Abstract
Purpose
To assess the dose-response relationship for stomach cancer following radiotherapy for cervical cancer.
Methods and Materials
We conducted a nested, matched case-control study of 201 cases and 378 controls among 53,547 5-year survivors of cervical cancer diagnosed from 1943–1995, from five international, population-based cancer registries. We estimated individual radiation doses to the site of the stomach cancer for all cases and to corresponding sites for the matched controls (overall mean stomach tumor dose, 2.56 gray [Gy], range 0.03–46.1 and following parallel opposed pelvic fields, 1.63 Gy, range 0.12–6.3).
Results
Over 90% of women received radiotherapy, mostly with external beam therapy in combination with brachytherapy. Stomach cancer risk was non-significantly increased (odds ratios [ORs] 1.27–2.28) for women receiving between 0.5–4.9 Gy to the stomach cancer site and significantly increased at doses ≥5 Gy (OR=4.20, 95% confidence interval, 1.41–13.4, Ptrend=0.047) compared to non-irradiated women. A highly significant radiation dose-response relationship was evident when analyses were restricted to the 131 cases (251 controls) whose stomach cancer was located in the middle and lower portions of the stomach (Ptrend=0.003), whereas there was no indication of increasing risk with increasing dose for 30 cases (57 controls) whose cancer was located in the upper stomach (Ptrend=0.23).
Conclusions
Our findings showed for the first time a significant linear dose-response relationship for risk of stomach cancer in long-term survivors of cervical cancer.
Keywords: cervical cancer, stomach cancer, radiotherapy, case-control, second primary cancer
Introduction
Cervical cancer is the third most common cancer in women, with a worldwide burden of 530,000 cases and 275,000 deaths in 2008 (1). In the United States, there were 249,496 survivors of cervical cancer estimated to be alive in 2010 (2). The majority of these survivors were treated with pelvic radiotherapy, and several previous studies have reported that cervical cancer survivors are at increased risk of developing a second cancer related to their radiation exposure (3–7).
An estimated 17% (95% confidence interval [CI] 10%–23%) of second cancers after cervical cancer have been attributed to radiotherapy (8), with the highest risks reported for second cancers of organs that are in or near the radiation field, such as the bladder and rectum. However, the data are sparse on cancer risks for gastrointestinal sites that are farther away from the radiation field and typically receive low to moderate radiation doses (<5 gray [Gy]). In particular, cohort studies of women treated for cervical cancer have reported 1.2 to 2-fold significantly increased risks of stomach cancer following radiotherapy compared with women not receiving radiotherapy (3–5, 7), but these studies lacked detailed information on radiation dose. In a previous analytic study of women treated for cervical cancer that included individual radiation doses averaged across the entire stomach (mean dose, 2 Gy), Boice et al reported a significant two-fold risk of second stomach cancer compared to non-irradiated women, but there was no evidence that risk increased with increasing radiation dose (6). To clarify the association between radiation dose and stomach cancer risk among cervical cancer survivors, we conducted a case-control study nested in a large population-based international cohort that included detailed radiation dose to the site of the stomach cancer for individuals.
Materials and Methods
Study Population
Five population-based cancer registries (Denmark, Finland, Iowa, Ontario, and Sweden) identified 53,547 5-year survivors of invasive cervical cancer. The registries reported 289 cases of second primary stomach cancer diagnosed at least 5 years after cervical cancer with no previous invasive cancer other than a non-melanoma skin cancer or in-situ bladder cancer. Controls were individually matched 2:1 to cases by registry, race (Iowa only), birth date and cervical cancer diagnosis within 5 years, and survival without a subsequent primary cancer at least as long as the interval from date of cervical cancer diagnosis until date of stomach cancer diagnosis for the matched case. After exclusions due to unavailability of medical records (83 cases and 58 controls) and lack of matched controls (5 cases), the final study population consisted of 201 cases and 378 controls. Of these, 54 cases and 16 controls had been included in a previous case-control study of stomach cancer after cervical cancer (6).
Data Collection
Abstractors collected data from medical records up to the date of the stomach cancer for cases and equivalent date for matched controls. Data included demographic variables, diagnosis and treatment of cervical cancer including radiotherapy and chemotherapy, recurrence of cervical cancer, subsequent treatment, and diagnosis of stomach cancer. Records were also reviewed for general cancer risk factors such as tobacco use, alcohol, height and weight, family history of cancer, but data were too sparse to include in our analyses.
