Significance
Bacterial autotransporter proteins contain a large segment that is transported from the periplasm (the space between the inner and outer membranes) to the extracellular milieu. Because the periplasm lacks ATP, the energy required for the transport reaction is thought to be derived from the folding of this segment following its secretion. Contrary to the prevailing view, we found that a heterologous polypeptide that cannot fold was secreted efficiently when it was fused to an autotransporter. Reducing the negative charge of this polypeptide, however, strongly impaired secretion. Our results identify net charge as a potentially important factor that drives autotransporter secretion.
Keywords: bacterial pathogenesis, membrane protein assembly, type Va secretion
Abstract
Autotransporters are a large class of virulence proteins produced by Gram-negative bacteria. They contain an N-terminal extracellular (“passenger”) domain that folds into a β-helical structure and a C-terminal β-barrel (“β”) domain that anchors the protein to the outer membrane. Because the periplasm lacks ATP, the source of energy that drives passenger domain secretion is unknown. The prevailing model postulates that vectorial folding of the β-helix in the extracellular space facilitates unidirectional secretion of the passenger domain. In this study we used a chimeric protein composed of the 675-residue receptor-binding domain (RD) of the Bordetella pertussis adenylate cyclase toxin CyaA fused to the C terminus of the Escherichia coli O157:H7 autotransporter EspP to test this hypothesis. The RD is a highly acidic, repetitive polypeptide that is intrinsically disordered in the absence of calcium. Surprisingly, we found that the RD moiety was efficiently secreted when it remained in an unfolded conformation. Furthermore, we found that neutralizing or reversing the charge of acidic amino acid clusters stalled translocation in the vicinity of the altered residues. These results challenge the vectorial folding model and, together with the finding that naturally occurring passenger domains are predominantly acidic, provide evidence that a net negative charge plays a significant role in driving the translocation reaction.
The survival of bacterial pathogens in a host environment requires the transport of virulence factors to the cell surface and beyond. One of the most commonly used secretion pathways in Gram-negative pathogens is the autotransporter (type Va) pathway (1). Autotransporters consist of a signal peptide, an N-terminal extracellular (“passenger”) domain that typically mediates a virulence function, and a C-terminal β-barrel (“β”) domain that resides in the outer membrane (OM). Passenger domains are highly divergent in sequence but generally adopt a characteristic β-helical structure (2). The size of passenger domains is also variable, but they often exceed 100 kDa. β-domains are ∼30 kDa in size, and although they are also divergent in sequence, they fold into nearly superimposable 12-stranded β-barrel structures (3–5). After autotransporters are translocated across the inner membrane (IM) via the Sec pathway they interact with chaperones that presumably keep them in an assembly-competent state in the periplasm (6–8). Subsequently they interact with the Bam complex, an essential heterooligomer that catalyzes the insertion of β-barrel protein into the OM (7–12). Following their translocation across the OM, many passenger domains are released from the cell surface by one of several distinct proteolytic processing mechanisms (13).
Although it has been established that passenger domain translocation proceeds in a C- to N-terminal direction (7, 14), the mechanism of translocation has remained enigmatic. It was originally proposed that autotransporters contain all of the functional elements needed to mediate passenger domain secretion (15). At first glance, this hypothesis seems to be supported by X-ray crystallographic studies showing that the passenger and β-domains are linked by an α-helical segment that traverses the β-barrel pore (3–5). The same studies, however, show that the β-domain pore is only ∼10 Å in diameter. Given that the directionality of translocation presumably requires the formation of a C-terminal hairpin followed by the progressive sliding of more N-terminal segments past a static strand, both strands of the hairpin would need to be in a fully extended conformation. Small folded polypeptides, however, have been shown to be secreted efficiently by the autotransporter pathway, and some naturally occurring passenger domains acquire at least limited tertiary structure in the periplasm (16, 17). Furthermore, an α-helical segment likely resides inside the β-domain pore before the completion of translocation (18, 19). Finally, a component of the Bam complex (BamA) has been shown to interact with the passenger domain during the translocation reaction (7). Although the β-domain does appear to play a role in translocation (20, 21), available evidence suggests that the Bam complex promotes the membrane insertion of the β-domain and the secretion of the passenger domain in a concerted reaction (7, 8).
The energy source for passenger domain translocation has likewise remained unclear. Although protein translocation reactions generally require the input of external energy in the form of either ATP hydrolysis or a membrane potential, there is no ATP in the periplasm, electrochemical gradient across the OM, or obvious connection to the protonmotive force across the IM. To explain the energetics of autotransporter secretion it has been proposed that the progressive folding of small segments of the passenger domain in the extracellular space drives translocation forward while preventing retrograde movement into the periplasm (2, 22). This vectorial folding model is supported by the finding that mutations that impair the folding of C-terminal segments of two different autotransporter passenger domains significantly reduce the efficiency of secretion (19, 23). It should be noted, however, that an examination of the energetics of other types of protein translocation reactions has shown that moving proteins across membranes is often extremely costly. The equivalent of >10,000 ATP molecules is required to move a protein from the chloroplast stroma into the thylakoid lumen (24), and in the absence of a protonmotive force ∼5,000 ATP molecules are required to move a protein through the bacterial Sec machinery (25). Even the transport of proteins across the chloroplast envelope, which is considerably less costly, requires ∼650 ATP molecules (26). Given that even this lower value represents a ΔG = 30,000 kJ/mol whereas the ΔG associated with protein folding is typically in the ∼20–60 kJ/mol range (27), it seems likely that the folding of the passenger domain does not fully account for the energetics of secretion.
In this study, we tested the vectorial folding hypothesis by examining the fate of a conditionally disordered chimeric passenger domain. We fused the receptor-binding domain (RD) of the Bordetella pertussis toxin CyaA to a C-terminal fragment of EspP, an Escherichia coli O157:H7 autotransporter whose passenger domain undergoes proteolytic processing. CyaA is an ∼177-kDa toxin that is normally secreted through a type I pathway (28). The 675-residue RD consists of ∼40–45 glycine- and aspartate-rich repeat in toxin (RTX) motifs that are involved in calcium binding (29). In the absence of calcium the CyaA RD does not fold into a well-defined 3D structure, but instead exists in a premolten globule ensemble of conformations (30). Like other intrinsically disordered polypeptides it contains a high content of random coils and is highly hydrated (30–32). Upon binding calcium, the CyaA RD folds into a stable β-roll structure that facilitates interaction with a cell surface receptor and the translocation of an N-terminal adenylate cyclase domain across the plasma membrane (32–36). We found that the chimeric passenger domain was secreted efficiently even in the absence of calcium and thereby showed that translocation can proceed efficiently without passenger domain folding. Furthermore, site-directed mutagenesis experiments together with an analysis of naturally occurring passenger domain sequences unmasked a potentially important role of protein charge in the translocation reaction. Finally, by examining the secretion of a truncated version of the chimeric passenger domain in the presence of calcium we confirmed that small folded polypeptides can be secreted via the autotransporter pathway.
Results
An Intrinsically Disordered Polypeptide Can Be Secreted Efficiently via the Autotransporter Pathway.
