Abstract
The adaptation of an observer’s saccadic eye movements to artificial post-saccadic visual error can lead to perceptual mislocalization of individual, transient visual stimuli. In this study, we demonstrate that simultaneous saccadic adaptation to a consistent error pattern across a large number of saccade vectors is accompanied by corresponding spatial distortions in the perception of persistent objects. To induce this adaptation, we artificially introduced several post-saccadic error patterns, which led to a systematic distortion in participants’ oculomotor space and a corresponding distortion in their perception of the relative dimensions of a cross-figure. The results indicate a tight coupling between the oculomotor and visual–perceptual spaces that is not limited to misperception of individual visual locations but also affects metrics in the visual–perceptual space. This coupling suggests that our visual perception is continuously recalibrated by the post-saccadic error signal.
Keywords: visual perception, eye movements, saccadic adaptation, post-saccadic visual error, gaze-contingent stimuli, sensorimotor alignment
Introduction
During every second of our waking lives, we make approximately three to four saccadic eye movements to examine our visual environment. These movements are executed so quickly that their trajectories need to be entirely programmed prior to their onset. To keep saccadic targeting accurate, this programming quickly adapts to compensate for errors caused by natural pathologies. Such saccadic adaptation can also be artificially induced in the laboratory by repeatedly displacing the target of a saccade during its execution (e.g., Miller, Anstis, & Templeton, 1981; Straube & Deubel, 1995; see Hopp & Fuchs, 2004; Pelisson, Alahyane, Panouilleres, & Tilikete, 2010, for reviews). After several repetitions of moving the target by a constant vector either with or against the saccade direction, observers’ saccades start to over- or undershoot, respectively, the initial (pre-saccadic) target position (Hopp & Fuchs, 2004). While there is convincing evidence that visual error signals drive the adaptation process, the nature of these signals and the mechanisms mediating adaptation have not been clearly identified (e.g., Chen-Harris, Joiner, Ethier, Zee, & Shadmehr, 2008; Ethier, Zee, & Shadmehr, 2008; see Bahcall & Kowler, 2000; Pelisson et al., 2010, for a review).
Interestingly, saccadic adaptation seems to be accompanied by changes in visuospatial perception. Several studies have shown saccadic adaptation to be accompanied by changes in the localization of visual stimuli by subjective report (Awater, Burr, Lappe, Morrone, & Goldberg, 2005; Bahcall & Kowler, 1999; Bruno & Morrone, 2007; Collins, Doré-Mazars, & Lappe, 2007; Moidell & Bedell, 1988). Moidell and Bedell (1988) found a small (approximately 0.5°) change in perceptual localization of a briefly presented target during observer fixation following saccadic adaptation to a target at the same location. Four additional studies have since reported significant mislocalization of stimuli presented at locations similar to the adapted target; however, in every report since Moidell and Bedell, mislocalization during fixation has either not been tested (Bahcall & Kowler, 1999; Bruno & Morrone, 2007) or has failed to show an effect (Awater et al., 2005; Collins et al., 2007; Georg & Lappe, 2009). The only exception is a recent study by Zimmermann and Lappe (2010) that found mislocalization during fixation after inducing particularly large and enduring post-saccadic visual error. While the above work provides converging evidence for changes in visual perception following motor adaptation of saccadic eye movements, the nature of these changes and their underlying neurophysiology are far from clear. Given that saccades can be adapted differentially along multiple vectors (Frens & van Opstal, 1994; Hopp & Fuchs, 2004; Noto, Watanabe, & Fuchs, 1999), it seems possible that the changes in visual perception are not limited to a single translational shift but could match any pattern of saccadic adaptation across the visual field. In this context, it is important to notice that such error can be indicative of inaccuracies in not only the oculomotor map but also the visual–perceptual map and thus may require adaptation to one or both (cf., Awater et al., 2005; Collins et al., 2007). Such remapping of visual perception would be indicated by distortions in the perceived shape of persistent objects during fixation. In the present study, we investigate whether, following the systematic, anisotropic adaptation of a large portion of observers’ central oculomotor space, their visuospatial perception of a large, centrally positioned object is distorted in a manner closely following the oculomotor adaptation pattern during fixation. Such a result would demonstrate that saccadic adaptation is accompanied by changes in visuospatial perception that not only affect the perception of an object’s location but also the perception of the object itself.
To test this hypothesis, we devised a variant of a recently introduced saccadic adaptation paradigm (Garaas, Nieuwenhuis, & Pomplun, 2008), in which we adapted only the horizontal or vertical component of all saccades made during a visual search task. Such an anisotropic adaptation of oculomotor space across the entire central visual field was necessary to create a consistent, whole-field change in visuospatial perception that could be measured without any external, unadapted reference. Including such a reference into the measurement procedure would have divided the observers’ visual field into adapted and unadapted areas. This situation could have weakened the perceptual changes, and comparisons between adapted and unadapted areas may rely on a distinct set of processes unaffected by saccadic adaptation.
Methods
Subjects
Thirty-seven naive students and one author (T. G.) from the University of Massachusetts Boston participated in the experiment; all had normal or corrected-to-normal vision. A total of eight sessions were run for each of the six experimental conditions. Experimental procedures were approved by the University of Massachusetts Boston IRB. Prior to data collection, all participants signed an informed consent form.