Stomach Tumor Location
Anatomic location of the stomach cancer was based on all available information, such as diagnostic pathology, radiology (barium studies, computed tomography scans) esophagoduodenoscopy and surgical reports. We assumed a typical J-shaped stomach for a patient in the treatment (recumbent) position (9). We classified the stomach tumor sites as proximal (upper stomach) or body and distal (middle and lower stomach). There were 40 cases with unknown tumor site (1 overlapping, not otherwise specified and 39 unknown site).
Radiation Treatment and Dosimetry
Radiotherapy for cervical cancer consisted primarily of a combination of external beam and brachytherapy. External beam treatments typically were given in 1.5–3.0 Gy fractions over 4–6 weeks for a total dose of 30–50 Gy, depending upon stage of disease. External beam energies included orthovoltage (160 – 400 kVp) (47.9%), cobalt-60 (19.1%), megavoltage photons (4 to 45 MV) (21.3%), combined energies (4.3%) and unknown energy (7.4%).The majority of women were treated with parallel-opposed anterior/posterior pelvic fields (AP/PA pelvic fields) with typical heights between 10 and 18 cm. Less frequently used external beam fields included abdominal fields (above the umbilicus and below the diaphragm), perineal fields, lateral and rotational pelvic fields. Brachytherapy was primarily low-dose rate, and delivered an average total of 6000 mghr (range 1000 – 13,000 mghr). Five cases and 16 controls received high-dose rate brachytherapy.
Radiation physics experts reviewed individual radiation treatment records and estimated radiation doses using a custom-designed dose program, based on water phantom measurements, which calculated doses to the stomach for each cervical cancer treatment field based on field location, size, laterality, and dose delivered (10). The dose program takes into account three major external out of beam dose contributions, which include radiation leakage through the head of the machine, scatter off the beam collimators and within the patient from the primary beam (see (10) for dosimetry details). Mean doses to the stomach tumor location were estimated for 182 cases and 337 controls. In addition, a radiation quality score was assigned to each patient, based on the completeness of radiotherapy data ranging from 1 to 4 (1=complete; 2=not complete, omission not serious; 3= not complete, omission serious; and 4=inadequate for dosimetry). Individual radiation data were complete or nearly complete for 84.9% of cases and 83.4% of controls.
Statistical Analysis
We used conditional logistic regression to estimate the relative odds (OR) of stomach cancer by comparing the exposure history of cases to their matched controls (11). For radiation dose-response analyses, we compared dose to the site of the stomach cancer (and equivalent location for controls) to the referent category of non-irradiated women (Tables 2–4). For stomach tumors that involved more than one region (e.g., antrum/pylorus), we assigned a dose based on the mean dose to each specified site of involvement that was weighted according to the size of the stomach region. For those cases with overlapping (n=1), multifocal (1), entire stomach (4) or unknown tumor location within the stomach (n=39), mean dose to the entire stomach was assigned. Missing exposure data were handled by including an indicator variable (1=missing dose, 0= not missing) in the models. The OR for continuous radiation dose was expressed as a linear function [1+β x radiation dose in Gy, where β is the excess odds ratio per Gy (EOR/Gy)]. Linear quadratic and linear exponential models were also tested. Two-sided likelihood ratio P-values and profile likelihood 95% CIs were calculated. The attributable risk was calculated by summing the quantities [dose×EOR/Gy.] / [1+ (dose×EOR/Gy)] over cases with known dose.
Table 2.
Risk of stomach cancer by cervical cancer treatment
| Cervical cancer treatment | Mean Dose* (Gy) | Cases | Controls | OR | 95% CI | |
|---|---|---|---|---|---|---|
| N=201 | N=378 | LL | UL | |||
| Type of treatment | ||||||
| No radiation or chemotherapy | 0.00 | 9 | 28 | 1.00 | ref | |
| Radiation only | 2.56 | 191 | 343 | 1.83 | 0.85 | 4.31 |
| Chemotherapy† and radiation | 10.81 | 1 | 7 | 0.44 | 0.02 | 3.28 |
| Type of radiation | ||||||
| No radiotherapy | 0.00 | 9 | 28 | 1.00 | ref | |
| Brachytherapy only | 0.64 | 17 | 37 | 1.53 | 0.59 | 4.16 |
| External beam and brachytherapy | 2.95 | 171 | 307 | 1.85 | 0.85 | 4.36 |
| External beam only | 1.68 | 4 | 6 | 2.5 | 0.5 | 12.1 |
| Mean radiation dose to tumor site category (Gy)‡ | ||||||
| 0 | 0.00 | 9 | 28 | 1.00 | ref | |
| >0–0.49 | 0.31 | 4 | 22 | 0.51 | 0.10 | 2.04 |
| 0.50–0.99 | 0.75 | 24 | 36 | 2.28 | 0.86 | 6.34 |
| 1.0–1.99 | 1.45 | 57 | 98 | 1.87 | 0.80 | 4.71 |
| 2.0–2.99 | 2.43 | 50 | 94 | 1.63 | 0.71 | 4.05 |
| 3.0–3.99 | 3.4 | 21 | 54 | 1.27 | 0.48 | 3.51 |
| 4.0–4.99 | 4.37 | 9 | 17 | 1.91 | 0.53 | 6.88 |
| 5.0–46.1 | 12.1 | 17 | 16 | 4.20 | 1.41 | 13.4 |
| P trend | 0.047§ | |||||
| Unknown dose | --- | 10 | 13 | 2.83 | 0.85 | 9.74 |
Abbreviations: Gy, gray; OR, odds ratio; CI, confidence interval; LL, lower limit; UL, upper limit
Mean dose to stomach cancer site
5 of 8 patients treated with chemotherapy received alkylating agents: cyclophosphamide (1 case, 1 control), cisplatin (2 controls) or Trenimon (1 control). Three other patients were treated with 5-fluorouracil (1 control) and bleomycin (2 controls).
