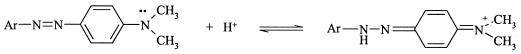Abstract
Several model azo dyes are reductively cleaved by growing cultures of an ascomycete yeast species, Issatchenkia occidentalis. In liquid media containing 0.2 mM dye and 2% glucose in a mineral salts base, more than 80% of the dyes are removed in 15 h, essentially under microaerophilic conditions. Under anoxic conditions, decolorization does not occur, even in the presence of pregrown cells. Kinetic assays of azo reduction activities in quasi-resting cells demonstrated the following: (i) while the optimum pH depends on dye structure, the optimum pH range was observed in the acidic range; (ii) the maximum decolorizing activity occurs in the late exponential phase; and (iii) the temperature profile approaches the typical bell-shaped curve. These results indirectly suggest the involvement of an enzyme activity in azo dye reduction. The decolorizing activity of I. occidentalis is still observed, although at a lower level, when the cells switch to aerobic respiration at the expense of ethanol after glucose exhaustion in the culture medium. Decolorization ceased when all the ethanol was consumed; this observation, along with other lines of evidence, suggests that azo dye reduction depends on cell growth. Anthraquinone-2-sulfonate, a redox mediator, enhances the reduction rates of the N,N-dimethylaniline-based dyes and reduces those of the 2-naphthol-based dyes, an effect which seems to be compatible with a thermodynamic factor. The dye reduction products were tested as carbon and nitrogen sources. 1-Amino-2-naphthol was used as a carbon and nitrogen source, and N,N-dimethyl-p-phenylenediamine was used only as a nitrogen source. Sulfanilic and metanilic acids did not support growth either as a carbon or nitrogen source.
Over the last two decades, considerable work has been done with the goal of using microorganisms as bioremediation agents in the treatment of wastewater containing textile dyes. These contaminants contribute a minor fraction to the usually high load of dissolved organic matter in textile effluents (35), but they are highly visible and must be removed in order to comply with the regulations concerning effluent discharge.
Azo aromatic dyes are the major group of textile dyestuffs. These structures can be reductively cleaved into colorless amines by several bacterial species (for reviews, see references 2, 9, 41, and 43); nevertheless, azo dye reduction occurring in the presence of living matter can be an abiotic process. An example of this is the reduction of acid orange 7 (called dye IV in the present study) and reactive red 2 in anaerobic sludge, where sulfide, produced by sulfate-reducing microorganisms, can reduce azo bonds (45). In most of the reported processes of azo dye bioreduction, however, the participation of an enzymatic activity is assumed. Since the products of azo dye reduction, with few exceptions (4, 11, 26), cannot be used by bacteria as carbon and energy sources, the cleavage of azo bonds is a gratuitous process which can occur when the microorganisms use a reduced carbon compound as the growth substrate.
Azo dye reduction occurring in the presence of living matter poses additional problems. Sulfonic azo dyes are impermeant to the cell membranes. NAD(P)H is also impermeant to the cell membranes and is believed to be the primary source of electrons. This is why reduction of highly polar azo dyes is usually postulated to take place outside the cell (43). This fact would be compatible either with an abiotic reduction mediated by some extracellular reductant species (45) or with the involvement of an externally directed reductase activity in the plasma membrane that was capable of transferring reducing equivalents to acceptor species in the extracellular medium. So far, several bacterial cytoplasmic azoreductases have been isolated and characterized (18, 33, 37, 38). However, as shown by two recent studies (5, 42), the putative azoreductases had, in fact, insignificant in vivo activity.
Independently of the intracellular location of the azoreductase, theoretically, a redox mediator could facilitate the transfer of reducing equivalents from intracellular NAD(P)H to the substrate dye. This hypothesis was proposed as early as 1975 in a study describing the decolorization of azo food dyes by Proteus vulgaris (15). The participation of redox mediators in the decolorization of azo dyes by bacterial species is now generally accepted, and several mediators (usually quinonoid compounds) have been described as effectively enhancing decolorization processes (7, 22, 23, 40). The redox mediators studied are usually exogenous compounds added to the culture medium, but in a recent study (22), Keck and coworkers observed that aerobic preincubation of a Sphingomonas sp. strain with 2-naphthalenesulfonate strongly stimulated the subsequent anaerobic reduction of azo dyes by the same strain. In a later study (23), Keck et al. identified 4-amino-1,2-naphthoquinone and 4-ethanolamino-1,2-naphthoquinone, formed by spontaneous oxidation of 1,2-dihydroxynaphthalene (an intermediate of the 2-naphthalenesulfonate degradation), as putative redox mediators in the decolorization reaction. Another study, observing an autocatalytic effect in the anaerobic reduction of acid orange 7, demonstrated that 1-amino-2-naphthol, one of the dye reduction products, stimulated decolorization (46). The presence of redox mediators in humic substances naturally found in soils has also been reported to enhance decolorization and other redox processes (10, 16, 23).
Azo dye reduction occurs preferentially under anoxic or oxygen-limited conditions (6, 20, 24, 28, 32, 34, 40, 48). Under these circumstances, azo dyes act as terminal electron acceptors during microbial respiration. Aerobic azo dye reduction processes in the presence of additional carbon sources were recently questioned by Stolz (43), since the experimental conditions of decolorization have not unequivocally shown the presence of oxygen in the culture medium, being more consistent, in fact, with oxygen-limited conditions.
The few reports on bioremediation of colored effluents by yeasts usually mention biosorption as the major cause for decolorization (14, 31). Martins et al., however, isolated a strain of Candida zeylanoides, which efficiently decolorizes several azo dyes (30). Further work demonstrated that a reductive cleavage of the azo bond was involved in this process and described some characteristics of the corresponding dye reducing activity (39). In this work, we examined the decolorizing activity of a novel yeast strain, Issatchenkia occidentalis. This species is even more efficient than C. zeylanoides in decolorizing the previously tested dyes. Therefore, we decided to investigate, in more detail, the effects of several parameters on the performance of I. occidentalis as an azo dye reducer. The evidence obtained from this study is expected to provide a sound basis for the development of a biotreatment process for azo dye-containing wastewaters.
MATERIALS AND METHODS
Chemicals and culture medium components.
Peptone, yeast extract, yeast nitrogen base (YNB), and yeast carbon base (YCB) were obtained from Difco. Other chemicals were commercially available, analytical grade reagents.
Dyes.