Apparatus
Participants sat in a dark room in front of a computer screen where a chin rest was used to restrict head movements. Eye movements were recorded using the SR Research Eye-Link II eye-tracker system. The average error of visual angle in this system is 0.5°, and its sampling frequency is 500 Hz. Stimuli were presented on a 21-inch Dell P1130 monitor using a resolution of 1024 × 768 and a refresh rate of 100 Hz. The maximum delay between participants’ eye movements and the subsequent display shift was approximately 11 ms. Participants sat approximately 36 cm from the screen resulting in horizontal and vertical viewing angles of 44° and 33°, respectively. Responses were obtained using a standard PC mouse.
Adaptation materials
Adaptation displays were prefabricated and consisted of either six or twelve randomly arranged items (average luminance of 22.0 cd/m2) on a display (22° × 22°) with a black background (0.2 cd/m2), with half of the displays containing a target item. The minimum distances between neighboring items in the displays were set to 9.3° and 5.2° in the six- and twelve-item displays, respectively. Our use of two different display sets was motivated by the desire to concentrate saccadic gain changes around saccade vectors that are similar in amplitude to an arm of the cross-figures used during the perceptual judgment trials (7.5 ± 1.0°) while maintaining a range of adapted vector amplitudes. In order to facilitate the measurement of saccadic adaptation, target and distracter items were very similar so that participants had to foveate them to recognize the difference. All items measured approximately 1.0° in diameter and consisted of four colored lines of equal length that were radially aligned at angles of 0°, 90°, 180°, and 270° (see Figure 1). Target items were identical to the distracter items except that two of their lines were of the same color, whereas the distracter items contained lines of four different colors. The colors present on each item were randomly chosen to be either pink (CIE chromaticity coordinates: x = 0.348, y = 0.229), light brown (x = 0.378, y = 0.386), blue (x = 0.254, y = 0.264), or green (x = 0.296, y = 0.480).
Figure 1.
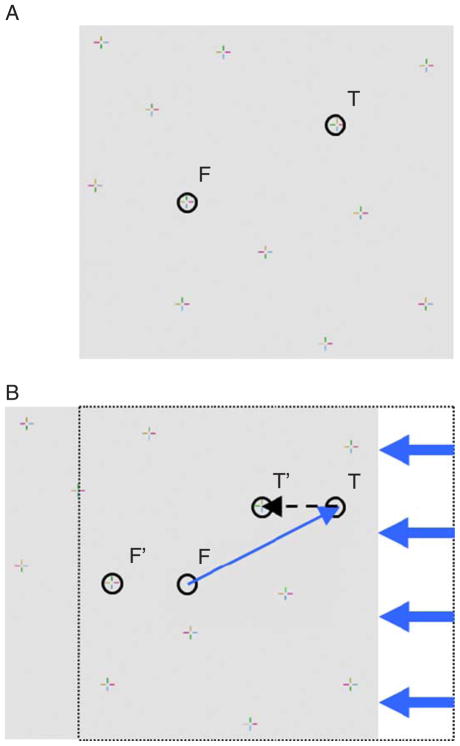
Illustration of an unadapted saccade during the horizontal amplitude reduction condition. (A) Prior to the saccade, the participant is fixating on the item at position F and intends to shift his or her gaze to the item at position T. (B) During the saccade (thin blue arrow), the entire stimulus panel (gray area) is shifted horizontally by half of the saccade’s horizontal amplitude (indicated by the thick blue arrows). Once the participant’s saccade has landed at the intended absolute position T, the intended item will be located at T′, resulting in a horizontal visual error. A corrective saccade, indicated by the dashed arrow, is then necessary to fixate the intended item, which would cause another display shift (not illustrated).
Adaptation procedure
Participants performed three adaptation blocks of 75 trials each in which they were to indicate whether the present display contained a target item. Amplitude reduction or augmentation was accomplished by uniformly shifting all search items against the adapted component of the saccade by half its amplitude or with the adapted component by a third of its amplitude, respectively (see Figure 1). Thus, four experimental conditions (horizontal reduction, vertical reduction, horizontal augmentation, vertical augmentation) were administered to individual groups of participants, inducing anisotropic compression or expansion of observers’ oculomotor map across the central visual field. At least 24 h separated each experimental condition for a single participant.
Adaptation analysis
Saccadic gain during the visual search task was measured for the first saccade of a series of one or more saccades that started within 2° from the center of an item and eventually landed within 2° of another item, which usually included one or more corrective saccades. Additionally, for a saccade to be included in the measure, its direction had to be within 20° of either the saccade target’s initial or shifted position. These criteria were necessary to focus analysis on saccades for which we could confidently designate the target item. The measurement of absolute saccadic adaptation in degrees was additionally restricted to saccades that could potentially influence participants’ visual perception of the cross-figures during perceptual judgment trials. Specifically, absolute adaptation was restricted to saccades that contained a vertical component between 3.5° and 11.5° during the vertical adaptation conditions and was equally restrictive on the horizontal component during horizontal adaptation conditions. These restrictions were motivated by the results of previous research on saccadic adaptation fields (Frens & van Opstal, 1994; Noto et al., 1999).