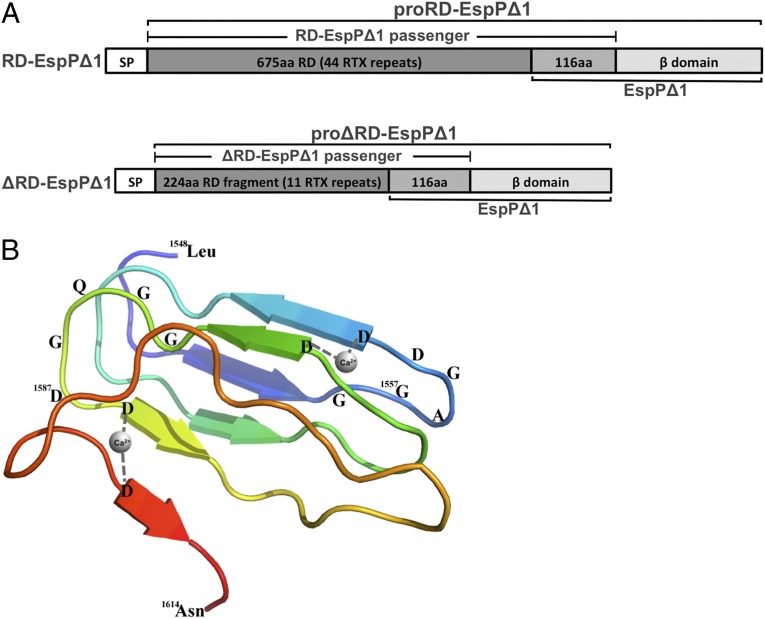
In the absence of an obvious external energy source, it has been proposed that the free energy released upon the folding of the passenger domain in the extracellular space might provide a driving force for efficient translocation across the OM (2, 22). To test this hypothesis, we fused the CyaA RD (residues 1,006–1,680) to EspPΔ1, a previously characterized derivative of EspP that contains the last 116 aa of the passenger domain and the β-domain (16), to generate a chimera designated RD-EspPΔ1 (Fig. 1A). Because the EspP passenger domain is released in an intrabarrel proteolytic reaction, the C-terminal ∼28 residues are embedded in the pore of the β-domain before cleavage (37, 38). The CyaA RD moiety contains all of the RTX repeats and a flanking sequence that has been shown to be essential for calcium responsiveness (39, 40), but not the C-terminal targeting peptide required for secretion through the type I pathway. The RD is organized into five blocks of about eight nonapeptide RTX motifs [the prototype of which is GGXG(N/D)DX(U)X, where U represents any large hydrophobic residue] separated by linkers of 23–49 residues (36). In the absence of calcium, the RD is intrinsically disordered due to the paucity of hydrophobic residues that can form a hydrophobic core and to strong electrostatic repulsions between the aspartate residues (30). In the presence of low millimolar concentrations of calcium, however, the RD folds into a β-roll structure (Fig. 1B) (30, 32). Because the passenger domain fragment of EspPΔ1 is too short to fold into a stable structure (19), the chimeric RD-EspPΔ1 passenger domain would be expected to remain disordered in the absence of calcium.
Fig. 1.
Illustration of RD-EspP chimeras used in this study. (A) The RD-EspPΔ1 and ΔRD-EspPΔ1 fusion proteins are shown. In RD-EspPΔ1, the entire RD (CyaA residues 1,006–1,680) was fused to EspP∆1, a derivative of EspP containing the last 116 residues of the passenger domain and the β-domain (16). In ΔRD-EspPΔ1, the C-terminal 224 residues of the RD were fused to EspPΔ1. Both chimeras contained the 55-residue EspP signal peptide (SP). The pro form of the protein, which contains covalently linked passenger and β-domains, is observed before the secretion and proteolytic processing of the passenger domain. (B) The structure of the C-terminal seven RTX repeats of the RD in the presence of calcium was predicted using the Phyre server (62). Calcium ions bridge adjacent loops of the β-roll structure by binding to the carboxyl groups of aspartates located at the sixth position of the GGxG(N/D)D motif. For clarity, only two calcium ions are shown.
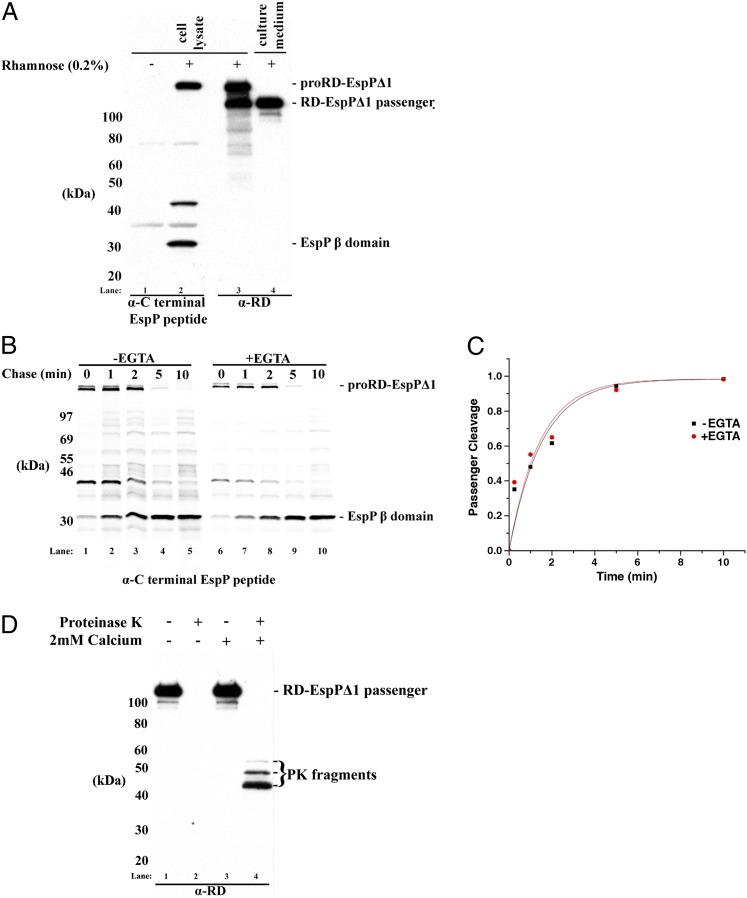
Initial studies showed that the RD-EspPΔ1 passenger domain is translocated across the OM efficiently. In these experiments AD202 were transformed with pWK1, a plasmid that encodes RD-EspPΔ1 under the control of the rhaB promoter. Cultures were grown in LB, and rhamnose was added to a portion of the cells to induce synthesis of the chimeric protein. Cells were isolated by centrifugation, and both the cell pellet and the culture medium were analyzed by Western blot. In the presence of the inducer, a high molecular weight protein that corresponds to proRD-EspPΔ1 (the form of the protein that contains covalently linked passenger and β-domains) and the free EspP β-domain were observed in cell extracts when blots were probed with an antiserum raised against a C-terminal EspP peptide (Fig. 2A, lanes 1 and 2). Because cleavage of the passenger domain is contingent on the completion of translocation (7), the accumulation of the β-domain strongly suggested that the chimeric passenger domain was secreted. Consistent with this interpretation, both proRD-EspPΔ1 and the cleaved RD-EspPΔ1 passenger domain were detected by a monoclonal antibody raised against a Cya RD peptide (Fig. 2A, lane 3). Whereas all of the proRD-EspPΔ1 was cell associated, about half of the passenger domain was found in the external milieu (Fig. 2A, lane 4). As previously observed (41, 42), polypeptides containing the RD moiety ran noticeably slower than their calculated molecular weight. The ∼84-kDa RD-EspPΔ1 passenger domain, for example, migrates at ∼105 kDa. The aberrant migration is presumably a consequence of the high net negative charge of the RTX repeats.
Fig. 2.
The RD-EspP∆1 passenger domain is secreted efficiently as an unfolded polypeptide. (A) AD202 transformed with a plasmid encoding RD-EspP∆1 (pWK1) were grown in LB, and synthesis of the fusion protein was induced by the addition of rhamnose. The cells were pelleted, and both the cell pellet and the culture medium were analyzed by Western blot using an antiserum generated against a C-terminal EspP peptide (lanes 1 and 2) and a monoclonal antibody generated against the RD domain (lanes 3 and 4). (B) AD202 transformed with pWK1 were grown in M9 medium and subjected to pulse-chase labeling after the addition of rhamnose. EGTA was added to one portion of the culture before radiolabeling. Immunoprecipitations were performed using the anti-EspP C-terminal antiserum. (C) The fraction of the passenger domain molecules that were cleaved at each time point in B is shown. (D) AD202 transformed with pWK1 were grown in LB, and synthesis of the fusion protein was induced by the addition of rhamnose. The culture medium was then divided into two portions, one of which was incubated with calcium (2 mM). One-half of each sample was then treated with PK, and Western blotting was performed using the anti-RD antibody.
To examine the kinetics of secretion of the chimeric passenger domain, AD202 containing pWK1 were grown in M9 medium and subjected to pulse-chase labeling after the addition of rhamnose. Immunoprecipitations were then performed using the anti-EspP C-terminal antiserum. Within 1 min about half of the proRD-EspPΔ1 was cleaved, and by 5 min essentially all of the protein was processed (Fig. 2B, lanes 1–5). Remarkably, the data imply that the chimeric passenger domain is secreted and released from the cell surface nearly as efficiently as the native EspP passenger domain (for comparison, see ref. 7), at least when cells are grown in minimal medium. Because the concentration of calcium in M9 medium (100 µM) is below the threshold at which native RD fragments acquire appreciable tertiary structure (500 µM) (30), it is likely that the RD-EspP passenger domain remained intrinsically disordered during the translocation reaction. To confirm that the secretion of the chimeric passenger domain does not require stable folding, we added 5 mM EGTA to half of the cells in the experiment described above. The data show that the kinetics and efficiency of translocation were unaffected by the complete absence of free calcium ions [Fig. 2 B (lanes 5–10) and C].