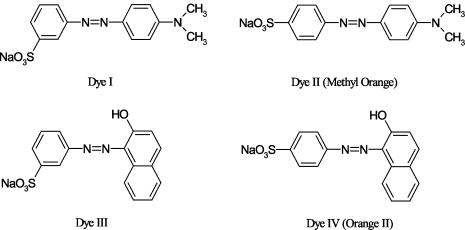
The structures of the dyes tested in the present work are depicted in Fig. 1. Dye II (methyl orange, CI 13025) and dye IV (orange II, CI 15510), both ca. 85% dye content, were purchased from Sigma-Aldrich and used without further purification. The other two dyes (minimum 90% dye content) were synthesized by the conventional method of coupling the diazonium salt of metanilic acid with either N,N-dimethyl-p-phenylenediamine or 1-amino-2-naphthol (17). The structures of the isolated dyes, as sodium salts, were confirmed by 1H nuclear magnetic resonance spectroscopy in dimethyl sulfoxide.
FIG. 1.
Structures of the azo dyes used in this work: m-[(4-dimethylamino)phenylazo]benzenesulfonic acid (dye I), p-[(4-dimethylamino)phenylazo] benzenesulfonic acid (CI 13025 methyl orange or orange 52) (dye II), m-[(2-hydroxy-1-napthyl)azo]benzenesulfonic acid (dye III), and p-[(2-hydroxy-1-napthyl) azo]benzenesulfonic acid (CI 15510 orange II or acid orange 7) (dye IV).
Microorganism and maintenance conditions.
The ascomycete yeast I. occidentalis (PYCC 5770) (deposited in the Portuguese Yeast Culture Collection) was isolated on the basis of its capacity to decolorize YEPD (yeast extract-peptone-dextrose) agar plates containing 0.5% [wt/vol] yeast extract, 1% [wt/vol] peptone, 2% [wt/vol] glucose and the azo dye orange II, as described in a previous publication (30). The identification of the yeast strain followed the usual methods described for yeast taxonomy (44): testing of defined carbon compounds as fermentation substrates or as the sole carbon and energy sources, assimilation tests of nitrogen compounds, temperature tolerance for growth (30, 35, and 40°C), growth in the presence of 0.01 and 0.1% (wt/vol) cycloheximide, growth in the presence of 50 and 60% (wt/vol) d-glucose, and hydrolysis of urea. Morphological characteristics were also considered, i.e., vegetative reproduction only by budding, formation of pseudohyphae, and formation of persistent asci containing round ascospores on yeast malt agar. A commercial software (3) was used as an aid for species identification. The strain was routinely maintained on slants of YEPD agar.
Analytical methods.
Cell growth in liquid medium was monitored by attenuance measurements (47) at 640 nm (D640). Blanks for these readings were prepared from aliquots of centrifuged medium. At high cell densities, both sample and blank were diluted with distilled water by the same factor. A linear correlation between attenuance readings (D640) and cell weight (dry weight) was established by the standard gravimetric method (cell weight [dry weight] in grams · liter−1) = 1.1289(D640 − 0.0487); r2 = 0.996]. Dye concentration was monitored by absorbance readings of aliquots of centrifuged medium at the dye λmax. The assay cuvette contained 0.3 ml of 1 M acetate buffer (pH 4.0), sample, and water to a volume of 3.0 ml; the blank was prepared with the same dilution of buffer in distilled water. Dissolved oxygen was measured as partial oxygen pressure using a Clark-type polarographic electrode and an ATI RUSSEL model RL 400 instrument according to the manufacturer's instructions (detection level, 0.1 mg · liter−1). Metanilic and sulfanilic acids, formed by reduction of dye I or III and dye II or IV, respectively, were detected and quantified by high-pressure liquid chromatography (HPLC) analysis, using a reversed-phase column (RP-18) and an eluent containing the ion-pair reagent tetrabutylammonium phosphate (TBAP), as previously described (39). Under these conditions, metanilic and sulfanilic acids had retention times of 10.0 ± 0.5 and 12.4 ± 0.5 min, respectively. Glucose and ethanol concentrations in the growth media were also determined by HPLC (retention times of 11.1 ± 0.2 and 24.5 ± 0.2 min, respectively), using a Polyspher OAKC (Merck) column, refractive index detection, and arabinose as the internal standard (27).
Decolorization in liquid media.
Decolorization experiments by growing cultures of I. occidentalis were typically performed in 250-ml cotton-plugged Erlenmeyer flasks with 100 ml of sterile medium (normal decolorization medium [NDM]) containing 2% glucose as a carbon and energy source and the test dye (0.2 mmol · liter−1) in a mineral salts base, as previously described (39). The cultures were inoculated at a low cell density with a cell suspension prepared from a freshly grown slant. The flasks were incubated under orbital shaking (120 rpm) at 26°C, except where stated otherwise. Biomass and concentrations of dye, sulfanilic or metanilic acid, glucose, and ethanol were measured as described above, in samples of the incubation mixture collected at defined times. Dissolved oxygen was measured directly in the incubation media.
When testing the effect of adaptation of the strain to the dyes on the decolorization process, the cells were pregrown in NDM with or without dye, until the mid-exponential growth phase was reached. The cells were then harvested by centrifugation at 5,000 rpm in an Eppendorf Centrifuge 584OR, washed twice in cold sterile distilled water, and used to inoculate fresh sterile NDM containing 0.2 mmol of dye · liter−1. The cultures were started at high cell densities (1.4 D units; 1.6 g [dry wt] of cells · liter−1). In control experiments, the inocula consisted of unadapted cells.
Assimilation of azo dye reduction products.
Minimal media with all essential nutrients and vitamins except a carbon source (YNB) or a nitrogen source (YCB) were prepared according to the manufacturer's instructions and supplemented with 0.5 mM metanilic or sulfanilic acid. Appropriate volumes of these media (100 ml) in 250-ml Erlenmeyer flasks were inoculated with freshly grown cells and incubated at 26°C and 120 rpm. Cell growth was monitored as described above. Control experiments were performed with 0.5 mM ammonium sulfate as the sole nitrogen source in YCB or with 0.5 mM glucose as the sole carbon and energy source in YNB. Using the same method, 1-amino-2-naphthol and N,N-dimethyl-p-phenylenediamine, both 0.5 mM, were tested as nitrogen sources. The initial pH was adjusted to 5.2 ± 0.2.
Activity assays with resting cells.
The decolorization activities of nongrowing cells were determined at various pH values, with cells harvested in different growth stages and with or without redox by using the redox mediator anthraquinone-2-sulfonic acid (AQS). To investigate the effect of pH or temperature on decolorization activity, the cells were grown for ca. 11 h on NDM, harvested by centrifugation, washed, and resuspended in 0.05 M phosphate buffer solution of the required pH. The assay mixture contained, in a total volume of 20 ml, 10.4 mM glucose and 0.047 mM dye; 0.1 mM AQS was included in activity assays of dyes I and II and omitted in the assays of dyes III and IV. The assay mixtures typically contained 7.1 ± 0.3 g of cells (dry wt) · liter−1. The assays were performed in 2 h in 50-ml cotton-plugged Erlenmeyer flasks shaken at 120 rpm and at 26°C. Within this period, the dye concentration decreased linearly with time. The same procedure was followed with cells harvested in different growth phases.
