Significance
Von Hippel–Lindau (VHL) is a tumor suppressor protein. Through its ubiquitin E3 ligase activity, VHL controls hypoxia-inducible factor (HIF) protein levels. We show that VHL is degraded upon expression or induction of proteins containing BC box-Suppressor of Cytokine Signaling (SOCS) domains. These proteins, ubiquitously expressed, are important for proper cellular cytokine signaling and are often exploited for viral benefit. We also show that cellular consequences of VHL degradation in response to stressors, such as viral infection, cytokines, and viral reactivation, afford distinct survival advantages in terms of increased metabolic and proangiogenic markers. The impairment of the VHL/HIF pathway upon viral infection reinforces the notion that this process may be linked to regulation of pathogenesis by viruses associated with human cancers.
Keywords: ubiquitylation, oncoviral proteins, hypoxia
Abstract
The tumor suppressor VHL (von Hippel–Lindau) protein is a substrate receptor for Ubiquitin Cullin Ring Ligase complexes (CRLs), containing a BC-box domain that associates to the adaptor Elongin B/C. VHL targets hypoxia-inducible factor 1α to proteasome-dependent degradation. Gam1 is an adenoviral protein, which also possesses a BC-box domain that interacts with the host Elongin B/C, thereby acting as a viral substrate receptor. Gam1 associates with both Cullin2 and Cullin5 to form CRL complexes targeting the host protein SUMO enzyme SAE1 for proteasomal degradation. We show that Gam1 protein expression induces VHL protein degradation leading to hypoxia-inducible factor 1α stabilization and induction of its downstream targets. We also characterize the CRL-dependent mechanism that drives VHL protein degradation via proteasome. Interestingly, expression of Suppressor of Cytokine Signaling (SOCS) domain-containing viral proteins and cellular BC-box proteins leads to VHL protein degradation, in a SOCS domain-containing manner. Our work underscores the exquisite ability of viral domains to uncover new regulatory mechanisms by hijacking key cellular proteins.
Ubiquitylation is a posttranslational modification that involves the tagging of protein substrates with ubiquitin moieties. Ubiquitin transfer requires the coordinated and subsequent activities of the ubiquitin-activating E1 enzyme, ubiquitin-conjugating E2 enzymes, and ubiquitin E3 ligases. E3 ligases show substrate specificity and can be grouped into three families based on their different E2-docking domains. CRLs (Cullin RING Ligases) are the largest subfamily of RING domain ligases (1). They display the same modular structure: a scaffold subunit (Cullin), a RING subunit, an adaptor, and a substrate-receptor subunit to recruit the specific protein target to be ubiquitylated (2). Each Cullin associates with a specific adaptor subunit, except for Cullin2 and Cullin5, which both interact with the Elongin B and Elongin C (hereafter EloB/C) heterodimer (3). The EloB/C heterodimer is specifically bound by substrate receptors that contain a minimal consensus sequence (called BC-box motif) (4–6) usually included in the SOCS (Suppressor of Cytokine Signaling) domain of several substrate-receptor subunits (7, 8). The consensus sequence and the function of the BC-box motif were first described for SOCS proteins (5, 8, 9), a family of downstream effectors of cytokine signaling cascade, involved in the negative feedback regulation of this pathway.
Several viruses encode for their own substrate receptors possessing the BC-box motif and are thus able to interact with EloB/C cellular adaptor, thereby modifying the cellular ubiquitin E3 ligase complex and their target proteins selection (10–17). We have demonstrated that the CELO (Chicken Embryo Lethal Orphan) Adenovirus early protein Gam1 is one of such BC box-containing proteins that is able to associate with both Cullin2 and Cullin5 to reconstitute active E3 ligase complexes and target the SUMO E1 enzyme (SAE1/SAE2 heterodimer) for degradation (18, 19). Conversely, the BC-box mutant Gam1 L258, 265A shows severely impaired binding to EloB/C, resulting in the absence of SUMO E1 degradation and the other downstream effects of the wild-type Gam1 protein (18, 20, 21), supporting the idea that these effects may depend on Gam1 ability to act as substrate-receptor protein.
VHL (von Hippel–Lindau) protein is a cellular BC box-containing substrate receptor and associates with Cullin2-based E3 ligases (22–24). VHL is a tumor suppressor, and its loss leads to the von Hippel–Lindau syndrome that often develops into renal clear-cell carcinoma and other highly vascularized tumors (25, 26). In normoxic conditions, VHL binds and targets HIF (Hypoxia-Inducible Factor) 1α subunit for degradation whereas, in hypoxic conditions, this subunit is stabilized and accumulates (27–30). Together with HIF 1β, HIF 1α forms a transcription factor that promotes the expression of a plethora of genes involved in angiogenesis (31), erythropoiesis (32), and glucose metabolism (33).
Here, we investigate and characterize a CRL-dependent mechanism that drives VHL protein degradation. Data on the BC-box mutants of Gam1, SOCS1, and SOCS3 show that VHL protein decrease is indeed BC box-dependent. Cullin knockdown experiments further confirm that VHL ubiquitylation and degradation require Cullin2 and Cullin5. We also explore heat shock, an experimental state shown to reactivate viral replication (34), and infection with Adenovirus Ad5ΔE3 to show a decrease in VHL levels upon viral infection. The decrease in VHL levels is also evident upon infection of human keratinocytes with the HPV16 (Human Papilloma virus Type 16) E6 and E7 oncoproteins that interact with CRL complexes (13) whereas no change in VHL protein levels is observed upon infection with SV40 large T-antigen that lacks a SOCS domain. Thus, BC box-containing proteins decrease VHL with the consequent stabilization of HIF 1α in the context of a cellular response to viral infection. The impairment of VHL/HIF pathway caused by viral or cellular SOCS domain-containing proteins in response to viral infection might have detrimental consequences for the infected cell, leading to a favorable environment for tumor onset and cancer progression.
Results
VHL Protein Decreases and HIF 1α Is Stabilized in Cells Expressing Gam1 WT and Not the Gam1 LL/AA Mutant Protein.