We next sought to rule out the possibility that in the context of the EspP fusion the CyaA RD folds in the extracellular space in a calcium-independent fashion. To this end we grew cells in LB, induced synthesis of the fusion protein, and collected the culture medium containing the cleaved passenger domain. Calcium (2 mM) was added to half of the medium. Both calcium-containing and control samples were then divided in half, and one half was treated with proteinase K (PK). In the absence of calcium, the secreted passenger domain was completely degraded by PK (Fig. 2D, lanes 1 and 2). In the presence of calcium, however, a large fraction of the secreted protein yielded ∼45- to 50-kDa protease-resistant fragments (Fig. 2D, lanes 3 and 4). These results confirmed that the RD moiety of the fusion protein is disordered in the absence of calcium but retains the ability to fold in the presence of calcium. Furthermore, we found that deleting the entire EspP passenger domain (except small segments that are embedded inside the β-domain before cleavage and that are required for efficient proteolytic processing) had no effect on the secretion of the RD (Fig. S1). This observation shows that the EspP passenger domain component in the RD-EspPΔ1 chimera does not fortuitously facilitate the secretion of the disordered RD polypeptide.
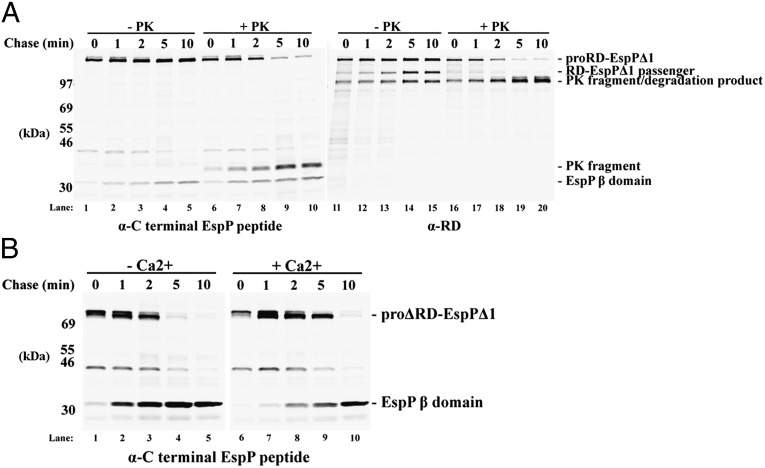
An examination of the effect of adding 2 mM calcium to cultures immediately before cells were subjected to pulse-chase labeling yielded further insight into the folding properties of the RD-EspPΔ1 passenger domain. In these experiments half of the radiolabeled cells were treated with PK to assess the exposure of the passenger domain on the cell surface. Nearly all of the proRD-EspPΔ1 was accessible to PK by 5 min (Fig. 3A, lanes 1–10). The disappearance of the pro form of the protein in the PK-treated samples correlated with the concomitant accumulation of an ∼33-kDa C-terminal fragment that has been observed previously (16). The ∼33-kDa fragment contains the EspP β-domain plus the small passenger domain segment that is embedded inside the β-domain before cleavage, and it is generated by PK digestion only after the passenger domain emerges in the extracellular space. These results indicate that the presence of calcium did not impair the initiation of passenger domain translocation. In contrast, calcium strongly inhibited the proteolytic processing of proRD-EspPΔ1 into discrete passenger and β-domain fragments (Fig. 3A, lanes 1–5 and 11–15). Because calcium has no effect on the cleavage reaction per se (Fig. S2), the cation presumably blocked the completion of translocation by promoting the intracellular folding of the RD moiety into a structure that, like other large folded structures, cannot be secreted efficiently by the autotransporter pathway (17, 43). Consistent with this interpretation, PK treatment led to the accumulation of an ∼100-kDa N-terminal fragment that likely corresponds to RD molecules that were trapped inside the periplasm and therefore resistant to protease digestion (Fig. 3A, lanes 16–20). The observation that the ∼100-kDa fragment was degraded by PK when cells were first permeabilized by sonication (Fig. S3) confirmed that it was located in the periplasm. These results provide additional evidence that the RD folds in a strictly calcium-dependent fashion in the context of the fusion protein and therefore remains in a predominantly unfolded state when it is secreted in the absence of calcium.
Fig. 3.
Intracellular folding of the RD impedes its secretion via the autotransporter pathway. (A) AD202 transformed with pWK1 were subjected to pulse-chase labeling following the addition of rhamnose. Calcium (2 mM) was added 2 min before radiolabeling. Half of the cells were treated with PK and immunoprecipitations were performed using the anti-EspP C-terminal antiserum (lanes 1–10) and RD antibody (lanes 11–20). (B) AD202 transformed with a plasmid encoding ΔRD-EspPΔ1 (pWK2) were subjected to pulse-chase labeling following the addition of rhamnose. Calcium (2 mM) was added to one-half of the culture 2 min before radiolabeling. Immunoprecipitations were performed using the anti-EspP C-terminal antiserum.
Finally, it is noteworthy that the secretion of a truncated CyaA-EspP chimera that contains only block V and a portion of block IV of the RD (ΔRD-EspPΔ1, Fig. 1A) was much less severely affected by the addition of calcium. Like the full-length RD, C-terminal RD fragments have been reported to fold in response to calcium (30). In the absence of calcium, ΔRD-EspPΔ1 was secreted and processed at about the same rate as RD-EspPΔ1 (Fig. 3B, lanes 1–5). In the presence of calcium the chimera was completely processed, but at a markedly slower rate (Fig. 3B, lanes 6–10). Presumably the delay resulted from the folding of the truncated RD into a short β-roll structure that pushes the size limit of polypeptides that can be secreted via the autotransporter pathway. Indeed another small folded polypeptide, the cholera toxin B subunit (CtxB), is also secreted by the autotransporter pathway somewhat slowly (16). The ΔRD-EspPΔ1 passenger domain appeared to be secreted even more slowly than that of a CtxB-EspPΔ1 fusion, but the ΔRD moiety is predicted to fold into a slightly larger structure than CtxB (Fig. S4). As a corollary, these results strongly suggest that each block of the RD folds independently in the context of the chimera as it does in the context of the native RD.
The Charge of the RD Influences Its Translocation Across the OM.
Given that stable folding of the RD does not appear to drive its translocation, we hypothesized that the amino acid composition of the nonapeptide RTX repeats might contribute to its efficient secretion. To test this idea we devised a series of experiments that were based on the finding that each block of the RD is an independent module. Because passenger domains are secreted in a C- to N-terminal direction, we reasoned that the introduction of mutations that compromise the secretion of a specific block would also impair the secretion of blocks that reside closer to the N terminus of RD-EspPΔ1, but not those that reside closer to the C terminus. Furthermore, because of the repetitive nature of the RD, we reasoned that multiple mutations might be needed to alter the character of any given block and to thereby impede its translocation across the OM.
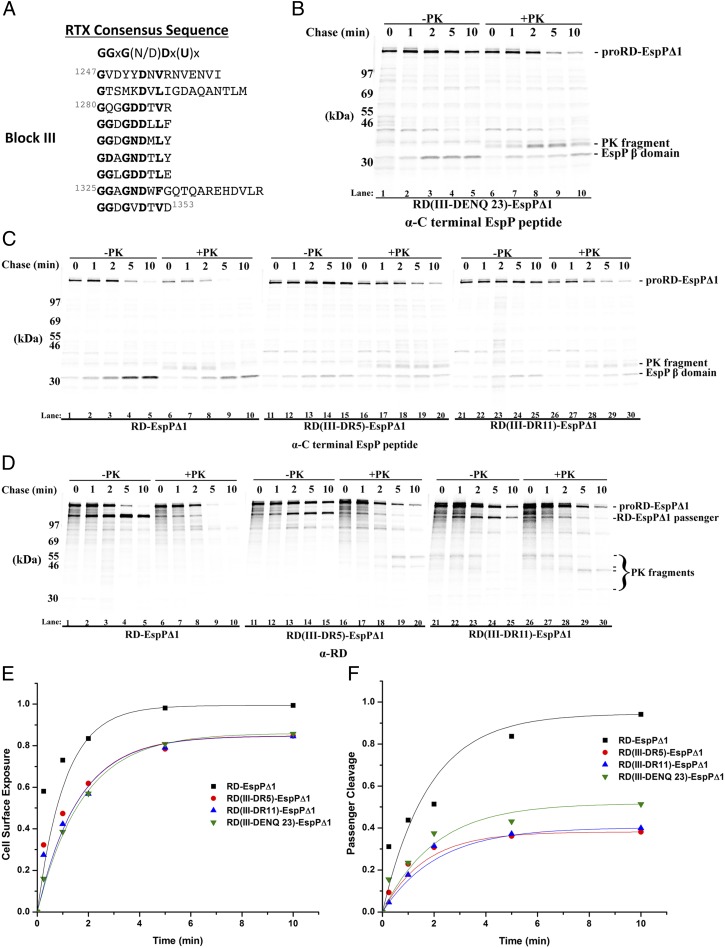
Consistent with our hypothesis, we found that the high negative charge of the RD is required for efficient secretion. In an initial experiment, we mutated all 23 aspartate and glutamate residues in block III to the corresponding neutral amino acid to create a construct designated RD(III-DENQ23)-EspPΔ1 (Fig. 4B and Table S1). AD202 transformed with a plasmid encoding the mutant protein were subjected to pulse-chase labeling, and half of each sample was treated with PK. Immunoprecipitations were then conducted using the anti-EspP C-terminal antiserum. The exposure of the passenger domain on the cell surface was assessed by monitoring the accessibility of the pro form of the protein to PK, and passenger domain processing was assessed by monitoring the accumulation of the free β-domain. Whereas the charge neutralization mutations only slightly impaired the cell surface exposure of the chimeric passenger domain, they exerted a considerably stronger effect on proteolytic processing (Fig. 4 B, E, and F). Because the mutations were located far away from the cleavage site, they most likely inhibited passenger domain cleavage indirectly by preventing the completion of translocation. If passenger domain translocation stalled in block III, as we expected, then a specific N-terminal fragment should have been retained inside the cell. We were unable to confirm this prediction, however, because the monoclonal anti-RD antibody that we used (which recognizes an unspecified epitope near the middle of the RD) did not recognize the mutant protein.