RESULTS
Dye removal by growing cultures.
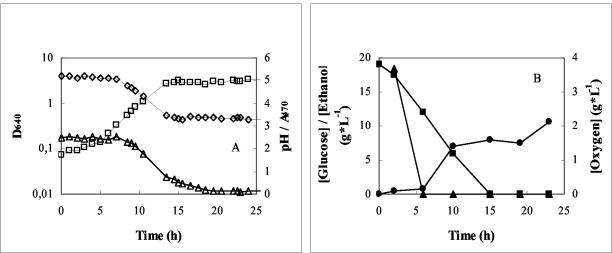
In decolorization assays performed with dyes I to IV, the exponential growth phase was observed between 5 and 12 h of incubation, following a lag phase of ca. 4 h. The fastest decrease in dye concentration occurred during the late exponential growth phase. After 20 h of incubation, the percentages of dye removal were >95% for dyes I and II and ca. 85% for dyes III and IV. Figure 2A shows a typical growth curve, as well as the evolution of the medium pH and dye II concentration versus time, whereas Fig. 2B displays the variation in glucose, ethanol, and dissolved oxygen concentrations within the same period.
FIG. 2.
Typical growth (□), pH (◊), and decolorization curves (▵) (A) and concentrations of glucose (▪), ethanol (•) and oxygen (▴) (B) during incubation of I. occidentalis on NDM containing 0.2 mM dye II. The initial cell density (D640) was 0.09.
HPLC analysis of supernatant samples for the presence of dye reduction products revealed the presence of metanilic acid or sulfanilic acid during decolorization of dyes I (or III) and II (or IV), respectively. These acids are stable under the experimental conditions, and as shown below, they are not used by this yeast as a carbon and energy source or as a nitrogen source within the experiment period. The maximum concentrations of these sulfonates in decolorized media approached the stoichiometric levels (0.17 ± 0.02 mM).
Ethanol starts to accumulate in the culture medium at the onset of the exponential growth phase, indicating that I. occidentalis is a fermentative yeast. Since the maximum yield of ethanol is 0.51 gg of glucose−1, the observed concentration of ethanol in the culture medium (Fig. 2B) approaches the expected value.
Effects of oxygen.
In the standard decolorization experiments described above, the incubation mixtures were contained in cotton-plugged Erlenmeyer flasks and subjected to agitation (120 rpm). Under these conditions, oxygen (from air) is admitted in the system, but CO2 evolution, resulting from glucose fermentation, contributes to decrease its concentration in the medium. In fact, analysis of Fig. 2B shows that, after 6 h of incubation, dissolved oxygen concentration is 0.2 mg · liter−1 or lower. Decolorization, which is faster during the late exponential phase, i.e., between 8 and 11 h of incubation (Fig. 2A), therefore occurs under low oxygen concentrations.
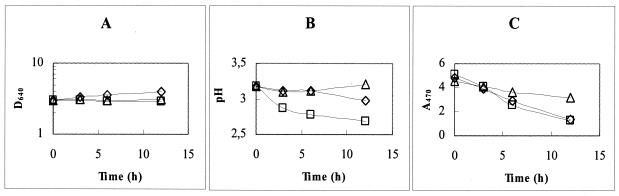
In an attempt to further elucidate the effect of dissolved oxygen in the decolorization process, additional decolorization experiments with cell suspensions inoculated at high densities (ca. 3.0 D units) were performed. Cells were grown for 10 h under the usual conditions and then harvested by centrifugation and washed. The combined pellets were resuspended in fresh NDM with 0.2 mM dye II, and 100-ml volumes of the resulting suspension were placed in separate Erlenmeyer flasks. The aeration conditions were as follows: one flask was continuously flushed with sterile air and incubated while being stirred magnetically, and a second flask was flushed with nitrogen for 15 min, tightly plugged with a rubber stopper, and placed in the orbital incubator. A control experiment, in our standard conditions, was simultaneously run. In the subsequent 12 h of incubation, a very slight increase in growth was detected (Fig. 3A), but in the flasks incubated under the standard conditions or with oxygen flushing, a slight decrease in the pH was observed (Fig. 3B). This was taken as evidence of metabolic activity, which supported a decolorization of ca. 70%, in both cases (Fig. 3C). The dissolved oxygen concentrations were 3.9 ± 0.4 mg · liter−1 in the aerated culture and <0.2 mg · liter−1 in the control culture. Under anoxic conditions, the color loss was only ca. 20%, as seen in Fig. 3, and oxygen concentration remained below the detection level.
FIG. 3.
Variation of cell density measured as D640 (A), pH (B), and dye concentration measured as A470 (C) in NDM preadjusted to pH 3.2 containing 0.2 mM dye II under different conditions: anoxic conditions (▵), air-flushed cultures (□), and normal incubation at 120 rpm (◊) (see text for details). The initial cell density was ≈3 D units.
The absence of metabolic activity in the anoxic conditions, obtained as described above, was further confirmed through the observation that a culture started at a low cell density and incubated for 24 h failed to grow (results not shown).
Effects of dye.
Possible toxic effects of xenobiotic compounds on a microorganism can be preliminarily assessed by comparing its growth curves in the presence and absence of the tested compounds. For dyes I to IV, it has been observed that neither the specific growth rate (0.32 ± 0.03 h−1) nor the biomass yield (0.16 ± 0.02 g [dry weight] · g of glucose−1) were significantly affected by the presence of the dyes.
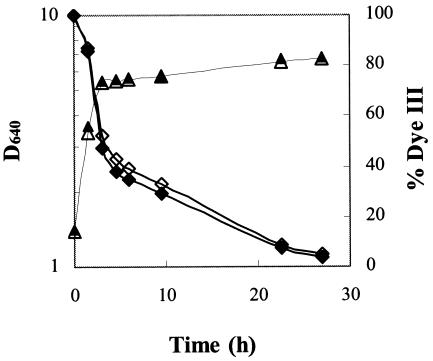
Having excluded the possibility that the dyes themselves were toxic, we also tested a possible enhancement of the decolorization process by using cell suspensions which had been grown overnight in NDM containing 0.2 mM dye as preinocula. The volume of each preinoculum was 20% of the final volume of the incubation mixture. Preinocula grown in the absence of dye were used in control experiments. For all the dyes tested, the decolorization curves obtained with adapted and nonadapted cells did not present any significant differences (Fig. 4), as observed earlier with C. zeylanoides (39).
FIG. 4.