For 152 histologically confirmed cases only, the corresponding ORs were 1.00, 0.83, 3.50, 2.33, 1.94, 1.11, 1.93 and 6.12 for dose groups 0, >0–0.49, 0.50–0.99, 1.0–1.99, 2.0–2.99, 3.0–3.99,4.0–4.99, 5.00+Gy, respectively. Ptrend, 0.064, EOR/Gy=0.098
EOR/Gy=0.11, 95% CI=0.001–0.48.
Table 4.
Risk of stomach cancers occurring in the body and distal stomach sites *
| Dose Category (Gy) | Mean† Dose (Gy) | Cases | Controls | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| N=131 | N=251 | LL | UL | |||
| 0 | 0.00 | 7 | 23 | 1.00 | ref | |
| >0.0–0.99 | 0.61 | 10 | 24 | 1.54 | 0.49 | 5.00 |
| 1.0–1.99 | 1.49 | 36 | 61 | 2.18 | 0.82 | 6.36 |
| 2.0–2.99 | 2.43 | 33 | 71 | 1.56 | 0.59 | 4.55 |
| 3.0–3.99 | 3.4 | 15 | 43 | 1.29 | 0.41 | 4.19 |
| 4.0–4.99 | 4.39 | 9 | 12 | 4.48 | 1.06 | 20.3 |
| ≥5.00 | 11.04 | 16 | 11 | 7.99 | 2.22 | 32.4 |
| P trend | 0.003‡ | |||||
| Unknown dose | -- | 5 | 6 | 3.52 | 0.74 | 17.2 |
Abbreviations: Gy, gray; CI, confidence interval; LL, lower limit; UL, upper limit, EOR, excess odds ratio
Body and distal sites include 29 body, 1 fundus/body, 50 antrum, 12 pylorus, 6 antrum/pylorus, 1 antrum/body, 4 entire stomach, 1 multifocal, 1 anterior wall, 18 lesser curvature and 8 greater curvature, and excludes 20 cardia, 5 fundus, 3 gastroesophageal junction, 2 stump, and 40 unknown stomach site (1 overlapping and 39 unknown).
Mean dose to stomach cancer site
EOR/Gy=0.281, 95% CI 0.04–1.08
Results
The median age at cervical cancer diagnosis was 54 years. Women were diagnosed over 5 decades from 1943–1995, with 65% of patients treated before 1970 (Table 1). The majority of women had squamous cell cancer of the cervix. Second stomach cancer cases were diagnosed a mean of 17 years (range 5–42) after cervical cancer. Histologic confirmation was available for 152 (75.6%) second stomach cancers, with most cases classified as adenocarcinoma.
Table 1.