Fig. 4.
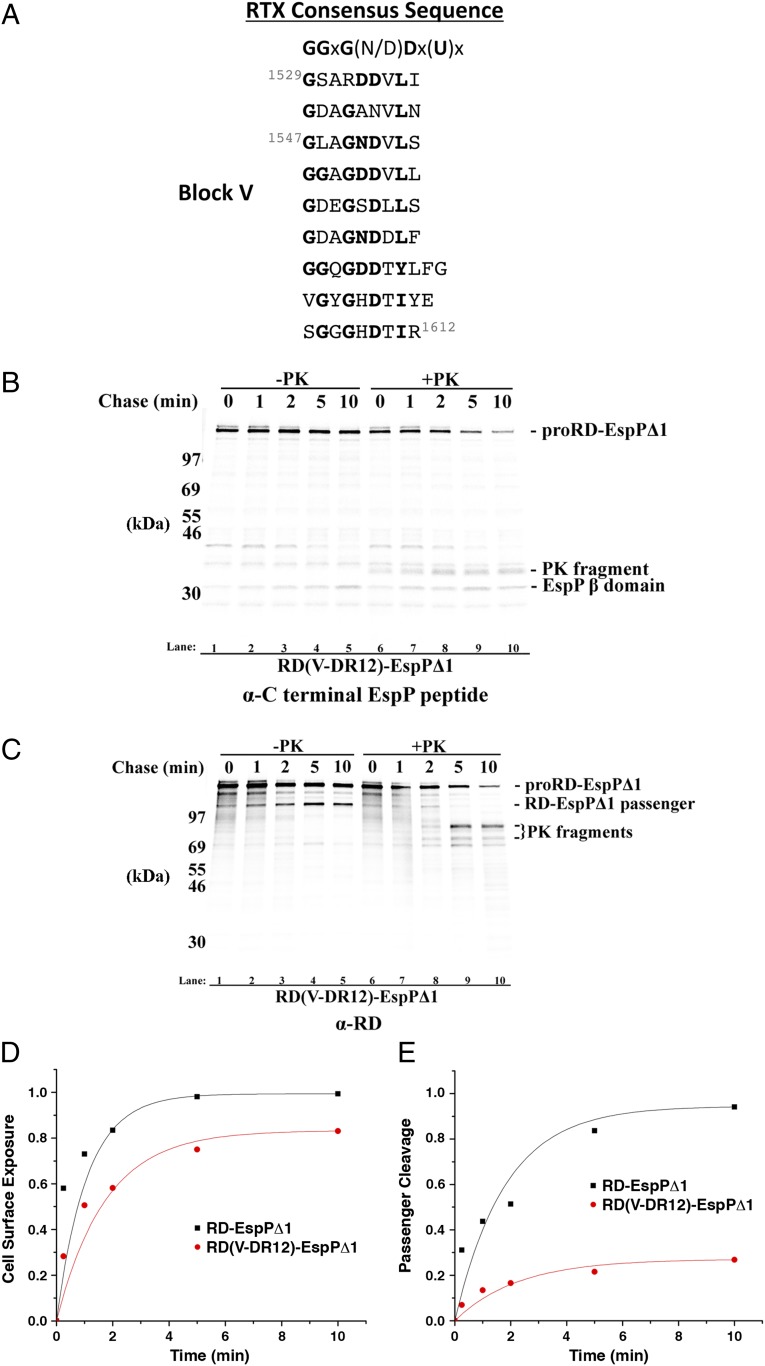
Charge neutralization or reversal in block III of the RD stalls translocation. (A) The sequence of the RTX repeats in block III of the RD is shown. Residues that conform to the consensus sequence are in boldface type. (B–D) AD202 transformed with a plasmid encoding RD-EspP∆1 or the indicated derivative were subjected to pulse-chase labeling after the addition of rhamnose. Half of the cells were treated with PK, and immunoprecipitations were conducted using the anti-EspP C-terminal antiserum (B and C) and the anti-RD antibody (D). (E and F) The fraction of the passenger domain molecules that were exposed on the cell surface (E) or cleaved (F) at each time point in B and C is shown.
Experiments in which we analyzed the biogenesis of RD-EspPΔ1 derivatives that have a smaller number of mutations in block III provided clearer evidence that passenger domain translocation stalls near the site of the charge alteration. We changed 5 aspartate residues near the C terminus of block III to asparagine or arginine to create RD(III-DN5)-EspPΔ1 and RD(III-DR5)-EspPΔ1, respectively, and 11 aspartate residues in the middle of block III to asparagine or arginine to create RD(III-DN11)-EspPΔ1 and RD(III-DR11)-EspPΔ1, respectively (Table S1). These mutations did not eliminate the epitope recognized by the anti-RD antibody. Although the charge neutralization mutants did not significantly affect protein biogenesis (Fig. S5A, lanes 1–10), immunoprecipitations using the anti-EspP C-terminal antibody showed that the two charge reversal mutants led to strong passenger domain cleavage defects (Fig. 4 C, E, and F). Immunoprecipitations using the anti-CyaA antibody also showed a reduced accumulation of the cleaved RD-EspPΔ1 passenger domain (Fig. 4D, lanes 11–15 and 21–25). Interestingly, N-terminal PK fragments that migrated at ∼55 kDa and ∼46 kDa were observed in cells that produced RD(III-DR5)-EspPΔ1 (Fig. 4D, lanes 18–20). Likewise, a major PK fragment that migrated at ∼43 kDa was observed in cells that produced RD(III-DR11)-EspPΔ1 (Fig. 4D, lanes 28–30). All of these fragments were degraded by PK when cells were first permeabilized by sonication (Fig. S6A). Given the marked tendency of the acidic RD to migrate slowly in SDS/PAGE, these molecular weight estimates are consistent with the retention of ∼357- and ∼328-residue N-terminal fragments inside the periplasm that would result from the stalling of translocation at the extreme C terminus of the mutated region. The detection of these fragments confirmed that the mutations inhibit the completion of passenger domain translocation rather than the cleavage reaction per se. Although we cannot exclude the possibility that the arginine residues in RD(III-DR5)-EspPΔ1 and RD(III-DR11)-EspPΔ1 form salt bridges that fortuitously stabilize secretion-incompetent folded structures, a variety of theoretical considerations (e.g., geometric constraints, the inherent weakness of salt bridges) suggest that this scenario is very unlikely. Indeed it is far more likely that the reduction in the net negative charge impedes translocation by another mechanism (Discussion).
To determine whether the large hydrophobic residues that are conserved in the RTX motif are also required for efficient translocation of the chimeric passenger domain, we mutated all 17 phenylalanine, leucine, isoleucine, and tyrosine residues in block III to alanine to generate RD(III-HYDA17) (Table S1). Pulse-chase analysis revealed that the mutation had no effect on protein biogenesis (Fig. S5B). These results provide evidence that charge mutations exert a specific effect on the fate of the passenger domain. Furthermore, the results show that the formation of a hydrophobic core, which often plays a key role in protein folding, is not required for the efficient secretion of the chimeric passenger domain.