Growth of I. occidentalis (triangles) and decolorization curves (diamonds) of dye III by preadapted cells (open symbols) and nonadapted cells (closed symbols) on NDM.
Standard dye reduction assay with resting cells.
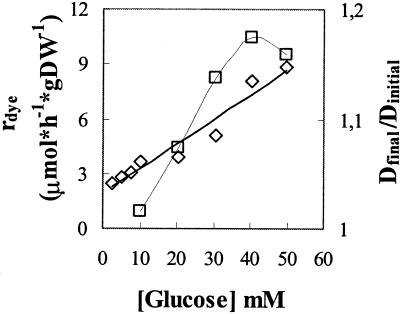
The apparent rates of dye removal by growing yeast cells are affected by increasing biomass and, presumably, by pH. A study of the parameters affecting the kinetics of decolorization therefore requires buffered media and a fixed amount of cells. This condition was approached by establishing the glucose concentration that allowed the best compromise between minimum cell growth and measurable dye reduction activity. Glucose concentrations of up to 20 mM in 0.05 M phosphate buffer (pH 4.0) produced a final D640 /initial D640 ratio of ≤1.1 in the course of 2 h, the maximum time for the assays (Fig. 5). For a glucose concentration of 10.4 mM in assay mixtures, as used henceforth, the final D640/initial D640 ratio was <1.05 and the measured decolorization specific activities therefore approached those of resting cells.
FIG. 5.
Effect of glucose concentration on specific decolorization rates, rdye (◊) and cell growth (final D640/initial D640 ratio) (□). rdye is given in micromoles · hour−1 · gram of cells (dry wt [DW])−1.
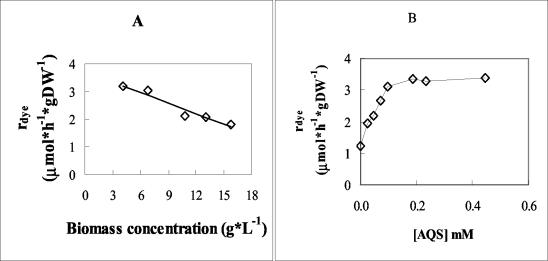
The dye concentration used (0.047 mM) allowed direct absorbance readings, thus minimizing errors related to sample dilution. The optimum biomass concentration was determined by performing assays with various cell concentrations from 0 to ca. 14 D units (0 to 15.8 g of cells [dry wt] · liter−1). The initial decolorization rates, as determined from plots of dye absorbance versus time, in each assay mixture, displayed saturation-like kinetics, due to substrate limitation at higher biomass concentrations. In terms of specific decolorization rates, the optimum cell concentration ranged between 4 and 6 D units (4.5 to 6.7 g of cells [dry wt] · liter−1), decreasing for D values above 6, as seen in Fig. 6A.
FIG. 6.
Effects of biomass (A) and AQS concentration (B) on specific decolorization rates (rdye) of dye II. rdye is given in micromoles · hour−1 · gram of cells (dry wt [DW])−1.
The effect of the redox mediator AQS on the specific decolorization rate of dye II was also analyzed. The assay mixtures contained optimum cell and glucose concentrations, as defined above, and AQS concentrations ranging from 0 to 0.445 mM. The decolorization rate increased linearly, reaching a maximum threefold increase with an AQS concentration of 0.1 mM. The effect of further concentration increase was negligible (Fig. 6B). For subsequent dye reduction assays with this dye, and also with dye I, which displayed the same type of response, a 0.1 mM concentration of AQS was therefore adopted. However, in decolorization assays of dyes III and IV, AQS was omitted because of its negative effect (results not shown).
Kinetic assays.
The assays for assessing the effects of dye concentration, pH, temperature, and growth phase on the decolorization rate were performed under the optimized conditions of glucose concentration and cell density, as described above, with added AQS in assays with dyes I and II only. Except when studying the effect of pH, cells were suspended in 0.05 M phosphate buffer, pH 4.0. The cells used in the assays had previously been grown for 10 to 11 h as described in Materials and Methods. However, in the assays investigating dye reduction activities in different growth phases, the cells were harvested at the specified times.
The data from dye reduction assays performed with various concentrations of dye II as the substrate could be fitted adequately to the Michaelis-Menten equation, rdye = rdye,max [dye]/(Km + [dye]), where rdye and rdye,max are the initial and maximum decolorization rates, respectively, Km is the Michaelis constant (millimolar) and [dye] is the concentration of the dye (millimolar). The kinetic parameters obtained with the Hanes linearization of the experimental data were as follows: rdye,max = 3.2 μmol · h−1 · g−1 and Km = 0.034 mM (r2 = 0.995).
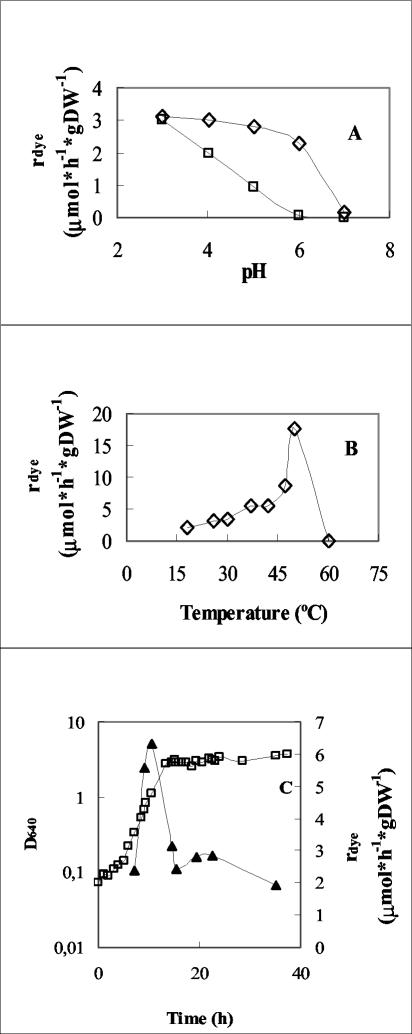
The effect of pH on the decolorization rate was studied by using cell suspensions in phosphate buffers of pH ranging from 3 to 7. Typically, for dyes I and II, the decolorization rate was virtually constant between pH values of 3 to 5, showing a fast decrease in the range 6 to 7. In contrast, for dyes III and IV, the decolorization rates decreased with pH increase in the range 3 to 6. Within the pH range tested, the maximum decolorization rate was observed, in both cases, at pH 3. Figure 7A illustrates this behavior for dyes II and III.
FIG. 7.
Effects of pH (A), temperature (B), and growth phase (C) on specific decolorization rates (rdye) (see text for details). (A) Effects of pH on dye II (◊) and dye III (□) rdye; (B) effect of temperature on dye II (⋄) rdye; (C) effect of growth phase on dye II (□) rdye and a typical growth curve (▴) for I. occidentalis in the presence of dye II. rdye is given in micromoles · hour−1 · gram of cells (dry wt [DW])−1.