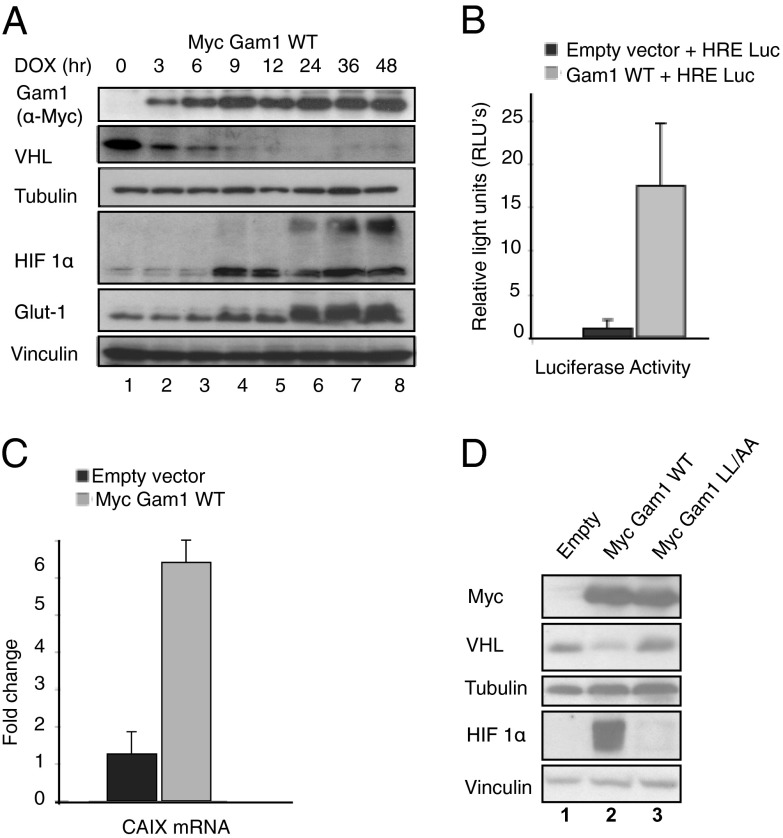
We have recently shown that Gam1 is a viral substrate receptor that recruits both Cullin2- and Cullin5-based E3 ligases (CRL) to target the SUMO E1 enzyme (SAE1/SAE2 heterodimer) for degradation (18, 19). VHL, a tumor suppressor, also forms part of a CRL complex (24) and acts as a cellular substrate receptor targeting HIF 1α for degradation (29). Interestingly, we observed a decrease in VHL protein levels upon the expression of doxycycline (DOX)-inducible Gam1 WT in Tet-on HeLa cells. The decrease in VHL levels with time coincided with HIF 1α stabilization and the accumulation of Glut1, a downstream target protein of HIF 1α (Fig. 1A). To assess the transcriptional activator function of HIF-1, we measured luciferase activity in cells cotransfected with the luciferase gene under the control of a bipartite Hypoxia Responsive Element (HRE) promoter. Luciferase activity increased 18-fold upon Gam1 WT protein expression (Fig. 1B). The expression of Carbonic Anhydrase IX (CAIX) gene, a direct transcriptional target of HIF-1 (35), showed a fourfold increase in Gam1 WT-expressing cells (Fig. 1C). We next sought to investigate whether the expression of Gam1 WT and a specific Gam1 BC-box mutant (Gam1 LL/AA), incapable of forming a functional CRL (18, 19), would differently affect cellular protein levels of VHL. In cells transiently transfected with Gam1 WT, VHL levels were significantly decreased compared with cells expressing the Gam1 BC-box mutant. We also found that HIF 1α levels were higher only in cells expressing Gam1 WT and not Gam1 LL/AA (Fig. 1D). These results collectively indicate that Gam1 WT, but not the mutant protein, decreases cellular levels of VHL and stabilizes and increases the transactivational function of HIF 1α, thus creating a hypoxic-like cellular state.
Fig. 1.
Gam1 WT decreases cellular levels of VHL, stabilizes HIF 1α, and increases the transactivational function of HIF 1α. (A) Immunoblotting (IB) showing Glut1 and HIF1α stabilization. HeLa Tet-on cells were induced (DOX) to express Myc Gam1 WT. Lysates were made in SDS lysis buffer at the indicated times and probed as indicated. Loading controls: Vinculin and Tubulin. (B) Luciferase assay. HeLa cells transfected with HRE (Hypoxia Responsive Element)-Luciferase reporter plasmid together with either empty vector or myc Gam1 wild-type (WT). Results are reported as fold induction of luciferase activity in relative light units per second (RLU/s) normalized to the control (empty vector) where n = 3 ± SD. (C) Quantitative RT-PCR. Results are reported as fold induction of CAIX mRNA relative to the control (empty vector) and normalized respectively to GADPH mRNA (to which the arbitrary value of 1 was assigned) where n = 3 ± SD. (D) IB of VHL and HIF 1α in HeLa cells transiently transfected with Myc Gam1 WT and Myc Gam1 LL/AA. Loading control: Tubulin and Vinculin.
VHL Protein Decrease upon Gam1 WT Protein Expression Is Ubiquitin/Proteasome-Dependent.