Finally, we sought to determine whether the effect of altering the net charge of block III on passenger domain translocation could be generalized to other parts of the RD. To this end we mutated 12 aspartate residues in block V to asparagine and arginine to generate RD(V-DN12)-EspPΔ1 and RD(V-DR12)-EspPΔ1, respectively. AD202 transformed with the mutant plasmids were subjected to pulse-chase analysis and PK treatment as described above. Neutralization of the aspartate residues only marginally affected the kinetics of passenger domain translocation and cleavage (Fig. S5A, lanes 11–15). Conversion of the aspartates to arginine only modestly impaired the exposure of the passenger domain, but led to a profound decline in passenger domain cleavage (Fig. 5 B, D, and E). Consistent with the prediction that the cleavage defect was an indirect effect of a translocation block, we observed the accumulation of a prominent ∼80-kDa PK fragment that was immunoprecipitated with the anti-RD antibody (Fig. 5C). Given the aberrant mobility of the RD on SDS/PAGE, this fragment likely contains most or all of the 616-aa segment that would be trapped in the periplasm if translocation stalls at the C terminus of the mutated region. The finding that the ∼80-kDa fragment was completely degraded by PK when the OM was first permeabilized by sonication or lysozyme/EDTA treatment confirmed that it was located in the periplasm and did not correspond to a folded form of the RD that was secreted (Fig. S6B). Furthermore, it is important to note that the block V charge reversal mutation caused a much stronger translocation defect than the stabilization of the native folded conformation of block V through the addition of calcium (Fig. 3B). Thus, it is unlikely that the mutation impeded secretion by fortuitously promoting the folding of the C terminus of the RD into a compact, translocation-incompetent conformation.
Fig. 5.
Charge reversal in block V of the RD stalls translocation. (A) The sequence of the RTX repeats in block V of the RD is shown. Residues that conform to the consensus sequence are in boldface type. (B and C) AD202 transformed with a plasmid encoding RD(V-DR12)-EspP∆1 were subjected to pulse-chase labeling after the addition of rhamnose. Half of the cells were treated with PK, and immunoprecipitations were conducted using the anti-EspP C-terminal antiserum (B) and the anti-RD antibody (C). (D and E) The fraction of the passenger domain molecules that were exposed on the cell surface (D) or cleaved (E) at each time point in B is shown.
Naturally Occurring Passenger Domains Are Predominantly Acidic.
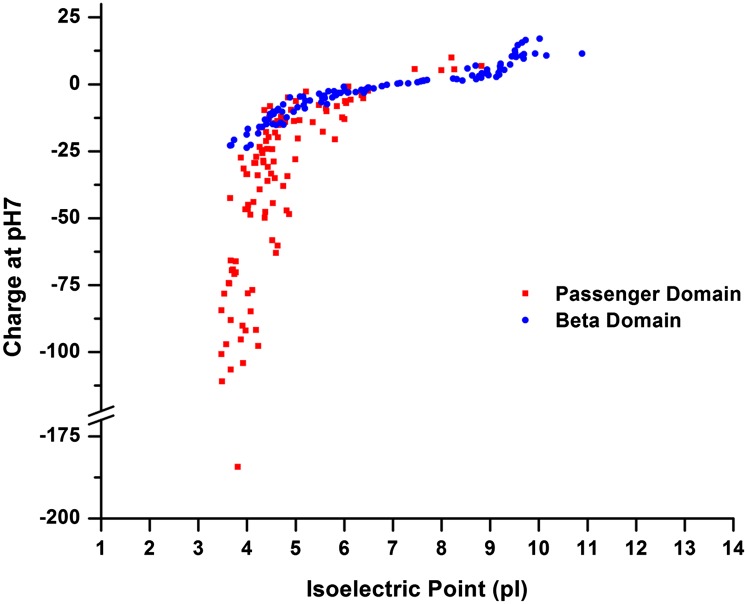
The link between the high negative charge of the RD and its efficient secretion via the autotransporter pathway prompted us to examine the net charge of naturally occurring passenger domains. In our analysis we determined the isoelectric point (pI) and net charge (at pH 7) of both passenger and β-domains of 111 autotransporters that were randomly selected from a list of over 1,500 putative autotransporters identified in diverse organisms in a recent bioinformatic study (44). We found that the passenger domains tend to have a pI below 6 and an unusually high negative charge (Fig. 6 and Table S2). In contrast, the pI range of the corresponding β-domains is very broad. The same pattern emerged when we analyzed the pI and net charge of a nonoverlapping set of 47 autotransporters that have been characterized experimentally (Fig. S7) (44). These results suggest that charge may be a significant factor in driving passenger domain secretion.
Fig. 6.
Autotransporter passenger domains are typically highly acidic. A plot of the net charge (at pH 7) and isoelectric point of the passenger and β-domains of 111 predicted autotransporters produced by a broad spectrum of different organisms (44) is shown.
In light of the bioinformatic data, an examination of the effect of mutating a set of 15 acidic amino acids located near the C terminus of the EspP passenger domain (between residues 899 and 986; Table S1) to either asparagine or arginine yielded intriguing results. Although the mutations only moderately impaired the exposure of the passenger domain on the cell surface, they strongly impaired the completion of the translocation reaction (Fig. S8 A–D). Like the F963A point mutation that has been characterized previously (19), the charge mutations impaired the folding of a C-terminal ∼17-kDa segment of the passenger domain. Because the folding of this segment is a rate-limiting step that drives the secretion of the remainder of the polypeptide, folding defects cause translocation to stall. Interestingly, whereas the F963A mutation caused translocation to stall after the entire ∼17-kDa fragment was exposed and led to the retention of an ∼90-kDa fragment in the periplasm (Fig. S8 B, lanes 27–30, and F, lanes 19–27) (19), the charge neutralization mutant appeared to cause translocation to stall sooner and led to the retention of slightly larger ∼92- to 94-kDa fragments (Fig. S8 B, lanes 7–10, and F, lanes 1–9). The charge reversal mutant blocked passenger domain translocation at an even earlier stage and led to the retention of an ∼103-kDa fragment [Fig. S8 B (lanes 17–20), E, and F (lanes 10–18)]. By strongly suggesting that that the mutations cause translocation to stall prematurely, these results provide evidence that the charge of a naturally occurring passenger domain can influence its movement across the OM.
Discussion
Because the periplasm lacks ATP, it was proposed many years ago that the secretion of polypeptides via the autotransporter pathway is driven by their folding into a native structure in the extracellular space. By examining the fate of RD-EspPΔ1, a chimera that was constructed by replacing most of the EspP passenger domain with a large polypeptide whose folding can be controlled experimentally, we obtained evidence that strongly challenges this hypothesis. We found that when the RD was in an intrinsically disordered conformation, it was secreted with essentially the same efficiency as the native EspP passenger domain. Although we cannot exclude the possibility that the formation of residual secondary structure (32) or some other structural elements in the absence of calcium promotes RD secretion, the data clearly show that the formation of a stable, native structure is not required for translocation. In a sense our results are surprising because there is strong evidence that the stable folding of the C-terminal segment of at least some native passenger domains is required for efficient secretion of the remainder of the polypeptide (19, 23). Furthermore, the repetitive β-helical structure of passenger domains suggests that they evolved to fold in a stepwise fashion. Nevertheless, the finding that the energy required to move proteins across biological membranes is much greater than the Gibbs free energy typically associated with protein folding seems at odds with the vectorial folding model. In addition, we recently found that the folding of medial and N-terminal segments of the EspP passenger domain plays a much smaller role in driving the secretion reaction to completion than the folding of the C terminus. Taken together, the available evidence strongly suggests that although the folding of specific segments may be required for the efficient secretion of native passenger domains, a large fraction of the energy required to transport both native and nonnative polypeptides across the OM via the autotransporter pathway is derived from sources other than protein folding.