Perceptual judgment materials
Sixteen cross-figures, labeled as zero through fifteen, with relative lengths ordered from most vertically extended (label 0, approximately 17° tall and 13° wide) to most horizontally extended (label 15, approximately 13° tall and 17° wide) were available for presentation (see Figure 4 for a sample cross-figure). The height–width difference changed by 0.55° between adjacent cross-figures; cross-figures 7 and 8 were closest to having equal-length bars. All cross-figures were white (70.2 cd/m2) on a black background (0.2 cd/m2) and contained a small black dot (diameter of 0.2°) in the center of the figure. Figures were presented in a central position on the screen, with slight, random horizontal and vertical offsets (up to ±1.18°) between trials; additionally, a 1500-ms blank period separated consecutive trials. Participants were instructed to press one of two buttons on the keyboard to indicate which bar of the cross-figure they perceived as longer while maintaining fixation on the small dot in the center of the cross-figure; participants were given a maximum of 5 s to make their choice.
Figure 4.
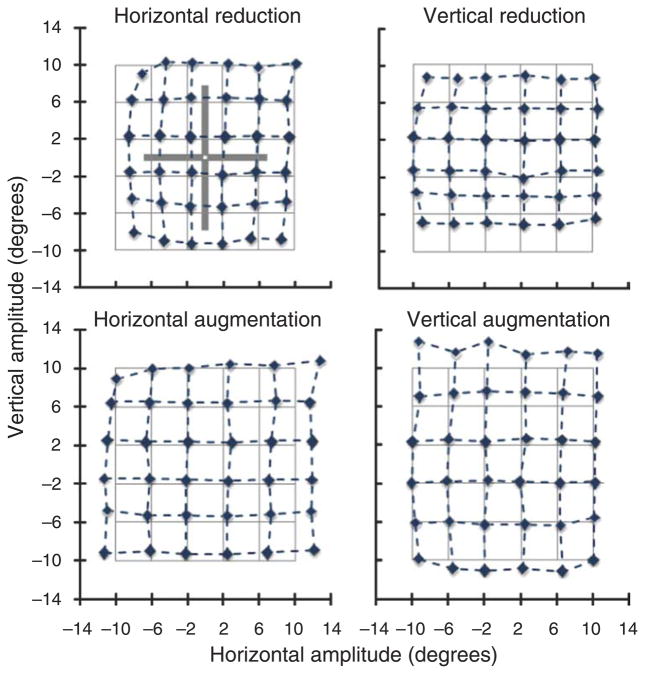
Adaptation effects on saccade vectors in the four experimental conditions. For each condition, the vectors from the currently fixated object to the target object of the next saccade were quantized into 36 spatial bins of 4 × 4 degrees. The grids show the subjects’ average saccade vectors for each of these bins, illustrating distortion in the adapted spatial dimension. In the upper left panel, a cross-figure from the perceptual judgment trials is shown for size comparison.
Perceptual judgment procedure
Alternating with the three adaptation blocks, participants performed four perceptual judgment blocks so that the experiment started and ended with one of them. They were designed to estimate participants’ current visuospatial perception of the relative lengths of the horizontal and vertical bars of the cross-figures. In a 60-trial staircase procedure, participants had to make a two-alternative forced-choice perceptual discrimination by declaring either the horizontal or the vertical bar as being longer, while fixating on the small dot located in the center of the cross-figure. The first block was used to determine a perceptual baseline against which changes in perception in later blocks could be referenced. Thus, between the first and fourth perceptual judgment blocks, changes in participants’ perception indicate how their perception was affected by saccadic adaptation.
Perceptual judgment analysis
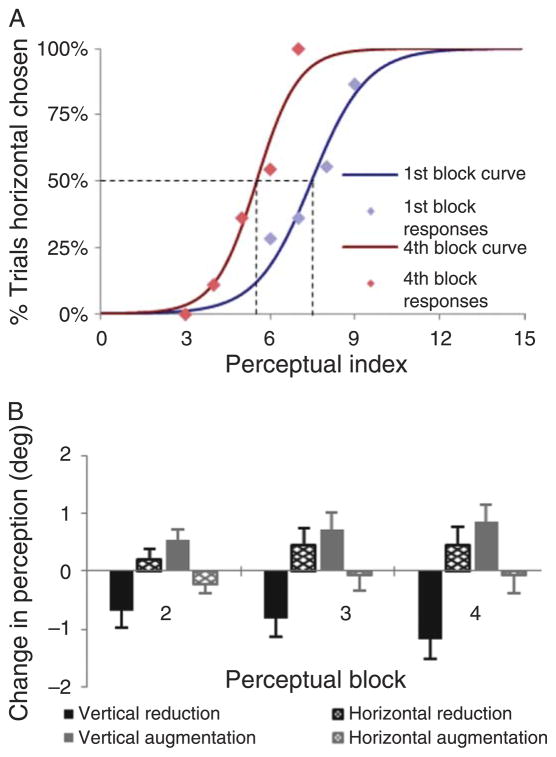
A trial was discarded from analysis if the participant’s fixation was further than 5 degrees from the center of the cross-figure. Participants that had 25% or more perceptual judgment trials discarded were excluded from analysis. The purpose of the perceptual judgment analysis was to estimate a subject’s current perceptual index, defined as the label (0 to 15) of the cross-figure that he or she perceived as having identical width and height. The basis for this estimation was the percentage of trials in which the subject reported a longer horizontal bar, for each of the sixteen cross-figures for which it was measured during a given block of perceptual judgment trials. A sigmoid function was fitted to these data, and the perceptual index for that block was then operationally defined as the cross-figure label at which the function crossed 50% (see Figure 3A for illustration); a perceptual index of 7.5 would represent veridical perception. Differences between perceptual judgment blocks were converted into degrees at a rate of 0.55° per unit difference in perceptual index (see Perceptual judgment materials section above).