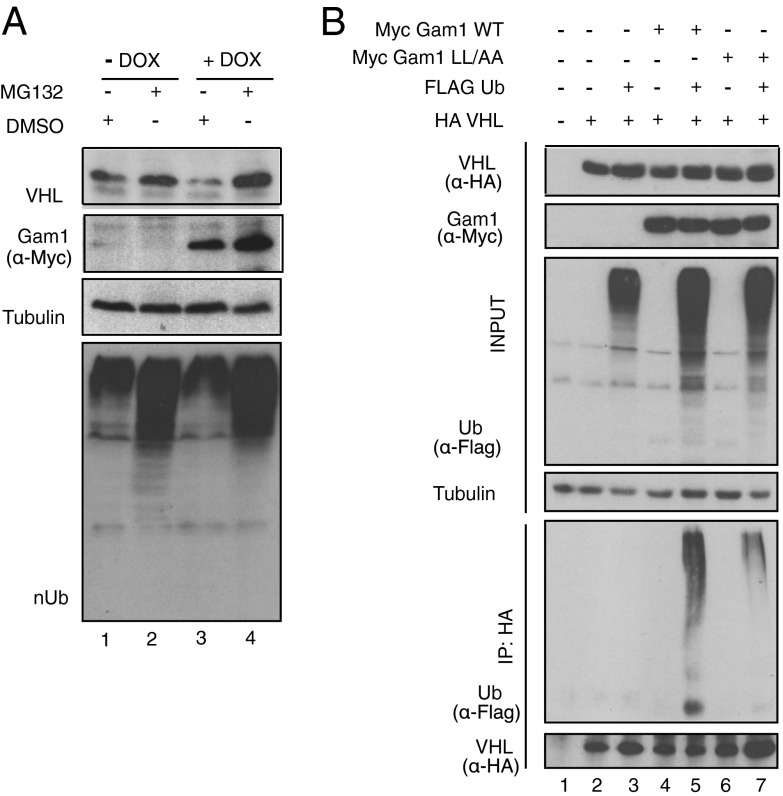
Under normal conditions, VHL is a stable protein (36) with a half-life of 24 h (Fig. S1). Instead, Gam1 WT shortens VHL half-life, with no significant changes in VHL mRNA levels (Fig. S2). To address whether the rapid and reproducible decrease of VHL protein levels may be due to proteasomal degradation, Tet-on Gam1 WT-inducible HEK 293T cells were treated with 10 μM proteasome inhibitor MG132 (Z-Leu-Leu-Leu-Al) to block protein degradation. Gam1 WT expression was induced by the addition of 1 μg/mL doxycycline, and, 1 h later, MG132 was added to the media. Cells were harvested 13 h after induction and analyzed. Proteasome inhibition efficiently rescued VHL protein levels, even upon expression of Gam1 WT (Fig. 2A, compare lanes 2 and lane 4). Because proteins targeted to the proteasome are usually ubiquitinated, we assessed whether VHL is subject to such modification upon Gam1 WT expression. To this end, we cotransfected Phoenix cells with HA-tagged VHL protein, Flag-tagged ubiquitin, and myc Gam1 (WT or LL/AA). Cells were then treated with 10 μM MG132 for 2 h before harvesting and lysed in a denaturing SDS-based lysis buffer to preserve only covalent bonds. VHL immunoprecipitation (IP) showed increased ubiquitylation in the presence of Gam1 WT (Fig. 2B, lane 5) whereas the Gam1 BC-box mutant induced only a slight VHL ubiquitylation (Fig. 2B, lane 7), most likely due to its extremely low affinity for the E3 ligase complexes (18). In the absence of Gam1, VHL protein did not display any ubiquitin modification (Fig. 2B, lane 3), indicating that Gam1 WT expression enhances VHL ubiquitylation and the consequent protein degradation. We then carried out the reciprocal IP in HeLa cells, immunoprecipitating Flag ubiquitin under the same experimental conditions. From the smear pattern, it was evident that VHL is ubiquitylated upon Gam1 WT protein expression and not when Gam1 BC-box mutant protein is expressed (Fig. S3, compare lanes 6 and 8), suggesting the requirement for an intact BC box in ubiquitylating VHL.
Fig. 2.
Gam1 WT and not Gam1 LL/AA cause ubiquitylation and proteasome-dependent decrease of VHL. (A) Tet-on HEK293T cells were induced (DOX). One hour after induction, MG132 or DMSO was added to the medium. nUb was used as a control for proteasome inhibition. Loading control: Tubulin. (B) Phoenix cells were transfected with HA VHL, Flag Ub, and myc Gam1 (WT or mutant LL/AA), harvested, and lysed in denaturing SDS-lysis buffer. One milligram of crude extract was immunoprecipitated (IP) against HA tag and analyzed by IB. Anti-Flag antibody detected ubiquitinated HA VHL. Loading control: Tubulin.
SOCS Domain Containing an Intact BC-Box Motif Is Sufficient to Decrease Cellular Levels of VHL.
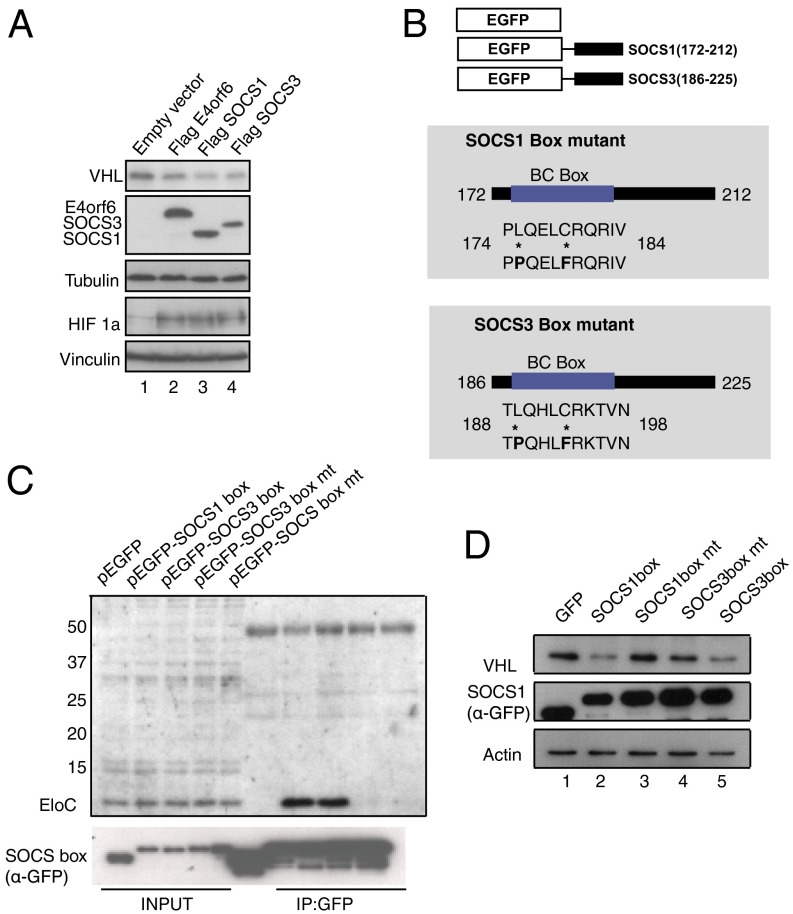
We then asked whether other BC box-containing proteins of viral and cellular origin could mediate the decrease in VHL protein. Thus, we transiently transfected cells with Adenoviral E4orf6 from Ad5, cellular SOCS1, and cellular SOCS3 proteins, which have been shown to contain a functional BC-box motif by virtue of its binding to EloB/C (8, 10, 12, 14, 15, 37). Interestingly, Ad5 E4orf6, SOCS1, and SOCS3, when independently and transiently expressed in U2OS cells, decreased levels of VHL concomitant with HIF 1α stabilization (Fig. 3A). To identify the minimum region required to mediate the decrease in VHL, we constructed EGFP fusions of the SOCS domain from full-length SOCS1 and SOCS3 constructs. SOCS1 and SOCS3 are well-studied negative regulators of cytokine signaling, having shown to function also as part of the signaling events that follow a viral infection. Previously published work has also shown that the SOCS domain binds EloB/C whereas the SOCS domain carrying a BC-box mutation is rendered defective in binding EloB/C (37). We created the SOCS-box domain and their respective BC-box mutants that have two amino acid substitutions L→P (175;SOCS1 and 189;SOCS3) and C→F (179;SOCS1 and 193;SOCS3) in their BC box (Fig. 3B) (6). HeLa cells were transiently transfected with the SOCS-box domain and the mutant proteins. Clearly, BC-box mutants unable to bind EloB/C (Fig. 3C, last two lanes) are unable to decrease protein levels of VHL (Fig. 3D, lanes 3 and 4). These data indicate that the BC-box region within the SOCS domain is necessary and sufficient to decrease cellular levels of VHL. However, it was likely that the VHL protein decrease that we observed upon the expression of BC box-containing proteins or the BC box itself may be nonspecific, potentially affecting substrates degraded by a CRL-mediated mechanism. To exclude this possibility, we transiently transfected U2OS cells with Gam1 WT, SOCS1 box, and Flag SOCS1. The levels of p53, a protein that is regulated by the CRL (14), remained unchanged (Fig. S4). Thus, it was more likely that VHL protein was a specific target of the BC box.