Selected characteristics of 5-year cervical cancer survivors by case-control status
| Characteristic | Cases (n=201) | Controls (n=378) | ||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Registry | ||||
| Denmark (1943–1999) | 51 | (25.4) | 79 | (20.9) |
| Finland (1953–2002) | 49 | (24.4) | 97 | (25.7) |
| Iowa (1973–2001) | 1 | (0.5) | 2 | (0.5) |
| Ontario (1964–2003) | 19 | (9.5) | 38 | (10.0) |
| Sweden (1958–2002) | 81 | (40.3) | 162 | (42.9) |
| Age at cervical cancer diagnosis (years) | ||||
| 26–44 | 50 | (24.9) | 96 | (25.4) |
| 45–54 | 57 | (28.3) | 107 | (28.3) |
| 55–64 | 59 | (29.4) | 111 | (29.4) |
| 65–83 | 35 | (17.4) | 64 | (16.9) |
| Year of cervical cancer diagnosis | ||||
| 1943–59 | 52 | (25.9) | 91 | (24.0) |
| 1960–69 | 81 | (39.3) | 151 | (39.9) |
| 1970–79 | 47 | (23.3) | 99 | (26.2) |
| 1980–95 | 21 | (10.5) | 37 | (9.8) |
| Cervical cancer histology* | ||||
| Squamous cell carcinoma | 147 | (73.1) | 269 | (71.2) |
| Adenocarcinoma | 7 | (3.5) | 29 | (7.7) |
| Other and unspecified | 47 | (23.4) | 80 | (21.1) |
| Cervical cancer stage† | ||||
| I | 86 | (42.8) | 183 | (48.4) |
| II | 74 | (36.8) | 144 | (38.1) |
| III/IV | 27 | (13.4) | 41 | (10.8) |
| Localized/regional | 14 | (7.0) | 10 | (2.7) |
| Cervical cancer treatment‡ | ||||
| No radiation or chemotherapy | 9 | (4.5) | 28 | (7.4) |
| Radiation | 191 | (95.0) | 343 | (90.7) |
| Chemotherapy and radiation | 1 | (0.5) | 7 | (1.9) |
| Type of radiation | ||||
| Brachytherapy only | 17 | (8.9) | 37 | (10.6) |
| External beam only | 4 | (2.1) | 6 | (1.7) |
| Brachytherapy and external beam | 171 | (89.0) | 307 | (87.7) |
| Type of external beam energy | ||||
| Orthovoltage | 77 | (44.0) | 157 | (50.2) |
| Cobalt-60 | 31 | (17.7) | 62 | (19.8) |
| Megavoltage | 22 | (12.6) | 33 | (10.5) |
| Betatron | 16 | (9.1) | 33 | (10.5) |
| Combined energies | 12 | (6.9) | 9 | (2.9) |
| Unknown | 17 | (9.7) | 19 | (6.1) |
| Type of isotope for brachytherapy | ||||
| Radium, any | 167 | (88.8) | 307 | (89.3) |
| Cesium | 15 | (8.0) | 24 | (7.0) |
| Cobalt | 4 | (2.1) | 12 | (3.5) |
| Unknown | 2 | (1.1) | 1 | (0.3) |
| Intervel from cervical cancer to stomach cancer or comparable date for controls | ||||
| 5–9 years | 50 | (24.9) | 94 | (24.9) |
| 10–14 years | 36 | (17.9) | 68 | (18.0) |
| 15–24 years | 64 | (31.8) | 119 | (31.4) |
| 25–42 years | 51 | (25.4) | 97 | (25.7) |
| Stage of stomach cancer§ | ||||
| I + II | 33 | (16.4) | - | |
| III + IV | 89 | (44.3) | - | |
| Localized/Regional | 52 | (25.9) | - | |
| Unknown | 27 | (13.4) | - | |
| Histology of stomach cancer¶ | ||||
| Adenocarcinoma | 159 | (79.1) | - | |
| Other specified and unspecified | 42 | (20.9) | - | |
| Site of stomach cancer, grouped** | ||||
| Proximal | 30 | (14.9) | ||
| Body | 30 | (14.9) | ||
| Lesser curvature | 18 | (9.0) | ||
| Greater curvature | 8 | (4.0) | ||
| Distal | 69 | (34.3) | ||
| Entire stomach, multi-focal, anterior wall | 6 | (3.0) | ||
| Unknown | 40 | (19.9) | ||
Includes other (3 cases) and not otherwise specified (44 cases, 80 controls).
Based on the Federation of International Gynecologic Oncology (FIGO) stage. Only 1 case and 1 control were diagnosed with stage IV cervical cancer. Cervical cancer patients not staged according to the FIGO scheme were categorized as having localized (10 cases, 9 controls), localized/regional (1 case) or regional (3 cases, 1 control) disease.
No radiation or chemotherapy included hysterectomy (9 cases, 23 controls) cervical conization (4 controls) and cerviectomy (1 control).
Includes stomach cancer cases with localized (n=25), or regional (n=27) disease.
Adenocarcinoma includes 131 cases with highly likely histologic confirmation, 27 cases based on registry data only and 1 case was indeterminate. Stomach cancer histology for other specified and unspecified includes 1 sarcoma, 4 neuroendocrine malignancies, 29 not otherwise specified, and 8 based on clinical data only.