Figure 3.
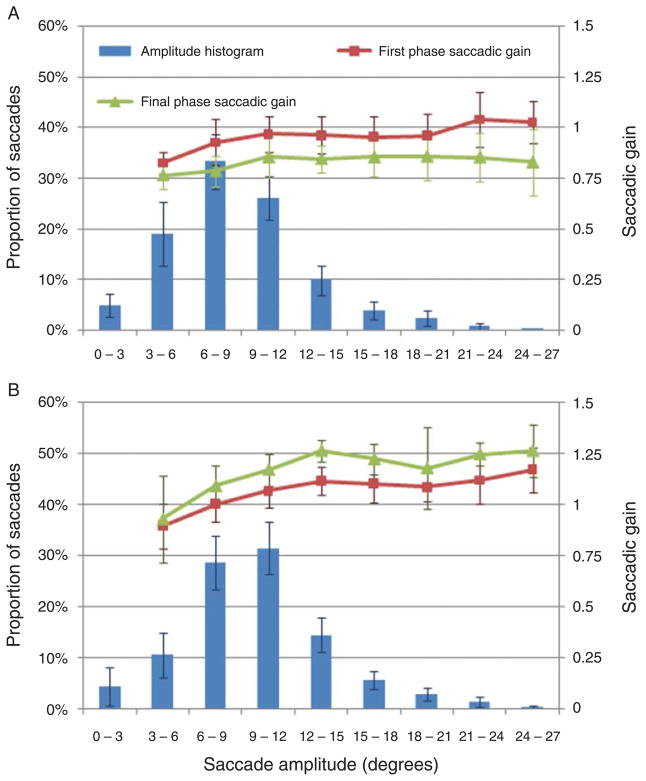
Histogram of saccade amplitude for saccades included in the calculation of saccadic gain, as well as the saccadic gain during the first and last adaptation phases as a function of saccade amplitude. Note that the gains during the first phase cannot be considered a baseline for unadapted saccade amplitude. (A) Plot for reduction conditions. (B) Plot for augmentation conditions. Error bars represent the standard deviation (SD).
Results
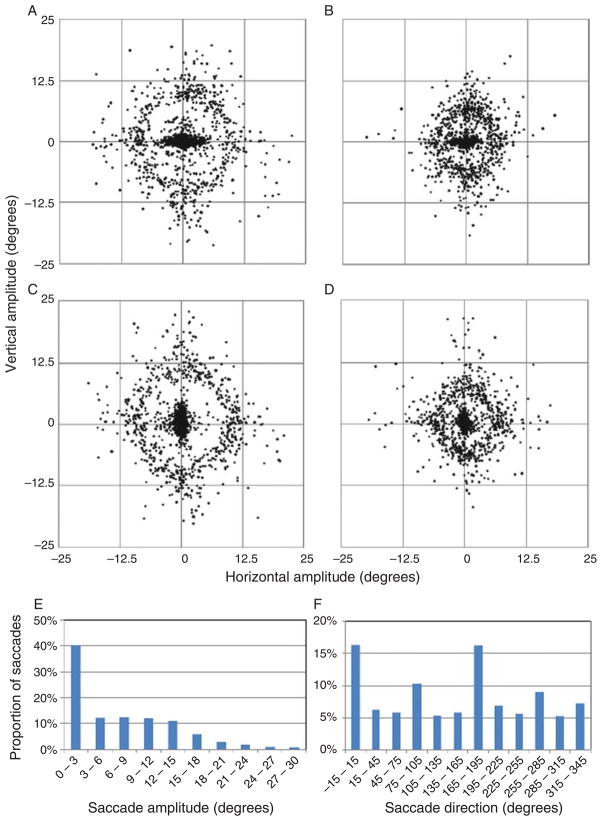
Saccades made by subjects during adaptation phases matched the expected distributions, based on the minimum separation of items in the six- and twelve-item displays of 9.3° and 5.2°, respectively. Table 1 lists for each experimental condition (1) the mean amplitude of all saccades made during the adaptation phases, (2) the mean amplitude of all saccades that contributed to the calculation of saccadic gain, and (3) the mean final saccadic gain (in this report, “±” indicates a mean value and its standard deviation across subjects). It is important to note that all saccades triggered simultaneous display shifts during the adaptation phases, but only saccades that could confidently be designated as inter-item saccades were used to calculate saccadic gain. Figure 2 illustrates the distribution of saccade amplitudes and directions that were measured during the adaptation phases.
Table 1.