Fig. 3.
SOCS domain BC-box mutant does not decrease VHL protein levels. (A) U2OS cells were transfected with Flag E4orf6, Flag SOCS1, or Flag SOCS3. Lysates were analyzed as depicted. Loading control: Tubulin and Vinculin. (B) Schematic representation of the EGFP-tagged SOCS domain, subcloned from full-length SOCS1 and SOCS3. Box mutants were constructed by specific mutations (*) within the BC box. (C) HeLa cells transfected with SOCS box, and their respective mutants were harvested and IP for GFP. IB for Elongin C shows mutant proteins unable to bind Elongin C. (D) Protein expression of SOCS box and their respective mutants in HeLa cells. Lysates were IB for VHL. Loading control: Actin.
BC Box-Dependent VHL Decrease Is Cullin-Dependent.
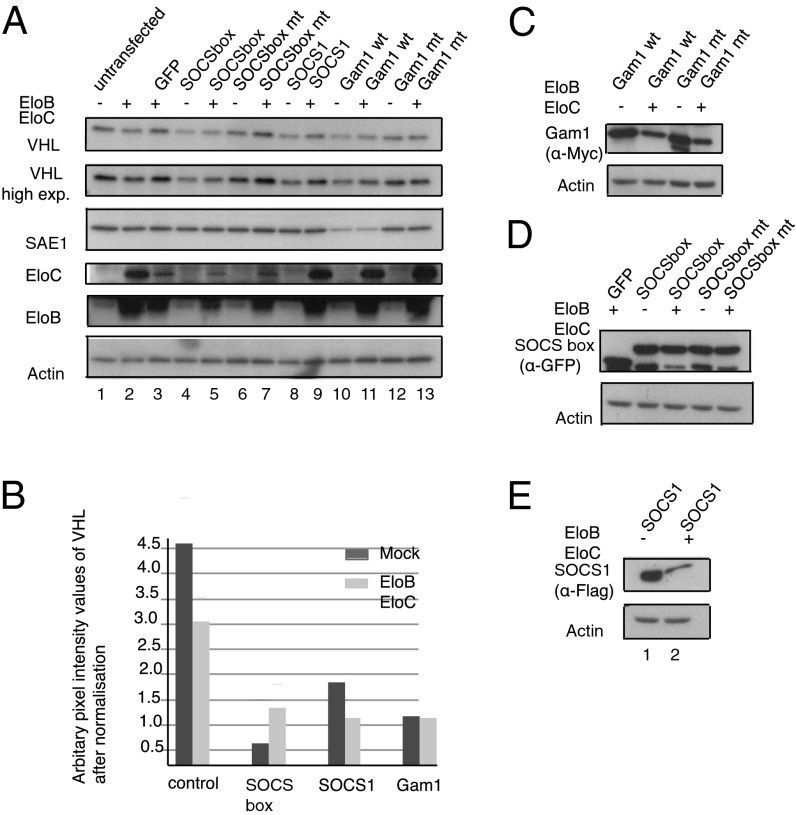
We next sought the mechanism behind the decrease of VHL protein. It has been shown that VHL mutants unable to bind EloB/C are prone to degradation (38). The transient expression of BC-box proteins could deplete cellular levels of EloB/C, thus rendering the VHL protein less stable as a consequence. To ensure sufficient levels of adaptor protein, we transiently transfected EloB/C along with a panel of SOCS domain and mutant proteins. Thirty hours after transfection, HeLa cell lysates were made and probed for VHL. From the immunoblot and the graphical representation of VHL levels, it was clear that transient expression of EloB/C did not rescue the BC box-dependent decrease of VHL (Fig. 4 A and B). We also found no appreciable increase in VHL over the 30 h in cells transfected with EloB/C versus the untransfected cells (Fig. 4A, compare lane 1 with lane 3). Expression of all transfected proteins was confirmed by immunoblotting (Fig. 4 C–E).
Fig. 4.
Cotransfection of EloB/C does not affect VHL protein decrease. (A) HeLa cells were cotransfected with a panel of SOCS domain proteins and their respective BC-box mutant proteins. EloB/C were cotransfected as indicated (+ or −). Lysates were probed against VHL, EloB/C, and SAE1. Loading control: Actin. Decrease in SAE1 is specific to Gam1 WT protein expression and is the positive control. (B) Graph of VHL protein levels (from immunoblot in A) based on pixel-intensity values measured from plot profiles of individual lanes using ImageJ. (C–E) Immunoblots of transfected proteins with loading controls.