Although we found that the folding of the RD-EspPΔ1 passenger domain does not drive its secretion, we obtained evidence that negative charge contributes to passenger domain secretion efficiency. We found that neutralizing all 23 of the acidic residues in RD block III or reversing the charge of a much smaller number of closely spaced aspartate residues in block III or block V inhibited protein translocation. The size of N-terminal fragments that were trapped in the periplasm strongly suggests that the translocation reaction proceeded normally until the altered segment reached the translocation channel. It is conceivable that the charge reversal mutations created salt bridges that stabilized the structure of RD-EspPΔ1 passenger domain segments and thereby trapped them in the periplasm, but this scenario is very unlikely. Salt bridges are generally an order of magnitude weaker than disulfide bonds (which are weaker than other covalent bonds), and long-range salt bridges are very weak (45). Geometry is also a critical factor in determining salt bridge stability (46). Given the close spacing of the mutations we introduced, it is difficult to imagine how more than a few geometrically favorable salt bridges could be formed. Indeed in the case of RD(III-DR5)-EspPΔ1, which contains only five additional arginine residues but exhibits a strong translocation defect, no more than one or two favorably positioned salt bridges would be expected. Furthermore, consistent with the idea that negative charge facilitates passenger domain translocation, an in silico analysis revealed that native passenger domains (but not β-domains) tend to be highly acidic. A large number of acidic residues are typically distributed throughout these repetitive polypeptide segments, as they are in the CyaA RD. Interestingly, the mutation of multiple acidic residues located in at least one region of the native EspP passenger domain appeared to impede its transport across the OM.
How might the negative charge of a polypeptide promote its secretion via the autotransporter pathway? It seems unlikely that acidic residues promote electrostatic interactions with residues situated in the β-domain pore because the net charge of the pore varies widely (3, 4), as our bioinformatic analysis might suggest. It is much more likely that passenger domain translocation is influenced by electrostatic interactions with either membrane proteins or lipids. One possibility is that charge repulsion between negatively charged passenger domains and LPS molecules promotes the movement of passenger domains away from the cell surface. Indeed changes in LPS structure have been shown to affect the biogenesis of Shigella flexneri and Haemophilus influenzae autotransporters (47, 48). Alternatively, the same factors that contribute to the marked tendency of positively charged regions of IM proteins to be retained on the cytoplasmic side of the membrane (the so-called “positive-inside” rule) (49) might favor a predominance of acidic residues in passenger domains. Although the physical basis for the positive-inside rule is not understood, it has been proposed that electrostatic effects arising from the net negative charge of phospholipid head groups might cause a pK shift that would selectively neutralize acidic residues and favor their translocation across the membrane (50).
Electrostatic interactions mediated by acidic residues may not completely account for the efficient translocation of either the RD-EspPΔ1 polypeptide or native passenger domains across the OM. Indeed as mentioned above, some passenger domains are not especially acidic, and a few are basic. If the insertion of β-domains into the OM is coupled to their folding, then it is conceivable that at least some of the energy for translocation is derived from the formation of a large number of hydrogen bonds during the folding process. It is unclear, however, whether the later stages of translocation, which may occur long after the β-domain is largely folded, could be driven by β-domain assembly. Conformational changes in the translocation machinery or conformational constraints imposed on the passenger domain might also play a role in promoting translocation. The pore of FimD was recently shown to facilitate the translocation of type I pilus subunits across the OM by imposing rotational and translational constraints that guide them along a low-energy pathway (51). Finally, it is formally possible that energy for passenger domain translocation is transduced from the cytoplasm by an IM protein that spans the periplasmic space. In this regard it was recently reported that a large IM protein called TamB promotes autotransporter assembly through an interaction with an OM protein called TamA (52). TamA is not required for the biogenesis of an E. coli autotranspoter called Hbp (9), however, and we have found that the disruption of either tamA or tamB has no effect on the secretion of the EspP passenger domain.
Finally, although the nature of the passenger domain translocation channel remains poorly understood, the results of our experiments on the ΔRD-EspPΔ1 fusion protein corroborate the conclusion that at least some small folded polypeptides can be secreted efficiently via the autotransporter pathway. The architecture of the ΔRD fragment is very different from that of CtxB, which is also secreted as a folded polypeptide when it is fused to EspPΔ1 (17). Whereas ΔRD forms a β-roll stabilized by calcium ions, CtxB forms a mixed α/β-structure stabilized by a disulfide bond. The finding that a folded polypeptide that has a distinct tertiary structure from CtxB is secreted helps to rule out the possibility that its translocation via the autotransporter pathway is an aberration. Nevertheless, there is evidence that some small folded polypeptides, including polypeptides that are smaller than CtxB and ΔRD, are not secreted when they are fused to other autotransporters (17, 53, 54). Indeed it appears that the secretion of folded polypeptides via the autotransporter pathway is highly dependent on the experimental system, and at this point it would be difficult to make generalizations. In any case, the slow biogenesis of CtxB-EspPΔ1 and ΔRD-EspPΔ1 suggests that the secretion of the folded moieties is limited by the size of the translocation pore or its ability to undergo a specific conformational change. Given that the translocation of folded polypeptides likely requires a greater input of energy than the translocation of unfolded polypeptides (24), the secretion of the ΔRD moiety strongly suggests that considerable energy can be harnessed to drive secretion via the autotransporter pathway. As a corollary, the finding that a polypeptide that folds in the periplasm can be secreted efficiently provides further evidence that folding in the extracellular space is not essential for translocation.
Materials and Methods
Bacterial Strains, Reagents, and Growth Conditions.
The E. coli strain AD202 (MC4100 ompT::kan) (55) was used in all experiments. Cultures were grown at 37 °C in LB broth or M9 medium containing 0.2% glycerol and all of the l-amino acids except methionine and cysteine (40 μg/mL). Trimethoprim (50 μg/mL) was used to maintain plasmids. An anti-EspP C-terminal peptide antiserum was described previously (56). A purified monoclonal antibody (9D4) that recognizes a CyaA RD epitope situated between residues 1,156 and 1,489 was obtained from Santa Cruz Biotechnology.
Plasmid Construction.
Plasmid pJH63, which encodes EspP∆1 (a 116-aa C-terminal fragment of the passenger domain plus the β-domain) has been described (56). To construct plasmid pWK1, which encodes the RD-EspPΔ1 chimera, the cyaA segment that encodes the RD (amino acids 1,006–1,680) was first amplified by PCR, using oligonucleotides 164 (5′-GATATAGAATTCACGCTCGAGCATGTGCAGCACATCATC-3′) and 198 (5′-CTATATGGATCCGTCCGGATACTGCGCCATTGCCTCGACCAGC-3′) and genomic DNA from B. pertussis strain Tahoma I as a template. The PCR fragment was then digested with EcoRI and BamHI and cloned into the cognate sites of pTRC99a (57). The EspP signal peptide was amplified using primers 162 (5′-GATATACCATGGATGAATAAAATATACTCTCTTAAATACAGCC-3′) and 199 (5′-CTATATGAATTCCGCAAAAGAATATGAGGATTGTAATAAGCC-3′) and pJH63 as a template and fused to the RD by cloning it into the NcoI and EcoRI sites of pTRC99a. Next, the mature region of EspP∆1 was amplified using primers 166 (5′-GATATAAAGCTTTATGCGATGCGTACAAACCTTTCTGAATCAG-3′) and 167 (5′-CTTTCAGGATCCTCAGAACGAGTAACGGAAATTAGCGTTGAC-3′) and pJH63 as a template and cloned into the BamHI and HindIII sites of pSCrhaB2 (58) to create plasmid pWK101. An NcoI-BamHI fragment encoding the EspP signal peptide and the RD was then moved from pTRC99a into pWK101 to create pWK102. Subsequently the EspP signal peptide was reamplified by PCR, using oligonucleotides 237 (5′-GATATACCATGGCATATGAATAAAATATACTCTCTTAAATACAGCC-3′) and 238 (5′-CTATATGAATTCTCTAGACGCAAAAGAATATGAGGATTGTAATAAGCC-3′), and cloned into the NcoI and EcoRI sites of pWK102. The resulting plasmid (pWK103) was digested with NdeI and religated to remove the NcoI site. To make the final plasmid, three methionine residues were inserted at the N terminus of the RD moiety, using oligonucleotide 264 (5′-GATATATCTAGAGATCAGGAAATGGAGATGATGACGCTCGAGCATGTGCAGCACATCATC-3′) and its complement. Plasmid pWK2, which encodes the ΔRD-EspPΔ1 chimera, was constructed in an analogous fashion. PCR fragments encoding CyaA residues 1,457–1,680 and the EspP signal peptide were generated using primer pairs 198 and 256 (5′-GATATATCTAGAGGCGAAGCCGGTGACGACTGGTTCTTCCAGGATGCCGCC-3′) and 162 and 238 (5′-CTATATGAATTCTCTAGACGCAAAAGAATATGAGGATTGTAATAAGCC-3′) and cloned into the XbaI/BamHI and NcoI/Xba sites of pTRC99a, respectively. The final plasmid was constructed by transferring an NcoI-BamHI fragment from pTRC99a into pWK101. To make the charge neutralization mutants RD(III-DENQ23)-EspPΔ1, RD(III-DN5)-EspPΔ1, RD(III-DN11)-EspPΔ1, and RD(V-DN12)-EspPΔ1 (plasmids pWK4, pWK5, pWK6, and pWK9); the charge reversal mutants RD(III-DR5)-EspPΔ1, RD(III-DR11)-EspPΔ1, and RD(V-DR12)-EspPΔ1 (plasmids pWK7, pWK8, and pWK10); and the multiple alanine substitution mutant RD(III-HYDA17)-EspPΔ1 (plasmid pWK3), DNA fragments containing the desired mutations were synthesized by Genescript or Genewiz (Table S1) and cloned into a plasmid. The fragments were then excised with appropriate restriction enzymes and used to replace the equivalent fragment in pWK1.