Results of a detailed analysis of saccades performed during the adaptation phases of the experiment. Differences between the amplitudes of “all saccades” and saccades that were used in the calculation of saccadic gain are due to the contribution of corrective saccades to the “all saccades” measures.
| Condition | All | 6-item | 12-item |
|---|---|---|---|
| All saccades horizontal reduction | 4.9 ± 0.9° | 5.4 ± 0.9° | 4.4 ± 0.8° |
| All saccades vertical reduction | 5.3 ± 0.7° | 5.7 ± 0.7° | 4.7 ± 0.6° |
| All saccades horizontal augmentation | 6.3 ± 1.1° | 6.8 ± 1.1° | 5.6 ± 1.1° |
| All saccades vertical augmentation | 5.6 ± 0.4° | 6.0 ± 0.4° | 3.7 ± 2.3° |
| Adaptation saccades horizontal reduction | 7.2 ± 0.4° | 8.9 ± 0.6° | 6.5 ± 0.3° |
| Adaptation saccades vertical reduction | 6.8 ± 0.4° | 8.3 ± 0.4° | 6.0 ± 0.4° |
| Adaptation saccades horizontal augmentation | 7.9 ± 0.4° | 10.3 ± 0.4° | 7.5 ± 0.3° |
| Adaptation saccades vertical augmentation | 8.0 ± 0.8° | 9.5 ± 0.4° | 7.0 ± 0.3° |
| Saccadic gain horizontal reduction | 0.85 ± 0.06 | 0.85 ± 0.06 | 0.83 ± 0.06 |
| Saccadic gain vertical reduction | 0.77 ± 0.05 | 0.77 ± 0.06 | 0.75 ± 0.04 |
| Saccadic gain horizontal augmentation | 1.20 ± 0.04 | 1.23 ± 0.08 | 1.16 ± 0.07 |
| Saccadic gain vertical augmentation | 1.16 ± 0.06 | 1.17 ± 0.06 | 1.11 ± 0.03 |
Figure 2.
Plots of saccade vectors for a single subject during the horizontal reduction condition with (A) six- and (B) twelve-item displays. Plots of saccade vectors for a single subject during the vertical augmentation condition with (C) six- and (D) twelve-item displays. (E) Histogram of saccade amplitudes for all subjects. (F) Histogram of saccade directions for all subjects. Both histograms are representative of the individual subjects’ histograms.
Amplitude reduction resulted in a final mean gain of 0.85 and 0.77 for the adapted component during the horizontal and vertical adaptation conditions, respectively (unadapted components remained near 1); these gains indicate adaptation to approximately 45% and 69% of the display shift, respectively. For amplitude augmentation, the final mean gains were 1.20 and 1.16, representing adaptation to 40% and 32% of horizontal and vertical display shifts, respectively. Absolute changes in saccade amplitude of the adapted component due to saccadic adaptation were −0.99 ± 0.58° and −1.84 ± 0.42° for horizontal and vertical amplitude reductions, respectively, and 0.71 ± 0.20° and 1.20 ± 0.42° for horizontal and vertical augmentations, respectively (see Methods section for measurement details). Figure 3 presents a histogram of saccade amplitudes during both conditions and shows saccadic gain at the start and end of the adaptation process for each amplitude interval. The average amplitude of the horizontal components during the horizontal reduction and augmentation conditions were 6.4 ± 0.8° and 5.3 ± 0.6°, respectively, and the average amplitude of the vertical components during the vertical reduction and augmentation conditions were 7.9 ± 1.4° and 6.9 ± 1.0°, respectively. Figure 4 illustrates observers’ pattern of adaptation across their central visual field, including a sample cross-figure used in the perceptual judgment trials to demonstrate their spatial relationship.
Between the first and fourth perceptual judgment blocks, changes in participants’ perception in each group matched the distortion of their oculomotor map (see Figure 5). Participants exhibited near-veridical perception prior to adaptation. However, during the final perceptual judgment block, participants tended to perceive—relative to their perceptual baseline—the horizontal bars of cross-figures to be shorter than the vertical bars in the horizontal reduction (mean perceptual change was 0.46 ± 0.87°) and vertical augmentation conditions (0.85 ± 0.98°) and revealed an opposite perceptual shift in the vertical reduction (−1.18 ± 0.94°) and horizontal augmentation (−0.08 ± 0.88°) conditions. While the perceptual changes in blocks 2 to 4 relative to the first (pre-adaptation) block were statistically significant for the horizontal reduction, t(23) = 2.44, p = 0.023, vertical reduction, t(23) = 4.97, p < 0.001, and vertical augmentation conditions, t(23) = 3.88, p = 0.001, they did not reach significance for the horizontal augmentation condition, t(23) = 0.86, p = 0.397.
Figure 5.
Perceptual judgment results. (A) Sample psychometric curves from the first and fourth perceptual judgment blocks in the vertical reduction condition, which represent perceptual indexes 7.5 (first block) and 5.5 (fourth block), respectively. (B) Perceptual change in degrees for each perceptual judgment block relative to the initial block during each of the four experimental conditions. Error bars represent the standard error (SEM).
Absolute changes in the perception of the relative bar lengths were smaller than absolute saccade amplitude changes for comparable target eccentricity (horizontal reduction: 46%; vertical reduction: 64%; horizontal augmentation: 11%; vertical augmentation: 71%). Correlations between changes in perception and changes in saccadic gain showed tendencies in the expected directions but reached significance only in the horizontal reduction condition. The correlation between saccadic gain changes in adaptation blocks one through three and perceptual changes in perceptual judgment blocks two through four was r = −0.44, p = 0.03 during the horizontal reduction condition; r = 0.32, p = 0.13 during the vertical reduction condition; r = 0.33, p = 0.12 during the horizontal augmentation condition; and r = −0.18, p = 0.41 during the vertical augmentation condition.