The effect of temperature on the decolorization rate was tested in the 18 to 60°C temperature range. As can be seen from Fig. 7B, the temperature profile of the decolorization reaction approaches the expected bell-shaped form characteristic of enzyme activity. The decolorization rates varied from 2.0 (at 18°C) to 17.7 μmol · h−1 · g−1 (at 50°C), indicating that within the assay period (2 h) and at temperatures up to 50°C, there was no apparent reduction in the decolorizing activity of the cells. Complete loss of activity was observed at 60°C.
Azo reduction assays were also performed with yeast cells harvested by centrifugation at different times, while growing in NDM. The cells were washed and then resuspended in the standard assay mixture. As seen in Fig. 7C, a sharp activity maximum is observed for cells in the late exponential phase, with the specific decolorization rate for dye II reaching the value 6.3 μmol · h−1 · g−1 at 10.5 h of incubation. During the early stationary phase, the rate remained approximately constant (2.8 μmol · h−1 · g−1), decreasing to 1.9 μmol · h−1 · g−1 after 35 h.
Assimilation of dye reduction products.
The persistence of metanilic or sulfanilic acids in decolorized supernatants of cultures incubated for a long period suggested that none of these compounds could be assimilated by the yeast strain (results not shown). This was confirmed by incubation of I. occidentalis in minimal medium (YCB or YNB) supplemented with a 0.5 mM concentration of the appropriate source. As seen in Table 1, none of the aminobenzenesulfonates supported yeast growth as a source of carbon and energy or nitrogen. When N,N-dimethyl-p-phenylenediamine was added to YCB minimal medium as a nitrogen source, yeast growth was observed. The other amine, 1-amino-2-naphthol, was used as a source of carbon and energy and of nitrogen by this yeast. Whenever assimilation occurred, an acidification of the medium was observed (final pH of 3.5 ± 0.5).
TABLE 1.
Biomass yields of I. occidentalis on sulfanilic and metanilic acids, 1-amino-2-naphthol, and N,N-dimethyl-p-phenylenediamine as the carbon or nitrogen source
| Minimal medium | Biomass yielda (g · mmol−1) (lag phase [h])
|
|||||
|---|---|---|---|---|---|---|
| Glucose | (NH4)2SO4 | Sulfanilic acid | Metanilic acid | 1-Amino-2-naphthol | N,N-dimethyl-p-phenylenediamine | |
| YCB | 2.77 | NG | NG | 0.89 (12) | 0.89 (2) | |
| YNB | 0.09 (2) | NG | NG | 0.43 | NG | |
NG, no growth.
Sequential batch decolorization of dye II.
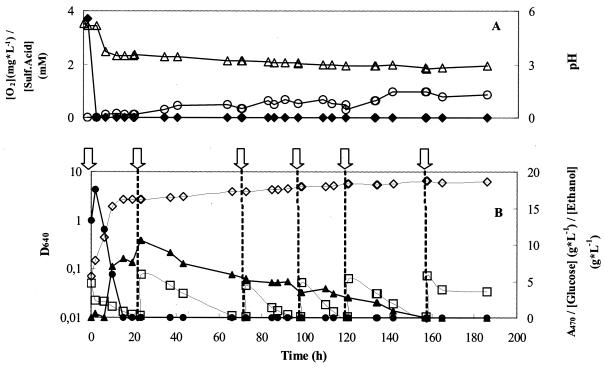
Standard decolorization experiments were usually monitored over a period up to 24 h. By this time, glucose was exhausted from the medium and dyes I and II had been completely removed; for dyes III and IV, a residual amount of 15 to 20% was still present. To examine the decolorization activity of the yeast cells after glucose depletion, a decolorized culture (300 ml), which had been incubated for 24 h under the standard conditions, was subjected to five successive additions of the required amounts of dye II stock solution to restore the initial dye concentration. Each dye addition was done after complete decolorization of the medium. As seen in Fig. 8, decolorization was complete during the first five cycles. During this process, ethanol concentration first increased to a maximum of 10 g · liter−1 in the course of the first cycle and then gradually decreased, disappearing at 160 h of incubation. By this time, decolorization also ceased. In the course of successive cycles, the concentration of sulfanilic acid gradually increased, as expected. Cell growth also continued, with an increase from 2.7 to 6.1 D640 units, showing that the yeast switched from a fermentative metabolism, with glucose, to aerobic respiration, with ethanol. The dissolved oxygen concentration consistently remained below the detection level until the end of the experiment. The decolorizing activity of the yeast cells, measured by the standard assay, slowly decreased with the decolorization-dye addition cycles but was still present in the last sample of cells, collected after 200 h of incubation.
FIG. 8.
Monitoring of several parameters during successive cycles of addition of dye to a culture of I. occidentalis on NDM, incubated at 120 rpm, without any further nutrient addition. Wide white arrows indicate the times of dye addition (see text for details). (A) pH (▵) and concentrations of oxygen (⧫) and sulfanilic acid [Sulf.Acid] (○); (B) cell growth (◊) and concentrations of glucose •, ethanol (▴), and dye II (□).
DISCUSSION
Decolorization of azo dyes by yeasts is much less studied than the homologous process mediated by bacterial species. In this work, an attempt was made to elucidate some basic physiologic aspects associated with azo dye destruction by I. occidentalis. This yeast, like a previously described ascomycete yeast strain (39), is capable of reducing several monoazo dyes to the corresponding amines and, therefore, promotes their decolorization through the cleavage of the azo bond.
The decolorizing activity of I. occidentalis was first studied in actively growing cultures in a glucose-containing culture medium with moderate shaking (Fig. 2). Under these conditions, ethanol was detected in the culture supernatant, showing that the microorganism was fermenting glucose.
The similarities of the decolorization profiles by dye-adapted and nonadapted cells (Fig. 4) indicate that the azo dye reduction activity in yeast cultures is a constitutive property of the cells, independent of their previous exposure to the dye. So far, we have not been able to detect any decolorizing activity in cell extracts or in the supernatant broth, even when high [NADH]/[dye] ratios were used. It thus appears that azo dye reduction activities are dependent on intact, active cells. Thus far, even in bacteria, the major enzyme responsible for azoreductase activity in vivo has not been positively identified. A recent report (42) described a high in vitro azoreductase activity of a cytoplasmic flavin reductase, part of the ribonucleotide reductase complex in Escherichia coli, but its overexpression in a Sphingomonas sp. strain failed to significantly increase the in vivo reducing activity of the bacterium. Earlier, Kudlich and coworkers (24) had also detected an azoreductase activity in a membrane fraction of the same Sphingomonas sp. strain. According to these researchers, the NADH:ubiquinone oxidoreductase was a likely candidate for the azoreductase activity.