Proximal includes 20 cardia, 5 fundus, 2 stump and 3 gastroesophageal junction; body includes 29 body and 1 fundus/body; distal includes 50 antrum, 12 pylorus, 6 antrum/pylorus and 1 antrum/body; and entire stomach includes 4 entire stomach, 1 multifocal, 1 anterior wall cancer.
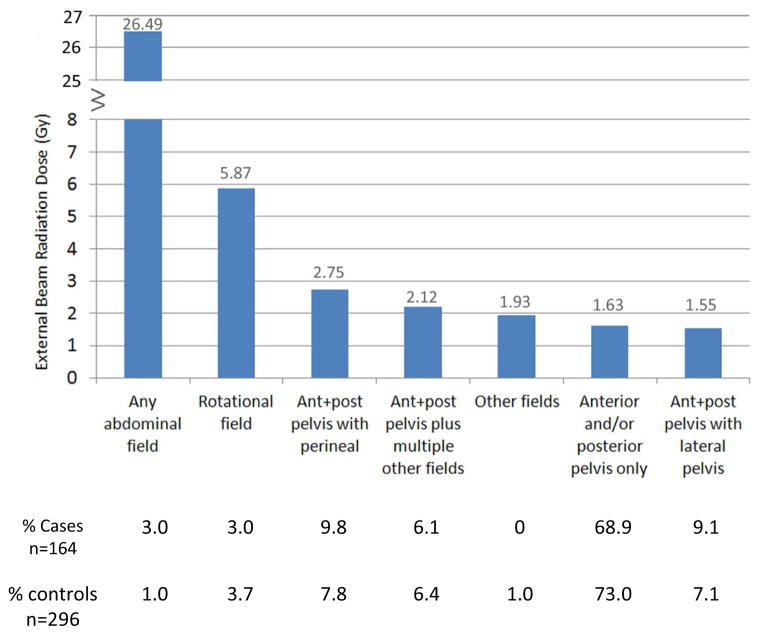
Over 90% of cases and controls were treated with radiation, and chemotherapy was rarely used. The mean dose to the stomach tumor site was 2.56 Gy (range, 0.3–46.1 Gy); the majority of cases (90.7 %) and controls (95.3 %) received less than 5 Gy. The most commonly used external beam field was AP/PA pelvis (113 cases and 216 controls) that delivered mean doses to the stomach tumor sites of 1.63 Gy (range, 0.12–6.3 Gy). The abdominal (mean, 26.5 Gy; 5 cases, 3 controls) and pelvic rotational fields (mean, 5.87 Gy; 5 cases, 11 controls) delivered the highest mean dose to the stomach tumor site, see Figure 1.
Figure 1.
External beam radiation doses (Gy) to stomach cancer sites by type of external beam radiation field used to treat cervical cancer (mean doses are indicated above each bar). Percent of cases and controls with known external beam fields are shown under each type of external beam field.
Abbreviations; ant, anterior; post, posterior
Stomach cancer risk was non-significantly increased in irradiated women compared to non-irradiated women (OR=1.83, 95% CI 0.85–4.31) (Table 2). Although risks were non-significantly elevated between 0.5–4.9 Gy, risk significantly increased for women receiving ≥5 Gy to the stomach cancer site (OR=4.20, 95%CI 1.41–13.4). Modeling the risk associated with stomach doses >0–4.9 Gy and ≥5 Gy yielded ORs= 1.73, 95%CI 0.79–4.09 and 4.47 (95%CI 1.52–14.0), respectively. When dose was measured on a continuous scale, we observed a borderline significant linear increase in risk with increasing dose to stomach cancer site (P trend=0.047, EOR/Gy=0.11, 95% CI 0.001–0.48). Alternative analyses utilizing a linear quadratic or linear exponential model did not improve the fit to these data (P trend >0.50). Because of the wide dose range (5–46.1 Gy) in the highest dose category, we evaluated a more detailed model and noted consistently elevated risks above 5 Gy with ORs of 3.6, 8.7, 3.5 and 4.7 for 5.0–6.9 Gy, 7.0–9.9 Gy, 10–14.9 Gy and 15+ Gy, based on small numbers in each dose category. Overall, we estimated that stomach cancers in 37/182 (20%) irradiated cervical cancer survivors in this study are attributed to radiotherapy.
The dose-response pattern for external beam therapy, adjusted for brachytherapy dose, was similar to analyses based on total dose with significantly increased risks for doses ≥5 Gy (OR=3.61, 95% CI 1.18 –12.1, Ptrend=0.052) (Table 3). We found no evidence that brachytherapy dose independently increased the risk of stomach cancer in analyses adjusted for external beam dose, with risks close to one in the highest dose group (≥1.0 Gy, OR=1.03, 95%CI 0.36–3.02, Ptrend ≥0.50).