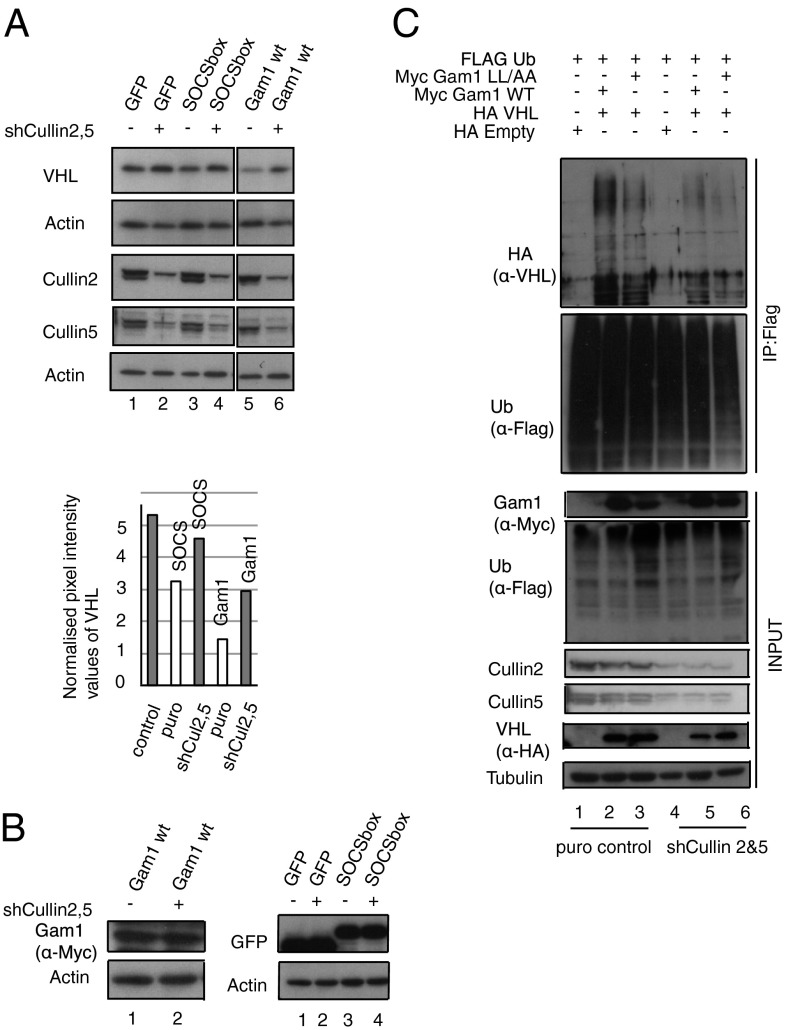
To investigate whether Cullin2 and Cullin5 were required to decrease VHL expression, we transfected HeLa cells with shRNA plasmids against Cullin2 and Cullin5. The shRNA plasmids were transiently cotransfected and selected in 1 μg/mL puromycin for 3 d. Following selection, the cells were transfected with the SOCS box, Gam1 WT, and the respective controls. Fig. 5 shows that, upon Cullin depletion, SOCS box and Gam1 WT are unable to decrease levels of VHL as efficiently as in cells containing the Cullins (Fig. 5A, compare lanes 3 and 4, lanes 5 and 6). We then tested whether Cullin knockdown specifically decreases VHL ubiquitylation. HeLa cells knocked down for Cullins were transfected with Flag Ub, Gam1 WT, Gam1 LL/AA, and HA VHL. Under SDS lysis conditions, which preserve only covalent protein modification, it was clear that there was a noticeable increase in the ubiquitylation of VHL upon Gam1 WT protein expression not reproduced in the absence of Cullins (Fig. 5C, compare lanes 2 and 5). Our data investigating the Cullin requirement for VHL decrease in BC box-expressing cells show that, firstly, the transient expression of the adaptor proteins EloB/C does not rescue the BC box-mediated decrease of VHL; secondly, Cullin2 and Cullin5 proteins participate in VHL protein degradation; and, thirdly, Cullin2 and Cullin5 depletion decreases VHL ubiquitylation and degradation.
Fig. 5.
Cullin2 and Cullin5 are involved in VHL decrease. (A) HeLa cells were transfected with shCul2 and shCul5 or control vector. VHL levels relative to Actin loading show a less significant decrease in VHL upon Cul2 and Cul5 knockdown in SOCS domain-transfected cells. (B) Transient expression of the transfected proteins is shown by IB. (C) HeLa cells selected as described in A were transfected as depicted. Flag Ub was IP under SDS lysis conditions (SI Materials and Methods). Loading control: Tubulin.
Virus Infection Decreases VHL Protein Levels.
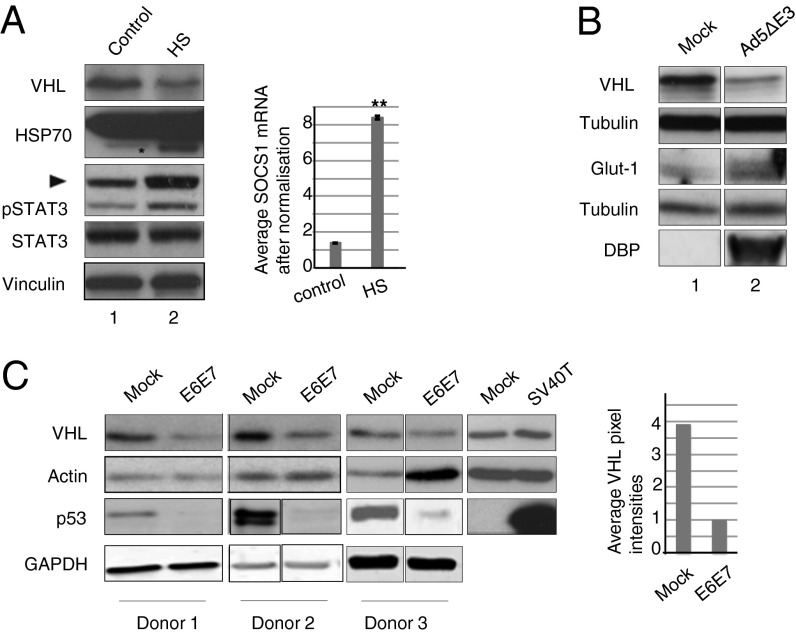
Heat shock is a well-documented experimental state that has been shown to cause viral reactivation (39, 40). More importantly, heat shock at 43 °C for 90 min causes viral reactivation of the replication incompetent CELO virus (lacking the Gam1 gene) (34). Thus, we used heat shock as an experimental setting to simulate viral infection and probe cellular levels of VHL. Fig. 6A shows that, when HeLa cells are heat shocked at 43 °C for 90 min, there is a significant decrease in VHL protein levels. Because viral infection and cytokine activation have been associated with STAT3 activation (41–43), we assessed the phosphorylated form of STAT3 (STAT3Y705), which was elevated in the heat-shocked samples versus the control (Fig. 6A, compare lanes 1 and 2). Heat-shock treatment also led to a threefold induction of SOCS1 mRNA transcription compared with control condition (Fig. 6A). To mimic a true viral infection, we infected HeLa cells with Ad5ΔE3 (Adenovirus dl327), a deletion mutant of Ad5 that has been shown to advance the time of inflammation and exhibit a significantly greater pathologic cellular response (44). Only in the deletion mutant infected cells there was a noticeably lower level of VHL protein, accompanied by increased Glut1, the downstream target of HIF 1 (Fig. 6B). To extend our observations of a viral infection and VHL protein decrease that was SOCS domain-dependent, we transduced human primary keratinocytes with recombinant retrovirus expressing E6 and E7 oncoproteins from human papillomavirus type 16 (HPV16). E7 has been shown to form a functional CRL (13), and E6/E7 infection was confirmed by the decrease in p53 levels (Fig. 6C) (45). A consistent and reproducible decrease in VHL protein upon infection was observed, with no significant effect on mRNA levels (Fig. S5). Infection of donor keratinocytes with SV40 large T-antigen, which lacks a SOCS domain, does not decrease cellular levels of VHL (Fig. 6C). Taken together, our results show that, upon infection, expression of BC box-containing viral proteins leads to VHL decrease.