Dissolved oxygen is repeatedly considered an inhibitor of the dye bioreduction process (36), since both molecules act as electron acceptors and oxygen is a much stronger oxidant. This is, apparently, the reason why azo dyes are more readily reduced under anaerobiosis. The effect of oxygen was particularly addressed in this work with I. occidentalis, because in the preliminary experiments of the decolorizing potential of the yeast strains that we have studied so far, no attempt was made to prevent the access of oxygen to the dye-containing incubation medium. The decolorizing experiments were usually performed in cotton-plugged flasks, shaken at 120 rpm. Under these standard conditions, faster decolorization rates were indeed observed at low oxygen concentrations (<0.2 mg · liter−1), which can be considered microaerophilic (21). However, our results indicate that oxygen does not significantly interfere with color loss, as seen in air-flushed cultures (Fig. 3). A likely explanation for this fact is the high kinetic barrier involved in the reduction of the triplet ground-state dioxygen. In contrast, under anoxic conditions, i.e., in a nitrogen-flushed culture incubated in rubber-stoppered flasks, the extent of decolorization was rather low (ca. 20%). Since pH (as well as cell density) essentially remained constant in the course of the experiment, a lack of metabolic activity was suspected. In a separate experiment that was also conducted under anoxic conditions in which the culture was inoculated at a low cell density, the yeast failed to grow. These observations show that I. occidentalis has an absolute requirement for oxygen, i.e., places it in the category of the aerobic-fermenting yeasts (1). Therefore, even pregrown cells were unable of performing azo dye reduction in the absence of oxygen. As far as we know, there is no previous report of a similar situation in decolorization processes mediated by facultative anaerobic bacteria.
The effects of pH, temperature, dye and redox mediator concentrations, and growth phase on decolorization rates suggest the participation (direct or indirect) of an enzyme activity in azo dye reduction, because (i) the temperature profile of the azo reduction activity cannot easily be interpreted on the grounds of a purely abiotic process and (ii) the decline of activity at the onset of the stationary phase is more compatible with a reduced metabolic activity of the cells. Additionally, azo dye reduction rate, like the intracellular formation of NAD(P)H, is a growth-dependent process, since it does not occur without a carbon and energy source. In fact, decolorization of dyes III and IV ceased upon glucose exhaustion in the culture medium, and decolorization of dye II, after the diauxic shift (Fig. 8), was linked to the presence of ethanol.
The assays involving a redox mediator, AQS, produced opposing effects in the decolorization rates of the N,N-dimethylaniline-based dyes and 2-naphthol-based dyes: reduction rates increased for dyes I and II but decreased for dyes III and IV in the presence of AQS. The explanation for these apparently contradictory findings is likely to be purely thermodynamic, depending on the relative reduction potentials of dyes and mediator. The role of redox mediators in the process of azo dye reduction by yeasts therefore requires further investigation, but its effective usefulness in this yeast process is doubtful considering that only high [AQS]/[dye] ratios (>20) produce significant rate enhancements.
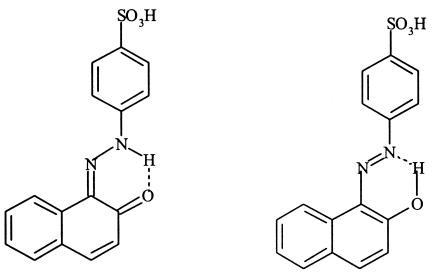
Concerning the effect of pH on the decolorization rates, there is also a considerable difference between N,N-dimethylaniline-based dyes (dyes I and II) and 2-naphthol-based dyes (dyes III and IV). Dyes with a hydroxyl group ortho to the azo bond, because they exist in the tautomeric azo-hydrazone forms (Fig. 9), have an abnormally high pKa2; for dye IV (and presumably for dye III), pKa2 is 11.4 (49). This means that the dye molecules will have an overall negative charge in the pH range 3 to 6. This fact alone is probably one factor making these dyes less susceptible to reduction than dyes I or II.
FIG. 9.
Azo-hydrazone tautomers of dye IV.
Additionally, since the global reaction for dye reduction consumes four H+ ions from the medium plus the two hydrogen atoms from NAD(P)H (reaction 1), a decrease in [H+] will have the effect of shifting the equilibrium in the direction of the reagents, thus making the free energy for the reaction less negative. The observed trend of an apparent dye reduction rate with increasing pH is consistent with the observation that the kinetics of redox reactions is usually determined by thermodynamics.
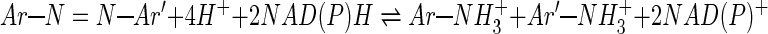 |
(1) |
Dyes I and II, in contrast, have a pKa2 of ∼3.5, and protonation occurs in an azo nitrogen (Fig. 10). This means that, up to pH ∼5, a still considerable fraction of the molecules will be protonated in one azo nitrogen, apparently in an amount allowing a fast decolorization rate.
FIG. 10.
Protonization of N,N-dimethylamino-based dyes at an azo nitrogen.
The above conclusions show that, in this respect also, the reduction by a yeast is considerably different from the reduction by bacteria, which has an optimum pH range close to neutrality (8, 33).
The decolorizing activity of I. occidentalis is not strictly related to a fermentative process, since it continued even after glucose exhaustion. Under these conditions, the yeast switches to aerobic respiration, at the expense of ethanol, which is the major carbon and energy source in the medium. Ethanol depletion, at 160 h of incubation, coincided with the disappearance of the decolorizing activity. Because the shake flasks were moderately agitated (120 rpm), the concentration of dissolved oxygen remained below the detection level. Therefore, the ethanol-dependent azo dye reduction equally occurred under microaerophilic conditions, although at a lower rate.
The assimilation assays with sulfanilic and metanilic acids revealed their inability to be used as carbon and energy sources and also as nitrogen sources. This fact is not surprising, because except for a few works describing the bacterial degradation of sulfanilic acid (12, 13), 6-aminonaphthalene-2-sulfonate (19, 25), and 2-aminobenzenesulfonate (29), arenesulfonates are usually described as xenobiotic compounds. The persistence of the other two amines expected from dye reduction, N,N-dimethyl-p-phenylenediamine (dyes I and II) and 1-amino-2-naphthol (dyes III and IV), could not be detected by HPLC analysis, since they are quickly oxidized in aerated aqueous solutions, forming colored products, and their elution profiles change considerably within a short period of time. However, the colors associated with the oxidation products of either N,N-dimethyl-p-phenylenediamine (deep pink) or 1-amino-2-naphthol (dark yellow to brown) were not seen in decolorized media. The assimilation tests revealed that N,N-dimethyl-p-phenylenediamine was used as nitrogen source whereas 1-amino-2-naphthol was both a source of nitrogen and a source of carbon and energy, thus accounting for the absence of their oxidation products in the decolorized supernatants.