We conducted a control experiment to verify that changes in perception were not simply due to the anisotropic motion of the search display. Again, separate sessions of horizontal and vertical display shifts were performed, but this time the shifts occurred randomly (per trial) either with or against the relevant component of saccades. As expected, no significant change in saccadic gain was observed (horizontal condition: 0.02 ± 0.08; t(23) = 1.12, p = 0.27; vertical condition: 0.03 ± 0.06; t(23) = 1.00, p = 0.33). Supporting our hypothesis, there was also no significant perceptual change (horizontal condition: −0.01 ± 1.23°; t(23) = 0.05, p = 0.958; vertical condition: −0.01 ± 0.64°; t(23) = 0.09, p = 0.928).
Discussion
Results from the present study show that motor adaptation of observers’ saccadic eye movements results in a change in the perception of the spatial dimensions of an object. In contrast to previous studies, the change in perception encountered here occurred during fixation of a large, persistent object, which was available for viewing until a decision was made. The results were robust, as they were significant in three out of the four adaptation patterns; the lack of significant perceptual changes during the horizontal augmentation condition is not surprising given the recurring asymmetry in results for horizontal versus vertical adaptation (Noto et al., 1999). Furthermore, the known asymmetries in the transfer of amplitude reduction versus amplitude augmentation to perceptual localization (Hernandez, Levitan, Banks, & Schor, 2008; Hopp & Fuchs, 2004; Kröller, De Graaf, Prablanc, & Péllison, 1999) may explain the stronger perceptual effects for amplitude reduction and the lack of a significant perceptual effect for horizontal augmentation.
The results of the present study may be important to unraveling the effect of saccadic adaptation on visual perception. Whereas all previous reports have focused on the perception of the visual location of individual point stimuli, the present study investigates the effect of saccadic adaptation on the perception of the size of a large, persistent object. Many earlier investigations (Goodale and Milner, 1992, Goodale, Milner, Jakobson, & Carey, 1991; Goodale & Westwood, 2004, Haxby et al., 1991, 1994; Lee & van Donkelaar, 2002; Luo, Garaas, Pomplun, & Peli, 2010; Milner et al., 1991; Perenin & Vighetto, 1988; Ungerleider & Mishkin, 1982) have noted potential anatomical and functional differences associated with the perception of location versus object attributes such as size; most notably are Ungerleider and Mishkin’s (1982) theory making the distinction of “what” versus “where” processing in the ventral and dorsal streams, respectively; and the theory by Goodale and Milner (1992) and Goodale and Westwood (2004) that functionally divides the respective areas into “vision for perception” and “vision for action.” As such, the present results may help identify the neurological loci for saccadic adaptation and the simultaneous change to visual perception.
Though purely speculative at this point, the perceptual judgments of horizontal versus vertical object size tested here—unlike the mislocalization of individual markers found in earlier research—may functionally rely on visual processing in ventral visual areas (e.g., Desimone & Schein, 1987). If this were the case, however, it would need to be explained how adaptation of observers’ saccadic eye movements could affect perceptual judgments involving areas that are unrelated to motor adaptation. Two potential sources immediately come to mind: first, saccadic adaptation could alter a visual sensory map that contributes to our perception of visual space, possibly through direct alteration of the receptive fields of neurons in striate areas, extrastriate areas, or both. However, if a visual sensory map were systematically altered by motor adaptation of saccadic eye movements, it is unclear why certain past investigations have found evidence of perceptual changes only in the presence of a saccadic eye movement. As suggested by Zimmermann and Lappe (2010), one potential explanation is that those experiments were either limited to amplitude reduction or used only small target steps in their adaptation procedure. In their study, Zimmermann and Lappe (2010) demonstrated that large post-saccadic visual errors are necessary to modify visual localization. In the case of amplitude reduction employing the standard back step paradigm, the quick adaptation process may reduce post-saccadic error too rapidly for changes in visual localization to occur.
Alternatively, the whole-field adaptation paradigm used in the present study may have facilitated modifications in the visual map. In our task, adaptation was enforced throughout a large portion of the visual field using a consistent pattern of adaptation, whereas previous studies have focused adaptation on only a single saccadic vector. Notably, using a similar adaptation method, Rolfs, Knapen, and Cavanagh (2010) found that when many saccadic vectors were adapted together, adaptation was faster than when a single saccadic vector was adapted independently. This finding suggests that the visual system’s experience of a consistent pattern of visual error may indicate a different pathology from the one associated with visual error occurring in a single saccadic vector and may signal the need for a distinct compensation mechanism.