Fig. 6.
Heat shock and viral infection decreases VHL protein. (A) Tet-on inducible HeLa cells were subjected to heat-shock treatment (HS) or kept at 37 °C as control. VHL protein decrease upon heat shock, as shown in the representative IB, is significant and consistent (P < 0.05, n = 3). Control: Hsp70 asterisk indicates the stress-induced band. A graphical representation of SOCS1 mRNA in control and HS cells shows a statistically significant (P < 0.006, n = 3) and reproducible increase of SOCS1 mRNA. (B) HeLa cells were either mock infected or infected with Ad5ΔE3. Twenty-four hours after infection, lysates were probed for VHL and Glut1. Infection was confirmed by probing with DBP. Loading control: Tubulin. (C) Primary Human Keratinocytes expressing viral proteins (SI Materials and Methods). Lysates were probed for p53 and VHL protein. Loading control: GAPDH.
Discussion
VHL targets HIF 1α to proteasome-dependent degradation. Key consequences of HIF1α activation in cancer are the effect on signaling to the vasculature due to the activation of angiogenic factors and on cancer-cell metabolism with advantages to proliferate and metastasize (46, 47). In this study, we investigate VHL protein decrease in the presence of the viral protein Gam1. Using the BC-box mutant of Gam1, we show that VHL decrease and the coincident HIF 1α stabilization depend on an intact BC box. It has been shown that VHL plays a role in the down-regulation of the expression of glucose transporter-1 (Glut1) and that cells lacking functional VHL overexpress hypoxia-inducible genes (46). In accordance with these data, in a kinetic study of Gam1 WT protein expression, it is evident that HIF 1α stabilization, consequent to VHL protein decrease, correlates with Glut1 protein levels, confirming that HIF 1α is stabilized and rendered transcriptionally active (Fig. 1). Incidentally, the Adenoviral protein E4orf6 and the cellular SOCS1 and SOCS3, which also form part of the CRL (8, 10, 12, 37), decrease VHL levels and cause HIF 1α stabilization. To investigate whether a common mechanism does exist in decreasing VHL protein, an otherwise stable protein observed from our cyclohexamide CHX chase (Fig. S1), we mutated the BC box within the SOCS domain to construct the SOCS1-box and SOCS3-box mutants. Transient expression of the mutant proteins did not decrease VHL, unlike their WT counterparts, pointing to the relevance of an intact BC box within the SOCS domain to induce the VHL decrease. Although substrate selection of SOCS box-containing proteins relies on regions upstream of the domain (for example Human Adenovirus protein E4orf6), it is interesting to note that transient expression of the SOCS domain, lacking upstream elements, mediates VHL protein decrease by default. This behaviour probably explains the similar, yet varying, degrees of VHL decrease when the Adenoviral protein E4orf6, Gam1 WT, and SOCS1 and SOCS3 are independently and transiently expressed (Fig. 3A). However, it must also be noted that transient expression of the SOCS domain does not affect cellular levels of p53 or SAE1, well-known targets of the CRL complex (Fig. S4 and Fig. 4A) (14, 19). Our experiments show that VHL decrease upon Gam1 WT expression is proteasome-dependent and that VHL is ubiquitylated in the presence of Gam1 WT and not the mutant. Because the SOCS domain forms part of the CRL (37), using Cullin2 and Cullin5 knockdown experiments, we show that VHL decrease requires Cullin2 and Cullin5. Indeed, VHL protein ubiquitylation was significantly reduced in Gam1-expressing cells when the Cullins are depleted. These results point to the requirement of the Cullins to mediate VHL protein decrease. Although it has been shown that sumoylated VHL is more stable (48), it is less likely that desumoylation plays a role in mediating VHL protein decrease upon expression of SOCS domain-containing proteins. In fact, although Gam1 WT has been shown to degrade the SUMO activating enzyme SAE1 (18, 19), we do not observe the decrease in SAE1 when other SOCS domain proteins are expressed (Fig. 4A). It is still unclear whether the SOCS domain CRL is the sole ligase that mediates the decrease of VHL. The possibility of additional ligases participating in VHL protein decrease arises from the observation that SOCS1 and SOCS3 bind with comparatively lower affinities to Cullin5 than the rest of the SOCS family of proteins (37). However, physiological effects not always rely on in vitro interaction strengths. Thus, a weaker Cullin5 interaction does not necessarily translate to reduced in vivo CRL activity of SOCS1 and SOCS3. To ensure that SOCS domain overexpression does not deplete endogenous levels of EloB/C (38), we cotransfected EloB and EloC along with a panel of SOCS-domain proteins. Although EloB/C overexpression reduces levels of SOCS1, as observed previously (8) and in our data (Fig. 4E), we found no change in our