This work shows that the ascomycete yeast I. occidentalis displays an effective azo dye reduction capacity, comparatively unspecific but, nevertheless, structure dependent in such aspects as glucose requirements for complete decolorization, pH-activity profiles, and effect of the tested redox mediator. All of these factors must be taken into account when considering the possible application of the yeast to a bioremediation process for azo textile dyes. Equally important is the effect of dissolved oxygen in the decolorization process. This particular strain, because it is an aerobic-fermentative yeast, has an absolute requirement of oxygen for growth and hence, for decolorization. While oxygen traces are sufficient for growth and allow dye reduction, our results also point to a noninhibitory effect of moderate levels of oxygen in the process. The decolorization process is also growth dependent, pointing to NAD(P)H availability as a crucial factor. This conclusion opens further perspectives in the search for more efficient yeast strains in azo dye decolorization.
Acknowledgments
P.A.R. gratefully acknowledges a Ph.D. scholarship from the European Project BIOEFTEX.
REFERENCES
- 1.Alexander, M. A., and T. W. Jeffries. 1990. Respiratory efficiency and metabolite partitioning as regulatory phenomena in yeasts. Enzyme Microb. Technol. 12:2-19. [Google Scholar]
- 2.Banat, I. M., P. Nigam, D. Singh, and R. Marchant. 1996. Microbial decolorization of textile dye-containing effluents: a review. Bioresour. Technol. 58:217-227. [Google Scholar]
- 3.Barnett, J. A., R. W. Payne, and D. Y. Yarrow. 1990. Yeast identification program, version 2. Cambridge University Press, Cambridge, United Kingdom.
- 4.Blümel, S., M. Contzen, M. Lutz, A. Stolz, and H.-J. Knackmuss. 1998. Isolation of a bacterial strain with the ability to utilize the sulfonated azo compound 4-carboxy-4′-sulfoazobenzene as the sole source of carbon and energy. Appl. Environ. Microbiol. 64:2315-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blümel, S., H.-J. Knackmuss, and A. Stolz. 2002. Molecular cloning and characterization of the gene coding for the aerobic azoreductase from Xenophilus azovorans KF46F. Appl. Environ. Microbiol. 68:3948-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bragger, J. L., A. W. Lloyd, S. H. Soozandehfar, S. F. Bloomfield, C. Marriott, and G. P. Martin. 1997. Investigations into the azo reducing activity of a common colonic microorganism. Int. J. Pharm. 157:61-71. [Google Scholar]
- 7.Cervantes, F. J., F. P. van der Zee, G. Lettinga, and J. A. Field. 2001. Enhanced decolourisation of Acid Orange 7 in a continuous UASB reactor with quinones as redox mediators. Water Sci. Technol. 44:123-128. [PubMed] [Google Scholar]
- 8.Chang, J.-S., C. Chou, Y.-C. Lin, J.-Y. Ho, and T. L. Hu. 2001. Kinetic characteristics of bacterial azo-dye decolourisation by Pseudomonas luteola. Water Res. 35:2841-2850. [DOI] [PubMed] [Google Scholar]
- 9.Chung, K.-T., and S. E. Stevens, Jr. 1993. Degradation of azo dyes by environmental microorganisms and helminths. Environ. Toxicol. Chem. 12:2121-2132. [Google Scholar]
- 10.Coates, J. D., K. A. Cole, R. Chakraborty, S. M. O'Connor, and L. A. Achenbach. 2002. Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Appl. Environ. Microbiol. 68:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coughlin, M. F., B. K. Kinkle, and P. L. Bishop. 1999. Degradation of azo dyes containing aminonaphthol by Sphingomonas sp. strain 1CX. J. Ind. Microbiol. Biotechnol. 23:341-346. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin, M. F., B. K. Kinkle, and P. L. Bishop. 2002. Degradation of Acid Orange 7 in an aerobic biofilm. Chemosphere 46:11-19. [DOI] [PubMed] [Google Scholar]
- 13.Coughlin, M. F., B. K. Kinkle, and P. L. Bishop. 2003. High performance degradation of azo dye Acid Orange 7 and sulfanilic acid in a laboratory scale reactor after seeding with cultured bacterial strains. Water Res. 37:2757-2763. [DOI] [PubMed] [Google Scholar]
- 14.Donmez, G. 2002. Bioaccumulation of the reactive textile dyes by C. tropicalis growing in molasses medium. Enzyme Microb. Technol. 30:363-366. [Google Scholar]
- 15.Dubin, P., and K. L. Wright. 1975. Reduction of azo food dyes in cultures of Proteus vulgaris. Xenobiotica 5:563-571. [DOI] [PubMed] [Google Scholar]
- 16.Field, J. A. 2002. Limits of anaerobic biodegradation. Water Sci. Technol. 45:9-18. [PubMed] [Google Scholar]
- 17.Furniss, B. S., A. J. Hannaford, P. W. G. Smith, and A. R. Tatchell. 1989. Vogel's textbook of practical organic chemistry, 5th ed., p. 951. Longman Group UK, Ltd., Harlow, Essex, United Kingdom.
- 18.Ghosh, D. K., A. Mandal, and J. Chaudhuri. 1992. Purification and partial characterization of two azoreductases from Shigella dysenteriae type 1. FEMS Microbiol. Lett. 98:229-233. [DOI] [PubMed] [Google Scholar]
- 19.Haug, W., A. Schmidt, B. Nörtmann, D. C. Hempel, A. Stolz, and H.-J. Knackmuss. 1991. Mineralization of the sulfonated azo dye Mordant Yellow 3 by a 6-amino-naphthalene-2-sulfonate-degrading bacterial consortium. Appl. Environ. Microbiol. 57:3144-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayase, N., K. Kouno, and K. Ushio. 2000. Isolation and characterization of Aeromonas sp. B-5 capable of decolorizing various dyes. J. Biosci. Bioeng. 90:570-573. [PubMed] [Google Scholar]
- 21.Isik, M., and D. T. Sponza. 2003. Effect of oxygen on the decolorization of azo dyes by Escherichia coli and Pseudomonas sp. and fate of aromatic amines. Process Biochem. 38:1183-1192. [Google Scholar]
- 22.Keck, A., J. Klein, M. Kudlich, A. Stolz, H.-J. Knackmuss, and R. Mattes. 1997. Reduction of azo dyes by redox mediators originating in the naphthalenesulfonic acid degradation pathway of Sphingomonas sp. strain BN6. Appl. Environ. Microbiol. 63:3684-3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keck, A., J. Rau, T. Reemtsma, R. Mattes, A. Stolz, and J. Klein. 2002. Identification of quinoide redox mediators that are formed during the degradation of naphthalene-2-sulfonate by Sphingomonas xenophaga BN6. Appl. Environ. Microbiol. 68:4341-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudlich, M., A. Keck, J. Klein, and A. Stolz. 1997. Localization of the enzyme system involved in anaerobic reduction of azo dyes by Sphingomonas sp. strain BN6 and effect of artificial redox mediators on the rate of azo dye reduction. Appl. Environ. Microbiol. 63:3691-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhm, A. E., A. Stolz, K. L. Ngai, and H.-J. Knackmuss. 1991. Purification and characterization of 1,2-dihydroxynaphthalene dioxygenase from a bacterium that degrades naphthalenesulfonic acids. J. Bacteriol. 173:3795-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulla, H. G., R. Krieg, T. Zimmermann, and T. Leisinger. 1984. Experimental evolution of azo dye-degrading bacteria, p. 663-667. In M. J. Klug and C. A. Reddy (ed.), Current perspectives in microbial ecology. American Society for Microbiology, Washington, D.C.
