the GSK3β-regulated +TIPs that contribute to the biology of infection. CLASPs mediate MT stabilization in response to GSK3β inactivation and MT orientation toward the leading edge of motile cells and neuronal growth cones (29, 41–43). Similar to HSV-1-infected cells, depletion of CLASPs results in MT looping (43) and reduced levels of Ac-MTs (29, 41, 43, 44). CLASPs link MTs to the cell cortex to control polarized trafficking, as well as persistent cell motility and migration (41, 44–49). Additional, more specialized properties of CLASPs may explain their specific importance to HSV-1. CLASPs regulate MT formation at the TGN, orienting TGN-derived MTs and facilitating post-Golgi transport to the cell front (26, 45, 50, 51). The TGN plays a central role in HSV-1 spread by functioning in viral glycoprotein trafficking, secondary envelopment, and egress of progeny virions (15, 16, 28, 52–55). Our findings suggest that MTs formed at the TGN and stabilized by CLASPs are important for efficient assembly of progeny virions and subsequent virus spread.
The fact that the TGN acts as an alternate MTOC (25) has further relevance, given that HSV-1 disrupts the centrosome. Centrosome disruption is induced by a number of viruses (24, 56–59) and may represent a strategy to gain control of host MT networks or a host response to infection. Our findings demonstrate that in the absence of a centrosome, the TGN acts as an alternate MTOC in HSV-1-infected cells, with MTs emanating from the TGN rapidly becoming acetylated. MTs have been described as “disorganized” and seemingly originating from multiple locations, rather than a singular site, during HSV-1 infection (17, 24, 27). The dispersed nature of the TGN and its ability to act as an MTOC in infected cells, illustrated here, offers an explanation for these previous observations. This is further supported by the impaired formation and clustering of Ac-MTs at the TGN in cells infected with Us3 mutant virus or in HSV-1-infected cells depleted of CLASPs. Overall, our findings uncover viral strategies to subvert host MT networks, exploiting the TGN as an MTOC and stabilizing MTs through the activity of a virus-encoded Akt mimic and highly specialized host +TIPs, CLASPs to facilitate virus spread.
Materials and Methods
Cell Culture and Viruses.
NHDFs, U20S, and HeLa cells were cultured and infected as described (36). Vero cells were used to grow and titer viruses (21, 36). Viruses were as described previously: HSV-1 WT and ΔUs3 (strain F) (23), K220A and Repair viruses (strain F) (22), and strain 17+ and HSV-1-GFP-Us11 (strain Patton) (20).
siRNAs, Plasmids, and Inhibitor Treatments.
The following siRNAs were from Applied Biosystems: control siRNAs (AM4635 and AM4637), CLASP1 (136866 and 136867), CLASP2 (261144 and 248843), CRMP2 (AM16704), GSK3β (14880), and Dia1 (242567 and 242568). Cultures were transfected on 2 consecutive days with 150 pmol/mL siRNA, using RNAiMax (Invitrogen), and infected 72 h after initial transfection. Plasmids expressing Flag-tagged Us3 WT or K220A were described previously (21); cells seeded in 12-well plates were transfected with 0.5 μg plasmid, using Lipofectamine 2000 (Invitrogen). For inhibitors, cultures were treated with DMSO or AKTVIII (Calbiochem) at 5 μM for 1 h before and during the course of infection. To depolymerize MTs, infected cultures were treated with 10 μM nocodazole for 3 h at 37 °C. For washout assays, nocodazole-treated cells were rapidly rinsed twice in medium and then incubated in growth medium for the indicated time before fixing.
Antibodies, Immunofluorescence, and Western Blotting.
Antibodies were from the following sources: EB1 (sc-47704), Santa Cruz Biotechnology; CLASP1 (ab108620), γ-tubulin (Ab27074), Abcam; CLASP2 (SAB1300828), Ac-tubulin (T6793), Sigma-Aldrich; Dia1 (610848), BD Transduction Laboratories; GSK3β (9832), S9-phosphorylated GSK3β (9336), Ac-tubulin (5335), CRMP2 (9393), Cell Signaling Technology. Infected cell protein ICP22 was from John Blaho, City College of New York. All other antibodies were described previously (36). For IF, cells were rinsed in PBS and then fixed in ice-cold methanol for 7 min and washed in PBS. Cultures were blocked in PBS containing 2.5% (vol/vol) FBS/0.25% Saponin for 40 min and then probed overnight at 4 °C with the indicated antibodies. Samples were washed in PBS/0.025% Saponin and then probed with Alexa Fluor-conjugated secondary antibody for 1 h at RT and then washed again. Where shown, nuclei were stained with Hoechst 33342. Images were acquired using a Zeiss Axioplan 2 microscope and OpenLab software. Images within experiments were acquired at the same settings, and all postacquisition adjustments to contrast and brightness for figure preparation were applied equally. WB analysis was performed as described previously (36).