Pulse-Chase Labeling and Kinetic Analysis of Protein Secretion.
AD202 transformed with an appropriate plasmid were grown overnight in M9 medium, washed, and diluted into fresh medium at OD550 = 0.02. When the cultures reached OD550 = 0.2, the expression of plasmid-borne genes was induced for 30 min by the addition of 0.2% (wt/vol) L(+) rhamnose. In some experiments, 2 mM CaCl2 or 5 mM EGTA was added immediately before pulse-chase labeling. Cells were pulse labeled for 30 s with 30 μCi/mL Trans 35S-label (MP Biomedical), and a chase was conducted by adding methionine and cysteine (1 mM). Aliquots of radiolabeled cultures were pipetted into tubes containing an equal volume of preformed ice at each time point. Spheroplast suspensions were prepared as previously described (59). In experiments that involved sonication, radiolabeled cells were collected by centrifugation (3,000 × g, 10 min, 4 °C), washed, resuspended in M9 medium at three-quarters the original concentration, and disrupted using a Misonix 3000 sonicator (microtip level 4) after intact cells were removed to perform controls. Where applicable, half of each sample (intact or sonicated cells or spheroplast suspensions) was treated with 200 μg/mL PK for 20 min on ice. PK digestion was stopped by the addition of 2 mM PMSF. Proteins in all samples were precipitated by the addition of 10% (wt/vol) TCA, and immunoprecipitations were conducted as previously described (59). Immunoprecipitated proteins were then resolved by SDS/PAGE on 8–16% minigels (Invitrogen). Radioactive proteins were visualized using a Fujifilm FLA-9000 phosphorimager. For quantitative analysis, cell surface exposure was defined as 1 − [proEspP (+PK)/(proEspP (−PK) + β-domain (−PK))] and passenger domain cleavage was defined as [β-domain (−PK)/[β-domain (−PK) + proEspP (−PK)] (7). The signal in each band was normalized to account for differences in the number of radioactive amino acids. Curves were fitted using Origin Pro software v. 8.5 (Origin Lab).
Steady-State Analysis of RD-EspP Fusion Protein Secretion.
Cells were grown in LB to OD550 ∼ 0.4, and fusion protein expression was induced for 30 min by the addition of rhamnose (0.2% wt/vol). Portions of the cultures were then chilled. In some experiments, cells were separated from the culture medium by centrifugation (3,000 × g, 10 min, 4 °C) and both the cell pellet and the supernatant were analyzed independently. In experiments in which only the secreted passenger domain was analyzed, cell pellets were discarded. One aliquot of the supernatant was incubated with 2 mM CaCl2 for 15 min at 37 °C, and an equal aliquot was kept on ice. Half of each aliquot was then treated with PK at 0 °C as described above. In all experiments proteins were TCA precipitated and resolved by SDS/PAGE. Western blotting was then performed using horseradish peroxidase-linked protein A (GE Healthcare) with the SuperSignal Pico Chemiluminescence kit (Thermo Scientific).
Bioinformatic Analysis.
To study the electrostatic properties of autotransporters, we first predicted the signal peptide–passenger domain and passenger domain–β-domain boundaries. Signal peptides were predicted using the SignalP 4.1 server (www.cbs.dtu.dk/services/SignalP) (60). Passenger–β-domain borders were determined via secondary structure predictions, using DomPred (http://bioinf.cs.ucl.ac.uk/psipred) (61) with the default settings as described previously (44). The pI and net charge (at pH 7) of each domain were then calculated using Editseq (Lasergene v.10; DNASTAR).
Supplementary Material
Acknowledgments
We thank Qing Chen (Center for Biologics Evaluation and Research/Food and Drug Administration) for helping to clone CyaA, Miguel Valvano for providing plasmid pSCrhaB2, Matt Wenham for generating the anti-EspP passenger domain polyclonal antibody, and Trevor Lithgow for sharing bioinformatic data. We also thank Olga Pavlova and Susan Buchanan for helpful comments on the manuscript. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310345110/-/DCSupplemental.
References
- 1.Leyton DL, Rossiter AE, Henderson IR. From self sufficiency to dependence: Mechanisms and factors important for autotransporter biogenesis. Nat Rev Microbiol. 2012;10(3):213–225. doi: 10.1038/nrmicro2733. [DOI] [PubMed] [Google Scholar]
- 2.Junker M, et al. Pertactin β-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc Natl Acad Sci USA. 2006;103(13):4918–4923. doi: 10.1073/pnas.0507923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oomen CJ, et al. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 2004;23(6):1257–1266. doi: 10.1038/sj.emboj.7600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard TJ, Dautin N, Lukacik P, Bernstein HD, Buchanan SK. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat Struct Mol Biol. 2007;14(12):1214–1220. doi: 10.1038/nsmb1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Berg B. Crystal structure of a full-length autotransporter. J Mol Biol. 2010;396(3):627–633. doi: 10.1016/j.jmb.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Perez F, et al. Roles of periplasmic chaperone proteins in the biogenesis of serine protease autotransporters of Enterobacteriaceae. J Bacteriol. 2009;191(21):6571–6583. doi: 10.1128/JB.00754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ieva R, Bernstein HD. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc Natl Acad Sci USA. 2009;106(45):19120–19125. doi: 10.1073/pnas.0907912106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ieva R, Tian P, Peterson JH, Bernstein HD. Sequential and spatially restricted interactions of assembly factors with an autotransporter β domain. Proc Natl Acad Sci USA. 2011;108(31):E383–E391. doi: 10.1073/pnas.1103827108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauri A, et al. The Bam (Omp85) complex is involved in secretion of the autotransporter haemoglobin protease. Microbiology. 2009;155(Pt 12):3982–3991. doi: 10.1099/mic.0.034991-0. [DOI] [PubMed] [Google Scholar]
- 10.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299(5604):262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 11.Wu T, et al. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121(2):235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Hagan CL, Kim S, Kahne D. Reconstitution of outer membrane protein assembly from purified components. Science. 2010;328(5980):890–892. doi: 10.1126/science.1188919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dautin N, Bernstein HD. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol. 2007;61:89–112. doi: 10.1146/annurev.micro.61.080706.093233. [DOI] [PubMed] [Google Scholar]
- 14.Junker M, Besingi RN, Clark PL. Vectorial transport and folding of an autotransporter virulence protein during outer membrane secretion. Mol Microbiol. 2009;71(5):1323–1332. doi: 10.1111/j.1365-2958.2009.06607.x. [DOI] [PubMed] [Google Scholar]
- 15.Pohlner J, Halter R, Beyreuther K, Meyer TF. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 16.Skillman KM, Barnard TJ, Peterson JH, Ghirlando R, Bernstein HD. Efficient secretion of a folded protein domain by a monomeric bacterial autotransporter. Mol Microbiol. 2005;58(4):945–958. doi: 10.1111/j.1365-2958.2005.04885.x. [DOI] [PubMed] [Google Scholar]
- 17.Jong WS, et al. Limited tolerance towards folded elements during secretion of the autotransporter Hbp. Mol Microbiol. 2007;63(5):1524–1536. doi: 10.1111/j.1365-2958.2007.05605.x. [DOI] [PubMed] [Google Scholar]
- 18.Ieva R, Skillman KM, Bernstein HD. Incorporation of a polypeptide segment into the β-domain pore during the assembly of a bacterial autotransporter. Mol Microbiol. 2008;67(1):188–201. doi: 10.1111/j.1365-2958.2007.06048.x. [DOI] [PubMed] [Google Scholar]