Table 3.
Risk of stomach cancer by type of radiotherapy for cervical cancer
| Dose Category | Mean Dose (Gy) | Cases | Controls | OR*,† | 95% CI | |
|---|---|---|---|---|---|---|
| N=201 | N=378 | LL | UL | |||
| 0 (no radiation) | 0.00 | 9 | 28 | 1.00 | ref | |
| External Beam Therapy (Gy)‡ | ||||||
| >0–0.49 | 0.35 | 7 | 14 | 1.45 | 0.35 | 5.89 |
| 0.50–0.99 | 0.74 | 37 | 59 | 1.57 | 0.75 | 3.37 |
| 1.0–1.99 | 1.45 | 66 | 124 | 1.23 | 0.63 | 2.43 |
| 2.0–2.99 | 2.39 | 28 | 66 | 0.92 | 0.43 | 1.99 |
| 3.0–3.99 | 3.35 | 11 | 22 | 1.24 | 0.46 | 3.26 |
| 4.0–4.99 | 4.56 | 6 | 8 | 1.77 | 0.45 | 6.67 |
| 5.0–45.8 | 14.3 | 12 | 11 | 3.61 | 1.18 | 12.12 |
| P trend | 0.052§ | |||||
| Unknown external beam dose | -- | 8 | 9 | 2.65 | 0.78 | 9.21 |
| Brachytherapy (Gy)¶ | ||||||
| >0–0.49 | 0.31 | 67 | 120 | 1.15 | 0.49 | 2.77 |
| 0.50–.99 | 0.71 | 94 | 168 | 1.19 | 0.52 | 2.83 |
| 1.0–2.00 | 1.18 | 24 | 51 | 1.03 | 0.36 | 3.02 |
| P trend | >0.50 | |||||
| Unknown brachytherapy dose | -- | 3 | 5 | 0.92 | 0.15 | 4.84 |
Abbreviations: Gy, gray; OR, odds ratio; CI, confidence interval; LL, lower limit; UL, upper limit
OR for external beam adjusted for brachytherapy dose.
OR for brachytherapy adjusted for external beam dose.
Mean dose from external beam to stomach cancer site.
EOR/Gy=0.106, 95%CI −0.0005–0.48
Mean dose from brachytherapy to stomach cancer site.
In further analyses by type of external beam field, stomach cancer risk was low among women treated with the commonly used AP/PA pelvic radiation fields only (OR=1.22, 95% CI 0.65–2.35), adjusted for brachytherapy dose. Women receiving ≥5 Gy with only AP/PA fields had an OR=2.93, 95% CI 0.68 –12.7 (6 cases, 8 controls). However, risks were higher among women who received ≥5 Gy to the tumor site who were treated with other fields (e.g., abdominal fields, rotational or lateral pelvic or perineal) (OR=5.91, 95% CI 1.68–23.0 (11 cases, 8 controls).
In analyses by stomach cancer site, we observed differences in risk for proximal stomach sites compared with body and distal sites. Among the 131 body or distal stomach cancers, we observed significantly increased risks following doses of 4–4.9 Gy (OR=4.48, 95%CI 1.06–20.3) and even greater increased risks for doses ≥5 Gy (OR=7.99, 95%CI 2.22–32.4, Ptrend=0.003) (Table 4). The EOR/Gy for the body and distal stomach sites (0.281, 95% CI 0.04–1.08) was more than twice that for all stomach sites (EOR/Gy=0.11). Although numbers were small, there was no indication of increasing risk with increasing dose in separate analyses of 30 cases (and 57 matched controls) whose cancer was located in the proximal stomach (EOR/Gy=−0.024, 95% CI=<−0.024, upper bound=0.067, Ptrend=0.23). None of the 30 proximal stomach cancer cases, but 5 of 57 controls were exposed to doses ≥4 Gy. The linear dose-response for the body and distal stomach significantly differed from the proximal stomach (P=0.017), however, this difference appeared to be influenced by two controls who received high-dose radiation (30 Gy and 40 Gy) to the proximal stomach.