- 27.Lages, F., and C. Lucas. 1997. Contribution to the physiological characterization of glycerol active uptake in Saccharomyces cerevisiae. Biochem. Biophys. Acta 1322:8-18. [DOI] [PubMed] [Google Scholar]
- 28.Laszlo, J. A. 2000. Regeneration of azo-dye-saturated cellulosic anion exchange resin by Burkholderia cepacia anaerobic dye reduction. Environ. Sci. Technol. 34:167-172. [Google Scholar]
- 29.Mampel, J., J. Ruff, F. Junker, and A. M. Cook. 1999. The oxygenase component of the 2-aminobenzenesulfonate dioxygenase system from Alcaligenes sp. strain O-1. Microbiology 145:3255-3264. [DOI] [PubMed] [Google Scholar]
- 30.Martins, M. A., M. H. Cardoso, M. J. Queiroz, M. T. Ramalho, and A. M. Oliveira-Campos. 1999. Biodegradation of azo dyes by the yeast Candida zeylanoides in batch aerated cultures. Chemosphere 38:2455-2460. [DOI] [PubMed] [Google Scholar]
- 31.Meehan, C., I. M. Banat, G. McMullan, P. Nigam, F. Smyth, and R. Marchant. 2000. Decolorization of Remazol Black-B using a thermotolerant yeast, K. marxianus IMB3. Environ. Int. 26:75-79. [DOI] [PubMed] [Google Scholar]
- 32.Moir, D., S. Masson, and I. Chu. 2001. Structure-activity relationship study on the bioreduction of azo dyes by Clostridium paraputrificum. Environ. Toxicol. Chem. 20:479-484. [PubMed] [Google Scholar]
- 33.Moutaouakkil, A.,Y. Zeroual, F. Z. Dzayri, M. Talbi, K. Lee, and M. Blaghen. 2003. Purification and partial characterization of azoreductase from Enterobacter agglomerans. Arch. Biochem. Biophys. 413:139-146. [DOI] [PubMed] [Google Scholar]
- 34.Nigam, P., I. M. Banat, D. Singh, and R. Marchant. 1996. Microbial process for the decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochem. 31:435-442. [Google Scholar]
- 35.Park, J., and J. Shore. 1984. Water for the dyehouse—supply, consumption, recovery and disposal. J. Soc. Dyers Colourists 100:383-399. [Google Scholar]
- 36.Pearce, C. I., J. R. Lloyd, and J. T. Guthrie. 2003. The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigments 58:179-196. [Google Scholar]
- 37.Rafii, F., and C. E. Cerniglia. 1990. An anaerobic non denaturing gel assay for the detection of azoreductase from anaerobic bacteria. J. Microbiol. Methods 12:139-148. [Google Scholar]
- 38.Rafii, F., and T. Coleman. 1999. Cloning and expression in Escherichia coli of an azoreductase gene from Clostridium perfringens and comparison with azoreductase genes from other bacteria. J. Basic Microbiol. 39:29-35. [PubMed] [Google Scholar]
- 39.Ramalho, P. A., H. Scholze, M. H. Cardoso, M. T. Ramalho, and A. M. Oliveira-Campos. 2002. Improved conditions for the aerobic reductive decolorisation of azo dyes by Candida zeylanoides. Enzyme Microb. Technol. 31:848-854. [Google Scholar]
- 40.Rau, J., H. J. Knackmuss, and A. Stolz. 2002. Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ. Sci. Technol. 36:1497-1504. [DOI] [PubMed] [Google Scholar]
- 41.Robinson, T., G. McMullan, R. Marchant, and P. Nigam. 2001. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 77:247-255. [DOI] [PubMed] [Google Scholar]
- 42.Russ, R., J. Rau, and A. Stolz. 2000. The function of cytoplasmic flavin reductases in the reduction of azo dyes by bacteria. Appl. Environ. Microbiol. 66:1429-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stolz, A. 2001. Basic and applied aspects in the microbial degradation of azo dyes. Appl. Microbiol. Biotechnol. 56:69-80. [DOI] [PubMed] [Google Scholar]
- 44.van der Walt, J. P., and D. Y. Yarrow. 1984. Methods for the isolation, maintenance, classification and identification of yeasts, p. 45-104. In N. J. Kreger-van Rij (ed.), Yeasts—a taxonomic study. Elsevier, Amsterdam, The Netherlands.
- 45.van der Zee, F. P., I. A. E. Bisschops, V. G. Blanchard, R. H. M. Bouwman, G. Lettinga, and J. A. Field. 2003. The contribution of biotic and abiotic processes during azo dye reduction in anaerobic sludge. Water Res. 37:3098-3109. [DOI] [PubMed] [Google Scholar]
- 46.van der Zee, F. P., G. Lettinga, and J. A. Field. 2000. The role of (auto)catalysis in the mechanism of an anaerobic azo reduction. Water Sci. Technol. 42:301-308. [Google Scholar]
- 47.Verhoven, J. W. 1996. International Union of Pure and Applied Chemistry, Organic Chemistry Division Commission on Photochemistry: glossary of terms used in photochemistry (IUPAC recommendations 1996). Pure Appl. Chem. 68:2223-2286. [Google Scholar]
- 48.Yu, J., X. Wang, and P. L. Yue. 2001. Optimal decolorization and kinetic modeling of synthetic dyes by Pseudomonas strains. Water Res. 35:3579-3586. [DOI] [PubMed] [Google Scholar]
- 49.Zollinger, H. 1991. Color chemistry. Syntheses, properties and applications of organic dyes and pigments, 2nd ed. VCH, Weinheim, Germany.