Another explanation involves a two-factor theory of visual space perception placed forth by Musseler and Van der Heijden (2004) and Van der Heijden, Musseler, and Bridgeman (1999). In this theory, it is argued that our perception of visual space is established by two sources: a visual sensory map and a non-visual motor map. The visual sensory map essentially provides the “substance” of what the visual field is composed of, whereas the non-visual motor map provides the “metric” of the visual field that encodes all possible eye movements to bring various locations under foveal inspection. These two maps are inherently interwoven to establish the identities and positions of objects in the visual field. As described by Musseler and Van der Heijden (2004), “This can be taken to mean that the perceived positions result from the visual sensory map ‘enriched’ by the motor map about the spatial positions in the visual field in terms of realized and required eye positions” (p. 238). Accordingly, in the current study, when the non-visual motor map is distorted due to saccadic adaptation—and by consequence, the orienting of visual attention immediately preceding saccadic eye movements (Awater et al., 2005; Collins & Doré-Mazars, 2006; Doré-Mazars & Collins, 2005; McFadden, Khan, & Wallman, 2002)—perception of stimulus position relying on the motor map will also be altered. Such a system would offer many ecological benefits to an organism, as it could not only acquire detailed information regarding object identity and position but also provide flexible programming of saccades and—as evidenced by our results—visual perception on both short-term and long-term timescales to account for various pathologies (cf., Collins et al., 2007; Zimmermann & Lappe, 2010).
On a broader scale, the present results suggest that visual–perceptual space is continuously recalibrated by the post-saccadic error signal, and it can be speculated that this mechanism makes vision a particularly robust spatial sensory system. Such robustness could explain the general finding from prism adaptation studies, in which the spatial relationships between various sensorimotor maps are distorted, that visual perception remains stable while errors are resolved by realignment of non-visual sensory (Harris, 1963) and motor maps (Crawshaw & Craske, 1974; Harris, 1965; Zwiers, van Opstal, & Paige, 2003) to the—presumably most reliable—visual sensory map. Moreover, if visual–perceptual space is indeed used as a reference for sensorimotor adaptation, then our current results predict that artificial saccadic adaptation should introduce errors in motor movements. In fact, a number of studies have found that manual, blind pointing to visual stimuli following saccadic adaptation is also distorted in the direction of adaptation (Bekkering, Adam, van den Aarssen, Kingma, & Whiting, 1995; Hernandez et al., 2008; Kröller et al., 1999). Further research is necessary to test these speculations and determine the role of visual–perceptual space and its plasticity in sensorimotor adaptation.
Acknowledgments
The authors would like to thank Harold Bedell, Eileen Kowler, David Melcher, and Maria Concetta Morrone for their comments on earlier versions of this text. This research was funded by Grant R21EY019545 from the National Eye Institute to Marc Pomplun and by a Graduate Assistance in Areas of National Need (GAANN) Ph.D. scholarship from the U.S. Department of Education to Tyler W. Garaas.
Footnotes
Commercial relationships: none.
Contributor Information
Tyler W. Garaas, University of Massachusetts Boston, Boston, MA, USA
Marc Pomplun, University of Massachusetts Boston, Boston, MA, USA.
References
- Awater H, Burr D, Lappe M, Morrone MC, Goldberg M. Effect of saccadic adaptation on localization of visual targets. Journal of Neurophysiology. 2005;93:3605–3614. doi: 10.1152/jn.01013.2003. [DOI] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E. Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature. 1999;400:864–866. doi: 10.1038/23693. [DOI] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E. The control of saccadic adaptation: Implications for the scanning of natural visual scenes. Vision Research. 2000;40:2779–2796. doi: 10.1016/s0042-6989(00)00117-6. [DOI] [PubMed] [Google Scholar]
- Bekkering H, Adam JJ, van den Aarssen A, Kingma H, Whiting HT. Interference between saccadic eye and goal-directed hand movements. Experimental Brain Research. 1995;106:475–484. doi: 10.1007/BF00231070. [DOI] [PubMed] [Google Scholar]
- Bruno A, Morrone MC. Influence of saccadic adaptation on spatial localization: Comparison of verbal and pointing reports. Journal of Vision. 2007;7(5):16, 1–13 . doi: 10.1167/7.5.16. http://www.journalofvision.org/content/7/5/16. [DOI] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. Journal of Neuroscience. 2008;28:2804–2813. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Doré-Mazars K. Eye movement signals influence perception: Evidence from adaptation of reactive and volitional saccades. Vision Research. 2006;46:3659–3673. doi: 10.1016/j.visres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Collins T, Doré-Mazars K, Lappe M. Motor space structures perceptual space: Evidence from saccadic adaptation. Brain Research. 2007;1172:32–39. doi: 10.1016/j.brainres.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Crawshaw M, Craske B. No retinal component in prism adaptation. Acta Psychologica. 1974;38:421–423. doi: 10.1016/0001-6918(74)90001-8. [DOI] [PubMed] [Google Scholar]