We also conducted a secondary dose-response analysis excluding stomach cancer cases (and matched controls) that occurred in more than one stomach region or had unknown specific stomach cancer site. The risk estimates for the remaining 148 cases/284 controls were OR=0.5 (95%CI=0.09–2.0, 4 cases/19 controls), 1.8 (95% CI 0.6–5.4, 20 cases/31 controls), 1.4 (95%CI 0.5–3.8; 42 cases/74 controls), 1.1 (95%CI 0.4–3.0; 31 cases/67 controls), 1.0 (95%CI=0.4–3.3; 17 cases/39 controls), 2.0 (95%CI=0.5–8.3; 8 cases/13 controls, and 2.7 (95%CI 0.8–9.8; 12 cases/13 controls) for radiation dose groups >0–0.49, 0.50–0.99. 1.0–1.9, 2.0–2.9, 3.0–3.9, 4.0–4.9, and ≥5 Gy, respectively, versus 0 Gy.
Stomach cancer risk (comparing 0.5–4.9 Gy and ≥5 Gy versus <0.5 Gy) appeared higher for women treated with radiotherapy for the 5–14.9 year time period following initial cancer diagnosis versus 15+ years (Phomogeneity = 0.10) (Table 5). There was no evidence that radiation-related risks varied by calendar year of cervical cancer diagnosis or by age at diagnosis of either cervical cancer or stomach cancer.
Table 5.
Risk of stomach cancer following cervical cancer according to age and year of cervical cancer, latency and attained age by mean dose to stomach cancer site
| Mean radiation dose (Gy) to stomach cancer site
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–0.49 Gy | 0.50–4.99 Gy | ≥5.00+ Gy | ||||||||
|
| ||||||||||
| Risk Factor | Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI)* | Cases | Controls | OR (95% CI)* | P homogeneity† |
| Total | 13 | 50 | 1.00 (ref) | 161 | 299 | 2.26 (1.18–4.62) | 17 | 16 | 5.61 (2.10–15.9) | |
| Age at cervical cancer diagnosis | ||||||||||
| <55 yrs | 10 | 25 | 1.00 (ref) | 81 | 160 | 1.59 (0.74–3.61) | 11 | 7 | 6.33 (1.84–24.6) | |
| 55+ yrs | 3 | 25 | 1.00 (ref) | 80 | 139 | 3.89 (1.49–12.0) | 6 | 9 | 5.43 (1.20–26.3) | 0.16 |
| Year of cervical cancer diagnosis | ||||||||||
| <1970 | 5 | 19 | 1.00 (ref) | 106 | 205 | 2.00 (0.84–5.34) | 14 | 13 | 5.67 (1.69–20.7) | |
| 1970+ | 8 | 31 | 1.00 (ref) | 55 | 94 | 2.24 (0.99–5.55) | 3 | 3 | 4.20 (0.68–26.6) | >0.50 |
| Latency (Interval from cervical cancer to stomach cancer) | ||||||||||
| 5–14 yrs | 6 | 26 | 1.00 (ref) | 75 | 125 | 4.72 (1.79 –15.2) | 5 | 5 | 8.72 (1.70–50.5) | |
| 15+ yrs | 7 | 24 | 1.00 (ref) | 86 | 174 | 1.35 (0.62–3.12) | 12 | 11 | 4.13 (1.25–14.6) | 0.1 |
| Attained age (Age at stomach cancer diagnosis) | ||||||||||
| <70 yrs | 9 | 23 | 1.00 (ref) | 63 | 118 | 1.86 (0.80–4.64) | 7 | 4 | 7.85 (1.74–43.9) | |
| 70+ yrs | 4 | 27 | 1.00 (ref) | 98 | 181 | 2.75 (1.18–7.29) | 10 | 12 | 5.07 (1.47–19.0) | >0.50 |
Abbreviations: Gy, gray; OR, odds ratio; 95% CI, confidence interval; ref, referent. The median value was used to form subgroups by age and calendar year of diagnosis, latency and year of stomach cancer diagnosis.
Risk estimates compared both dose groups 0.50–4.99 and ≥ 5.00 Gy to referent group of survivors who were not exposed to radiation or received <0.50 Gy
Homogeneity in radiation-related risks among various subgroups was evaluated under a multiplicative model by comparing the fit of a model with separate ORs for each subgroup to a model with a single estimate for all survivors.
Results from sensitivity analyses restricted to women with complete or nearly complete radiotherapy records, (163 cases, 292 controls) (EOR/Gy=0.099), and those with histologically confirmed stomach cancers (152 cases, 292 controls) (EOR/Gy=0.098) were similar to the full dose-response analyses based on 201 cases and 378 controls (EOR/Gy=0.11).