- 19.Peterson JH, Tian P, Ieva R, Dautin N, Bernstein HD. Secretion of a bacterial virulence factor is driven by the folding of a C-terminal segment. Proc Natl Acad Sci USA. 2010;107(41):17739–17744. doi: 10.1073/pnas.1009491107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saurí A, et al. Autotransporter β-domains have a specific function in protein secretion beyond outer-membrane targeting. J Mol Biol. 2011;412(4):553–567. doi: 10.1016/j.jmb.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Pavlova O, Peterson JH, Ieva R, Bernstein HD. Mechanistic link between β barrel assembly and the initiation of autotransporter secretion. Proc Natl Acad Sci USA. 2013;110(10):E938–E947. doi: 10.1073/pnas.1219076110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klauser T, Pohlner J, Meyer TF. Selective extracellular release of cholera toxin B subunit by Escherichia coli: Dissection of Neisseria Igaβ-mediated outer membrane transport. EMBO J. 1992;11(6):2327–2335. doi: 10.1002/j.1460-2075.1992.tb05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renn JP, Junker M, Besingi RN, Braselmann E, Clark PL. ATP-independent control of autotransporter virulence protein transport via the folding properties of the secreted protein. Chem Biol. 2012;19(2):287–296. doi: 10.1016/j.chembiol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alder NN, Theg SM. Energetics of protein transport across biological membranes. A study of the thylakoid DeltapH-dependent/cpTat pathway. Cell. 2003;112(2):231–242. doi: 10.1016/s0092-8674(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 25.Driessen AJ. Precursor protein translocation by the Escherichia coli translocase is directed by the protonmotive force. EMBO J. 1992;11(3):847–853. doi: 10.1002/j.1460-2075.1992.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi LX, Theg SM. Energetic cost of protein import across the envelope membranes of chloroplasts. Proc Natl Acad Sci USA. 2013;110(3):930–935. doi: 10.1073/pnas.1115886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lattman EE, Rose GD. Protein folding—What’s the question? Proc Natl Acad Sci USA. 1993;90(2):439–441. doi: 10.1073/pnas.90.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose T, Sebo P, Bellalou J, Ladant D. Interaction of calcium with Bordetella pertussis adenylate cyclase toxin. Characterization of multiple calcium-binding sites and calcium-induced conformational changes. J Biol Chem. 1995;270(44):26370–26376. doi: 10.1074/jbc.270.44.26370. [DOI] [PubMed] [Google Scholar]
- 30.Chenal A, et al. Calcium-induced folding and stabilization of the intrinsically disordered RTX domain of the CyaA toxin. Biophys J. 2010;99(11):3744–3753. doi: 10.1016/j.bpj.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6(3):197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 32.Chenal A, Guijarro JI, Raynal B, Delepierre M, Ladant D. RTX calcium binding motifs are intrinsically disordered in the absence of calcium: Implication for protein secretion. J Biol Chem. 2009;284(3):1781–1789. doi: 10.1074/jbc.M807312200. [DOI] [PubMed] [Google Scholar]
- 33.Baumann U, Wu S, Flaherty KM, McKay DB. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: A two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12(9):3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwaki M, Ullmann A, Sebo P. Identification by in vitro complementation of regions required for cell-invasive activity of Bordetella pertussis adenylate cyclase toxin. Mol Microbiol. 1995;17(6):1015–1024. doi: 10.1111/j.1365-2958.1995.mmi_17061015.x. [DOI] [PubMed] [Google Scholar]
- 35.Bejerano M, Nisan I, Ludwig A, Goebel W, Hanski E. Characterization of the C-terminal domain essential for toxic activity of adenylate cyclase toxin. Mol Microbiol. 1999;31(1):381–392. doi: 10.1046/j.1365-2958.1999.01183.x. [DOI] [PubMed] [Google Scholar]
- 36.Bauche C, et al. Structural and functional characterization of an essential RTX subdomain of Bordetella pertussis adenylate cyclase toxin. J Biol Chem. 2006;281(25):16914–16926. doi: 10.1074/jbc.M601594200. [DOI] [PubMed] [Google Scholar]
- 37.Dautin N, Barnard TJ, Anderson DE, Bernstein HD. Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J. 2007;26(7):1942–1952. doi: 10.1038/sj.emboj.7601638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnard TJ, et al. Molecular basis for the activation of a catalytic asparagine residue in a self-cleaving bacterial autotransporter. J Mol Biol. 2012;415(1):128–142. doi: 10.1016/j.jmb.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blenner MA, Shur O, Szilvay GR, Cropek DM, Banta S. Calcium-induced folding of a beta roll motif requires C-terminal entropic stabilization. J Mol Biol. 2010;400(2):244–256. doi: 10.1016/j.jmb.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 40.Sotomayor Pérez AC, et al. Characterization of the regions involved in the calcium-induced folding of the intrinsically disordered RTX motifs from the bordetella pertussis adenylate cyclase toxin. J Mol Biol. 2010;397(2):534–549. doi: 10.1016/j.jmb.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Hewlett EL, Gordon VM, McCaffery JD, Sutherland WM, Gray MC. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem. 1989;264(32):19379–19384. [PubMed] [Google Scholar]
- 42.Vojtova-Vodolanova J, et al. Oligomerization is involved in pore formation by Bordetella adenylate cyclase toxin. FASEB J. 2009;23(9):2831–2843. doi: 10.1096/fj.09-131250. [DOI] [PubMed] [Google Scholar]
- 43.Rutherford N, Charbonneau ME, Berthiaume F, Betton JM, Mourez M. The periplasmic folding of a cysteineless autotransporter passenger domain interferes with its outer membrane translocation. J Bacteriol. 2006;188(11):4111–4116. doi: 10.1128/JB.01949-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celik N, et al. A bioinformatic strategy for the detection, classification and analysis of bacterial autotransporters. PLoS ONE. 2012;7(8):e43245. doi: 10.1371/journal.pone.0043245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petsko GA, Ringe D. Protein Structure and Function. Sunderland, MA: Sinauer; 2003. [Google Scholar]
- 46.Kumar S, Nussinov R. Salt bridge stability in monomeric proteins. J Mol Biol. 1999;293(5):1241–1255. doi: 10.1006/jmbi.1999.3218. [DOI] [PubMed] [Google Scholar]
- 47.Spahich NA, Hood DW, Moxon ER, St Geme JW., III Inactivation of Haemophilus influenzae lipopolysaccharide biosynthesis genes interferes with outer membrane localization of the hap autotransporter. J Bacteriol. 2012;194(7):1815–1822. doi: 10.1128/JB.06316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teh MY, Tran EN, Morona R. Absence of O antigen suppresses Shigella flexneri IcsA autochaperone region mutations. Microbiology. 2012;158(Pt 11):2835–2850. doi: 10.1099/mic.0.062471-0. [DOI] [PubMed] [Google Scholar]
- 49.Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishtalik LI, Cramer WA. On the physical basis for the cis-positive rule describing protein orientation in biological membranes. FEBS Lett. 1995;369(2–3):140–143. doi: 10.1016/0014-5793(95)00756-y. [DOI] [PubMed] [Google Scholar]
- 51.Geibel S, Procko E, Hultgren SJ, Baker D, Waksman G. Structural and energetic basis of folded-protein transport by the FimD usher. Nature. 2013;496(7444):243–246. doi: 10.1038/nature12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selkrig J, et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat Struct Mol Biol. 2012;19(5):506–510, S1. doi: 10.1038/nsmb.2261. [DOI] [PubMed] [Google Scholar]
- 53.Leyton DL, et al. Size and conformation limits to secretion of disulfide-bonded loops in autotransporter proteins. J Biol Chem. 2011;286(49):42283–42291. doi: 10.1074/jbc.M111.306118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saurí A, Ten Hagen-Jongman CM, van Ulsen P, Luirink J. Estimating the size of the active translocation pore of an autotransporter. J Mol Biol. 2012;416(3):335–345. doi: 10.1016/j.jmb.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 55.Akiyama Y, Ito K. SecY protein, a membrane-embedded secretion factor of E. coli, is cleaved by the ompT protease in vitro. Biochem Biophys Res Commun. 1990;167(2):711–715. doi: 10.1016/0006-291x(90)92083-c. [DOI] [PubMed] [Google Scholar]
- 56.Szabady RL, Peterson JH, Skillman KM, Bernstein HD. An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proc Natl Acad Sci USA. 2005;102(1):221–226. doi: 10.1073/pnas.0406055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 58.Cardona ST, Valvano MA. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid. 2005;54(3):219–228. doi: 10.1016/j.plasmid.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Ulbrandt ND, Newitt JA, Bernstein HD. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88(2):187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- 60.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 61.Bryson K, Cozzetto D, Jones DT. Computer-assisted protein domain boundary prediction using the DomPred server. Curr Protein Pept Sci. 2007;8(2):181–188. doi: 10.2174/138920307780363415. [DOI] [PubMed] [Google Scholar]
- 62.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: A case study using the Phyre server. Nat Protoc. 2009;4(3):363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.