- Desimone R, Schein SJ. Visual properties of neurons in area V4 of the macaque: Sensitivity to stimulus form. Journal of Neurophysiology. 1987;57:835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- Doré-Mazars K, Collins T. Saccadic adaptation shifts the pre-saccadic attention focus. Experimental Brain Research. 2005;162:537–542. doi: 10.1007/s00221-005-2221-1. [DOI] [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. Journal of Neuroscience. 2008;28:13929–13937. doi: 10.1523/JNEUROSCI.3470-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frens MA, van Opstal AJ. Transfer of short-term adaptation in human saccadic eye movements. Experimental Brain Research. 1994;100:293–306. doi: 10.1007/BF00227199. [DOI] [PubMed] [Google Scholar]
- Garaas TW, Nieuwenhuis T, Pomplun M. A gaze-contingent paradigm for studying continuous saccadic adaptation. Journal of Neuroscience Methods. 2008;168:334–340. doi: 10.1016/j.jneumeth.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Georg K, Lappe M. Effects of saccadic adaptation on visual localization before and during saccades. Experimental Brain Research. 2009;192:9–23. doi: 10.1007/s00221-008-1546-y. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neuroscience. 1992;15:15–20. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD, Jakobson LS, Carey DP. A neurological dissociation between perceiving objects and grasping them. Nature. 1991;349:154–156. doi: 10.1038/349154a0. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. Evolving view of duplex vision: Separate but interacting cortical pathways for perception and action. Current Opinions in Neurobiology. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Harris CS. Adaptation to displaced vision: Visual, motor, or proprioceptive change? Science. 1963;140:812–813. doi: 10.1126/science.140.3568.812. [DOI] [PubMed] [Google Scholar]
- Harris CS. Perceptual adaptation to inverted, reversed, and displaced vision. Psychological Review. 1965;72:419–444. doi: 10.1037/h0022616. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady C, Horwitz B, Ungerleider L, Mishkin M, Carson R, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Sciences. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Horvitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: A PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez TD, Levitan CA, Banks MS, Schor CM. How does saccade adaptation affect visual perception? Journal of Vision. 2008;8(8):3, 1–16. doi: 10.1167/8.8.3. http://www.journalofvision.org/content/8/8/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Progress in Neurobiology. 2004;72:27–53. doi: 10.1016/j.pneurobio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kröller J, De Graaf JB, Prablanc C, Péllison D. Effects of short-term adaptation of saccadic gaze amplitude on hand-pointing movements. Experimental Brain Research. 1999;124:351–362. doi: 10.1007/s002210050632. [DOI] [PubMed] [Google Scholar]
- Lee JH, van Donkelaar P. Dorsal and ventral visual stream contributions to perception–action interactions during pointing. Experimental Brain Research. 2002;143:440–446. doi: 10.1007/s00221-002-1011-2. [DOI] [PubMed] [Google Scholar]
- Luo G, Garaas TW, Pomplun M, Peli E. Inconsistency between peri-saccadic mislocalization and compression: Evidence for separate “what” and “where” visual systems. Journal of Vision. 2010;10(12):32, 1–8 . doi: 10.1167/10.12.32. http://www.journalofvision.org/content/10/12/32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SA, Khan A, Wallman J. Gain adaptation of exogenous shifts of visual attention. Vision Research. 2002;42:2709–2726. doi: 10.1016/s0042-6989(02)00304-8. [DOI] [PubMed] [Google Scholar]
- Miller J, Anstis T, Templeton W. Saccadic plasticity: Parametric adaptive control by retinal feedback. Journal of Experimental Psychology. 1981;7:356–366. doi: 10.1037//0096-1523.7.2.356. [DOI] [PubMed] [Google Scholar]
- Milner AD, Perrett DI, Johnston RS, Benson PJ, Jordan TR, Heeley DW, et al. Perception and action in ‘visual form agnosia’. Brain. 1991;114:405–428. doi: 10.1093/brain/114.1.405. [DOI] [PubMed] [Google Scholar]
- Moidell BG, Bedell HE. Changes in oculocentric visual direction induced by the recalibration of saccades. Vision Research. 1988;28:329–336. doi: 10.1016/0042-6989(88)90161-7. [DOI] [PubMed] [Google Scholar]
- Musseler J, Van der Heijden AHC. Two spatial maps for perceived visual space: Evidence from relative mislocalizations. Visual Cognition. 2004;11:235–254. [Google Scholar]
- Noto CT, Watanabe S, Fuchs AF. Characteristics of simian adaptation fields produced by behavioral changes in saccade size and direction. Journal of Neurophysiology. 1999;81:2798–2813. doi: 10.1152/jn.1999.81.6.2798. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Alahyane N, Panouilleres M, Tilikete C. Sensorimotor adaptation of saccadic eye movements. Neuroscience and Biobehavioral Reviews. 2010;34:1103–1120. doi: 10.1016/j.neubiorev.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A. Optic ataxia: A specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain. 1988;111:643–674. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Knapen T, Cavanagh P. Global saccadic adaptation. Vision Research. 2010;50:1882–1890. doi: 10.1016/j.visres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Straube A, Deubel H. Rapid gain adaptation affects the dynamics of saccadic eye movements in humans. Vision Research. 1995;35:3351–3458. doi: 10.1016/0042-6989(95)00076-q. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. MIT Press; 1982. pp. 263–299. [Google Scholar]
- Van der Heijden AHC, Musseler J, Bridgeman B. On the perception of position. Advances in Psychology. 1999;129:19–37. [Google Scholar]
- Zimmermann E, Lappe M. Motor signals in visual localization. Journal of Vision. 2010;10(6):2, 1–11 . doi: 10.1167/10.6.2. http://www.journalofvision.org/content/10/6/2. [DOI] [PubMed] [Google Scholar]
- Zwiers MP, van Opstal AJ, Paige GD. Plasticity in human sound localization induced by compressed spatial vision. Nature Neuroscience. 2003;6:175–181. doi: 10.1038/nn999. [DOI] [PubMed] [Google Scholar]