earlier observation of SOCS domain-mediated VHL decrease. Thus, the observed VHL decrease is a less likely consequence of insufficient EloB/C proteins. It must also be noted that, in our experiments of Cullin depletion, carried out over a period of 3 d, we noticed no change in VHL levels. It is thus improbable that limited availability of the CRL components causes the decrease in levels of VHL. Similarly, EloB/C overexpression and VHL protein analysis over a period of 2 d also did not alter levels of VHL compared with controls, indicating that the decrease in VHL protein levels are SOCS domain-mediated (Fig. 4 A and B). From our CHX chase experiment, we also observed a decrease in VHL protein levels in SOCS domain-transfected cells (Fig. S6). We then asked whether VHL levels would be affected upon viral infection. Our data suggest that viral proteins carrying the SOCS domain decrease cellular levels of VHL (Fig. 3A). Indeed, infection with Adenovirus Ad5ΔE3, Human papilloma virus proteins E6 and E7, and heat-shock induction, which simulates a virus-infected state in cells, decreased VHL protein levels whereas infection with SV40 large T-antigen did not (Fig. 6). However, in a physiological setting such as heat shock, several factors could cooperate to decrease VHL protein levels despite our observation of an increase in mRNA levels of SOCS1 in HeLa cells (Fig. 6A). Our attempts of a SOCS1 and SOCS3 knockdown in HeLa cells resulted in massive cell death. Furthermore, the involvement of more than one member of the SOCS family of proteins renders it difficult to single out a select SOCS protein in mediating VHL decrease in a physiological setting. However, it is plausible that viral proteins containing a BC-box motif aim to achieve a hypoxic cellular state by altering the VHL HIF 1α axis (discussed in Fig. 7). This hypothesis is suggested from our time-course experiments expressing Gam1 WT (Fig. 1A) as well as from the transient overexpression of viral proteins (Fig. 3A). IFN-induced HIF 1α stabilization, as VHL loss, could cooperate in organizing host defenses and have cellular consequences that in some cases can inhibit viral replication (49, 50). However, in other instances of persistent viral infection, HIF-1 activation can lead to the activation of the proangiogenic program and oncogenic transformation as a consequence (11, 51–54). Therefore, considering the primary role of HIF 1α in immune defense, it is not surprising that cells have evolved auxillary mechanisms to rapidly sustain the stabilization of HIF 1α, for instance through the degradation of VHL protein. Recently, VHL has also been implicated in regulating protein synthesis by binding ribosomal proteins and preventing ribosome assembly (55). Because viral infection requires a functional protein synthesis apparatus, it makes VHL an attractive viral target. Once again, viruses have contributed to unveil a new regulatory mechanism, underlining their extraordinary role in advancing our understanding of cellular processes.
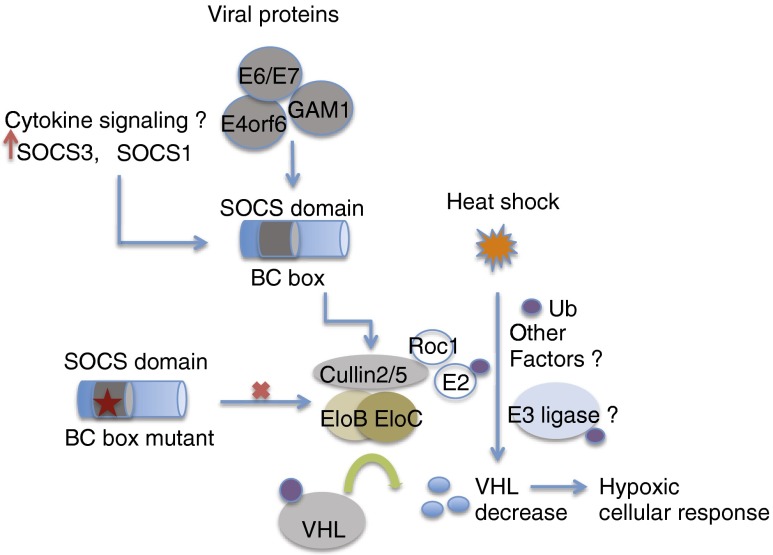
Fig. 7.
Model showing VHL protein decrease upon expression of SOCS domain-containing proteins of viral and cellular origin. Infection by viral proteins containing the SOCS domain or increased protein levels of cytokine signaling mediators SOCS1 and SOCS3 decrease VHL levels. BC-box mutants in the SOCS domain fail to assemble a functional Cullin2 and Cullin5 ligase complex, resulting in reduced VHL protein ubiquitination and its subsequent stabilization. VHL protein decrease might also be mediated by other unknown ligases as in the case of a heat-shock response or in cellular states when alternate pathways are activated. The consequent stabilization and activation of HIF 1 at the transcriptional level might provide a favorable environment for cell survival even under conditions of cellular stress.
Materials and Methods
SI Materials and Methods describes in detail cell lines used in this study. It also describes culture conditions, lysate preparations including buffers used for immunoprecipitation of ubiquitylated proteins, cycloheximide (CHX) and proteasome inhibition treatments. Plasmids, antibodies, primers, infections and transfections are also described.
Supplementary Material
Acknowledgments
We thank Drs. D. M. Katschinski, D. Hilton, W. H. Krek, M. Pagano, D. Pasini, and S. Polo for plasmids. We thank D. M. Katschinski, C. D. Lima, G. Ferbeyre, E. Saint-Germain, P. Murray, and M. Peter for helpful suggestions. We thank G. Taliento and G. Bonizzi at European Institute of Oncology biobank. This work is supported by grants from the Associazione Italiana per la Ricerca sul Cancro and European Commission-FP7-HPVAHEAD (to S.C.). M.E.P. was supported by the Umberto Veronesi Foundation. D.M. is supported by a Fondazione Italiana Ricerca Cancro fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311382110/-/DCSupplemental.