Supplementary Material
Acknowledgments
We thank Bernard Roizman, Richard Roller, and John Blaho for reagents and Ian Mohr (New York University School of Medicine) for critically reading the manuscript, helpful discussions, and support of this work through National Institutes of Health (NIH) Grant R01AI073898. This work was supported by NIH Grants R01GM101975 (to M.H.N.) and R01GM062939 (to G.G.G.). M.H.N. is a Schaefer Scholar.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310760110/-/DCSupplemental.
References
- 1.Li R, Gundersen GG. Beyond polymer polarity: How the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9(11):860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 2.Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol. 2011;22(9):968–974. doi: 10.1016/j.semcdb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 4.Gouveia SM, Akhmanova A. (2010) Cell and molecular biology of microtubule plus end tracking proteins: End binding proteins and their partners. Int Rev Cell Mol Biol. 285:1–74. [DOI] [PubMed]
- 5.Jouvenet N, Monaghan P, Way M, Wileman T. Transport of African swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin. J Virol. 2004;78(15):7990–8001. doi: 10.1128/JVI.78.15.7990-8001.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker JS, Broering TJ, Kim J, Higgins DE, Nibert ML. Reovirus core protein mu2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J Virol. 2002;76(9):4483–4496. doi: 10.1128/JVI.76.9.4483-4496.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren JC, Cassimeris L. The contributions of microtubule stability and dynamic instability to adenovirus nuclear localization efficiency. Cell Motil Cytoskeleton. 2007;64(9):675–689. doi: 10.1002/cm.20215. [DOI] [PubMed] [Google Scholar]
- 8.Husain M, Harrod KS. Enhanced acetylation of alpha-tubulin in influenza A virus infected epithelial cells. FEBS Lett. 2011;585(1):128–132. doi: 10.1016/j.febslet.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol. 2005;79(2):1191–1206. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott G, O’Hare P. Herpes simplex virus type 1 tegument protein VP22 induces the stabilization and hyperacetylation of microtubules. J Virol. 1998;72(8):6448–6455. doi: 10.1128/jvi.72.8.6448-6455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henning MS, et al. PDZD8 is a novel moesin-interacting cytoskeletal regulatory protein that suppresses infection by herpes simplex virus type 1. Virology. 2011;415(2):114–121. doi: 10.1016/j.virol.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Frampton AR, Jr, et al. Equine herpesvirus type 1 (EHV-1) utilizes microtubules, dynein, and ROCK1 to productively infect cells. Vet Microbiol. 2010;141(1-2):12–21. doi: 10.1016/j.vetmic.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naghavi MH, et al. Moesin regulates stable microtubule formation and limits retroviral infection in cultured cells. EMBO J. 2007;26(1):41–52. doi: 10.1038/sj.emboj.7601475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa Y, Cordeiro JV, Way M. F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection. Cell Host Microbe. 2007;1(3):213–226. doi: 10.1016/j.chom.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Roizman B, Knipe DM, Whitley RJ. (2007) Herpes simplex viruses. Field’s Virology, eds Knipe DM and Howley PM. (Lippincott, Williams, and Wilkins, Philadelphia), 5th Ed, pp 2501–2602.
- 16.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9(5):382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 17.Avitabile E, et al. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol. 1995;69(12):7472–7482. doi: 10.1128/jvi.69.12.7472-7482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotsakis A, Pomeranz LE, Blouin A, Blaho JA. Microtubule reorganization during herpes simplex virus type 1 infection facilitates the nuclear localization of VP22, a major virion tegument protein. J Virol. 2001;75(18):8697–8711. doi: 10.1128/JVI.75.18.8697-8711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yedowitz JC, Kotsakis A, Schlegel EF, Blaho JA. Nuclear localizations of the herpes simplex virus type 1 tegument proteins VP13/14, vhs, and VP16 precede VP22-dependent microtubule reorganization and VP22 nuclear import. J Virol. 2005;79(8):4730–4743. doi: 10.1128/JVI.79.8.4730-4743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benboudjema L, Mulvey M, Gao Y, Pimplikar SW, Mohr I. Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. J Virol. 2003;77(17):9192–9203. doi: 10.1128/JVI.77.17.9192-9203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuluunbaatar U, et al. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 2010;24(23):2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryckman BJ, Roller RJ. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol. 2004;78(1):399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purves FC, Longnecker RM, Leader DP, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61(9):2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasdeloup D, Labetoulle M, Rixon FJ. Differing effects of Herpes Simplex Virus 1 and Pseudorabies Virus infection on centrosomal function. J Virol. 2013;87(12):7102–7112. doi: 10.1128/JVI.00764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chabin-Brion K, et al. The Golgi complex is a microtubule-organizing organelle. Mol Biol Cell. 2001;12(7):2047–2060. doi: 10.1091/mbc.12.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efimov A, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12(6):917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campadelli G, et al. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 1993;90(7):2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turcotte S, Letellier J, Lippé R. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J Virol. 2005;79(14):8847–8860. doi: 10.1128/JVI.79.14.8847-8860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhmanova A, et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104(6):923–935. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura T, et al. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120(1):137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Meignier B, Longnecker R, Mavromara-Nazos P, Sears AE, Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988;162(1):251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 32.Coller KE, Smith GA. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic. 2008;9(9):1458–1470. doi: 10.1111/j.1600-0854.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benetti L, Roizman B. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol. 2006;80(7):3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deruelle MJ, Favoreel HW. Keep it in the subfamily: The conserved alphaherpesvirus US3 protein kinase. J Gen Virol. 2011;92(Pt 1):18–30. doi: 10.1099/vir.0.025593-0. [DOI] [PubMed] [Google Scholar]
- 35.Chuluunbaatar U, Roller R, Mohr I. Suppression of extracellular signal-regulated kinase activity in herpes simplex virus 1-infected cells by the Us3 protein kinase. J Virol. 2012;86(15):7771–7776. doi: 10.1128/JVI.00622-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh D, Mohr I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004;18(6):660–672. doi: 10.1101/gad.1185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu MJ, Wu CY, Chiang HH, Lai YL, Hung SL. PI3K/Akt signaling mediated apoptosis blockage and viral gene expression in oral epithelial cells during herpes simplex virus infection. Virus Res. 2010;153(1):36–43. doi: 10.1016/j.virusres.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Wagner MJ, Smiley JR. Herpes simplex virus requires VP11/12 to activate Src family kinase-phosphoinositide 3-kinase-Akt signaling. J Virol. 2011;85(6):2803–2812. doi: 10.1128/JVI.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc Natl Acad Sci USA. 2005;102(25):8990–8995. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van den Broeke C, et al. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci USA. 2009;106(21):8707–8712. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drabek K, et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol. 2006;16(22):2259–2264. doi: 10.1016/j.cub.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 42.Kumar P, et al. GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J Cell Biol. 2009;184(6):895–908. doi: 10.1083/jcb.200901042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hur EM, et al. GSK3 controls axon growth via CLASP-mediated regulation of growth cone microtubules. Genes Dev. 2011;25(18):1968–1981. doi: 10.1101/gad.17015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mimori-Kiyosue Y, et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005;168(1):141–153. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller PM, et al. Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat Cell Biol. 2009;11(9):1069–1080. doi: 10.1038/ncb1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lansbergen G, et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell. 2006;11(1):21–32. doi: 10.1016/j.devcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Tsvetkov AS, Samsonov A, Akhmanova A, Galjart N, Popov SV. Microtubule-binding proteins CLASP1 and CLASP2 interact with actin filaments. Cell Motil Cytoskeleton. 2007;64(7):519–530. doi: 10.1002/cm.20201. [DOI] [PubMed] [Google Scholar]
- 48.Wittmann T, Waterman-Storer CM. Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3beta in migrating epithelial cells. J Cell Biol. 2005;169(6):929–939. doi: 10.1083/jcb.200412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe T, et al. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J Cell Sci. 2009;122(Pt 16):2969–2979. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- 50.Rivero S, Cardenas J, Bornens M, Rios RM. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 2009;28(8):1016–1028. doi: 10.1038/emboj.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adachi A, et al. Golgi-associated GSK3beta regulates the sorting process of post-Golgi membrane trafficking. J Cell Sci. 2010;123(Pt 19):3215–3225. doi: 10.1242/jcs.063941. [DOI] [PubMed] [Google Scholar]
- 52.Antinone SE, Zaichick SV, Smith GA. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J Virol. 2010;84(24):13019–13030. doi: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harley CA, Dasgupta A, Wilson DW. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: Role for organelle acidification in assembly of infectious particles. J Virol. 2001;75(3):1236–1251. doi: 10.1128/JVI.75.3.1236-1251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee GE, Murray JW, Wolkoff AW, Wilson DW. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J Virol. 2006;80(9):4264–4275. doi: 10.1128/JVI.80.9.4264-4275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rémillard-Labrosse G, Mihai C, Duron J, Guay G, Lippé R. Protein kinase D-dependent trafficking of the large Herpes simplex virus type 1 capsids from the TGN to plasma membrane. Traffic. 2009;10(8):1074–1083. doi: 10.1111/j.1600-0854.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang W, McCrae MA. The rotavirus enterotoxin (NSP4) promotes re-modeling of the intracellular microtubule network. Virus Res. 2012;163(1):269–274. doi: 10.1016/j.virusres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Ploubidou A, et al. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 2000;19(15):3932–3944. doi: 10.1093/emboj/19.15.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bystrevskaya VB, Lobova TV, Smirnov VN, Makarova NE, Kushch AA. Centrosome injury in cells infected with human cytomegalovirus. J Struct Biol. 1997;120(1):52–60. doi: 10.1006/jsbi.1997.3897. [DOI] [PubMed] [Google Scholar]
- 59.Jouvenet N, Wileman T. African swine fever virus infection disrupts centrosome assembly and function. J Gen Virol. 2005;86(Pt 3):589–594. doi: 10.1099/vir.0.80623-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.