References
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6(1):9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 3.Yan Q, et al. Identification of Elongin C and Skp1 sequences that determine Cullin selection. J Biol Chem. 2004;279(41):43019–43026. doi: 10.1074/jbc.M408018200. [DOI] [PubMed] [Google Scholar]
- 4.Duan DR, et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995;269(5229):1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- 5.Kamura T, et al. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12(24):3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269(5229):1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 7.Kamura T, et al. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18(24):3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JG, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96(5):2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilton DJ, et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95(1):114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchette P, et al. Both BC-box motifs of adenovirus protein E4orf6 are required to efficiently assemble an E3 ligase complex that degrades p53. Mol Cell Biol. 2004;24(21):9619–9629. doi: 10.1128/MCB.24.21.9619-9629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai QL, Knight JS, Verma SC, Zald P, Robertson ES. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2006;2(10):e116. doi: 10.1371/journal.ppat.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng CY, Blanchette P, Branton PE. The adenovirus E4orf6 E3 ubiquitin ligase complex assembles in a novel fashion. Virology. 2007;364(1):36–44. doi: 10.1016/j.virol.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Huh K, et al. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol. 2007;81(18):9737–9747. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo K, et al. Adenovirus E4orf6 assembles with Cullin5-ElonginB-ElonginC E3 ubiquitin ligase through an HIV/SIV Vif-like BC-box to regulate p53. FASEB J. 2007;21(8):1742–1750. doi: 10.1096/fj.06-7241com. [DOI] [PubMed] [Google Scholar]
- 15.Querido E, et al. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 2001;15(23):3104–3117. doi: 10.1101/gad.926401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302(5647):1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Xiao Z, Ehrlich ES, Yu X, Yu XF. Selective assembly of HIV-1 Vif-Cul5-ElonginB-ElonginC E3 ubiquitin ligase complex through a novel SOCS box and upstream cysteines. Genes Dev. 2004;18(23):2867–2872. doi: 10.1101/gad.1250204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boggio R, Colombo R, Hay RT, Draetta GF, Chiocca S. A mechanism for inhibiting the SUMO pathway. Mol Cell. 2004;16(4):549–561. doi: 10.1016/j.molcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19. Boggio R, Passafaro A, Chiocca S (2007) Targeting SUMO E1 to ubiquitin ligases: A viral strategy to counteract sumoylation. J Biol Chem 282(21):15376–15382. [DOI] [PubMed]
- 20.Chiocca S, et al. Histone deacetylase 1 inactivation by an adenovirus early gene product. Curr Biol. 2002;12(7):594–598. doi: 10.1016/s0960-9822(02)00720-0. [DOI] [PubMed] [Google Scholar]
- 21.Colombo R, Boggio R, Seiser C, Draetta GF, Chiocca S. The adenovirus protein Gam1 interferes with sumoylation of histone deacetylase 1. EMBO Rep. 2002;3(11):1062–1068. doi: 10.1093/embo-reports/kvf213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13(14):1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohh M, et al. Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel-Lindau protein. J Clin Invest. 1999;104(11):1583–1591. doi: 10.1172/JCI8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pause A, et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci USA. 1997;94(6):2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen F, et al. Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: Correlations with phenotype. Hum Mutat. 1995;5(1):66–75. doi: 10.1002/humu.1380050109. [DOI] [PubMed] [Google Scholar]
- 26.Latif F, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 27.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 28.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 30.Ohh M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2(7):423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 31.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90(9):4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelzer E, et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17(17):5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glotzer JB, et al. Activation of heat-shock response by an adenovirus is essential for virus replication. Nature. 2000;407(6801):207–211. doi: 10.1038/35025102. [DOI] [PubMed] [Google Scholar]
- 35.Loncaster JA, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: Correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61(17):6394–6399. [PubMed] [Google Scholar]
- 36.Chou MT, Anthony J, Bjorge JD, Fujita DJ. The von Hippel-Lindau tumor suppressor protein is destabilized by Src: Implications for tumor angiogenesis and progression. Genes Cancer. 2010;1(3):225–238. doi: 10.1177/1947601910366719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babon JJ, Sabo JK, Zhang JG, Nicola NA, Norton RS. The SOCS box encodes a hierarchy of affinities for Cullin5: Implications for ubiquitin ligase formation and cytokine signalling suppression. J Mol Biol. 2009;387(1):162–174. doi: 10.1016/j.jmb.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenfeld AR, Davidowitz EJ, Burk RD. Elongin BC complex prevents degradation of von Hippel-Lindau tumor suppressor gene products. Proc Natl Acad Sci USA. 2000;97(15):8507–8512. doi: 10.1073/pnas.97.15.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YJ, et al. Heat shock protein 72 is associated with the hepatitis C virus replicase complex and enhances viral RNA replication. J Biol Chem. 2010;285(36):28183–28190. doi: 10.1074/jbc.M110.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez O, et al. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50(6):1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Punjabi AS, Carroll PA, Chen L, Lagunoff M. Persistent activation of STAT3 by latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells. J Virol. 2007;81(5):2449–2458. doi: 10.1128/JVI.01769-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses: Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281(20):14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 43.Tanabe Y, et al. Cutting edge: Role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol. 2005;174(2):609–613. doi: 10.4049/jimmunol.174.2.609. [DOI] [PubMed] [Google Scholar]
- 44.Ginsberg HS, et al. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci USA. 1989;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Münger K, Scheffner M, Huibregtse JM, Howley PM. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. [PubMed] [Google Scholar]
- 46.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 47.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67(2):563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 48.Cai Q, Robertson ES. Ubiquitin/SUMO modification regulates VHL protein stability and nucleocytoplasmic localization. PLoS ONE. 2010;5(9):e12636. doi: 10.1371/journal.pone.0012636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Hwang II, Watson IR, Der SD, Ohh M. Loss of VHL confers hypoxia-inducible factor (HIF)-dependent resistance to vesicular stomatitis virus: Role of HIF in antiviral response. J Virol. 2006;80(21):10712–10723. doi: 10.1128/JVI.01014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naldini A, Carraro F, Fleischmann WR, Jr, Bocci V. Hypoxia enhances the antiviral activity of interferons. J Interferon Res. 1993;13(2):127–132. doi: 10.1089/jir.1993.13.127. [DOI] [PubMed] [Google Scholar]
- 51.Nasimuzzaman M, Waris G, Mikolon D, Stupack DG, Siddiqui A. Hepatitis C virus stabilizes hypoxia-inducible factor 1alpha and stimulates the synthesis of vascular endothelial growth factor. J Virol. 2007;81(19):10249–10257. doi: 10.1128/JVI.00763-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Moon EJ, et al. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18(2):382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- 53.Tang X, et al. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin Cancer Res. 2007;13(9):2568–2576. doi: 10.1158/1078-0432.CCR-06-2704. [DOI] [PubMed] [Google Scholar]
- 54.Wakisaka N, et al. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2004;24(12):5223–5234. doi: 10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao WT, et al. The von Hippel-Lindau protein pVHL inhibits ribosome biogenesis and protein synthesis. J Biol Chem. 2013;288(23):16588–16597. doi: 10.1074/jbc.M113.455121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.