Discussion
This large international study of cervical cancer survivors showed for the first time that the risk of stomach cancer increased with increasing radiation dose from cervical cancer treatment. A unique feature of our international, population-based case-control study was estimation of the radiation dose to the anatomic location of the cancer within the stomach for individual cases (and equivalent location for matched controls), allowing more precise evaluation of the dose-response relationship. This study indicated that stomach cancer risk was non-significantly increased at doses from 0.5 to <5 Gy, and then increased to a 4-fold significant risk at doses ≥5 Gy to the stomach tumor site. The availability of data on stomach tumor location further allowed us to evaluate risk separately for cancers in different stomach locations. We observed a highly significant linear dose-response relationship for risk of stomach cancers occurring in the body and distal sites that were closest to the radiotherapy fields and received the highest doses.
Although increased risks for radiation-related stomach cancer have been reported in several cohort investigations of women with cervical cancer (3–5, 7), the relationship of radiation dose to risk has been evaluated in only one previous analytic case-control study (6). Boice et al. reported a significantly increased 2-fold risk of stomach cancer associated with radiotherapy, but risk did not clearly increase with increasing dose (Ptrend=0.20) (6). In that study, mean dose to the entire stomach was estimated for individuals, rather than dose to the specific location where the stomach cancer occurred. Since the mean dose to the stomach cancer location can vary as much as 5-fold across the stomach, using a mean dose for the entire organ could overestimate doses for the proximal stomach and underestimate doses for the body and distal stomach. The resulting dose misclassification would contribute to a flattening of the dose-response relationship. Our approach enabled us to identify a larger number of cases exposed to mean stomach tumor doses ≥3 Gy (47 cases) and ≥5 Gy (17 cases) compared to the Boice et al. study that reported only 35 cases ≥3 Gy. This allowed us to detect significantly increased risks at higher doses, particularly when we restricted the dose-response analysis to cases occurring in the body or distal stomach.
Our results add to the evidence of risk for radiation-related stomach cancer following high-dose radiotherapy from other medical studies (12, 14, 15) and from the atomic bomb survivors (13). In particular, significant radiation dose-response relationships were noted for stomach cancer after both Hodgkin lymphoma and testicular cancer patients, where mean stomach doses were substantially higher compared to our study, mainly due to abdominal field irradiation (mean dose=20.2 Gy for cases and mean=10.6 Gy for controls, P trend<0.0001) (12). In addition, incidence data from the atomic bomb survivors life span study (LSS) indicated a significant linear-dose response for stomach cancer for survivors exposed to lower doses, ≤ 4 Gy (P< 0.001) with no indication of non-linearity (P>0.5) following whole-body, acute exposures (13). The ERR/Gy was 0.47, 90% CI 0.29–0.68 for females in the LSS at attained age of 70 years with exposure at age 30 years. Analytic studies of patients irradiated for peptic ulcer (14) (mean stomach dose=14.8 Gy) and ankylosing spondylitis (15) (mean stomach dose=3.2 Gy), also demonstrated increased risks of stomach cancer, but neither study observed a significant dose-response relationship.
The size, shape, and location of the stomach are known to vary with body position, stomach contents, phase of respiration, abdominal muscle tone, and body type. A limitation of our study, as well as previous studies evaluating the association between radiotherapy and stomach cancer, was that the exact stomach position at the time of radiotherapy was unknown for individuals, and this position likely shifts over the course of radiation treatment. For cases where the anatomic location of the stomach tumor was unknown, or covered multiple regions (e.g., antrum/pylorus), the mean dose was averaged over these regions. This uncertainty in the stomach position and exact location of tumor origin could result in misclassification of dose estimates, although it was likely to be similar for cases and controls. Although our overall results are consistent with a linear increase in risk with increasing radiation dose, misclassification of exposure at lower doses, where the radiation-related risk is likely to be small, could account for the relatively flat dose-response we observed in this lower dose range (16).
Results for patients treated with the commonly used AP/PA fields were reassuring in that stomach cancer risk was relatively low across the dose range, and only 6 cases received doses ≥5 Gy. Although numbers were small, the use of other fields (abdominal, pelvic rotational, and some perineal fields) that delivered high doses to the cancer site appeared to account for much of the increased risk in the ≥5 Gy dose group, but some of these fields (e.g., pelvic rotational, perineal) are not frequently used currently.
This is the first study to evaluate separately the dose-related risk associated with brachytherapy and external beam radiation. We found no evidence that the low doses delivered to the stomach from brachytherapy significantly increased the already elevated risks of stomach cancer associated with external beam therapy. However, only 54 survivors received brachytherapy alone. Nonetheless, a previous study of uterine corpus cancer patients reported a significantly increased 2-fold risk for second stomach cancer associated with brachytherapy when used without external beam therapy (mean stomach dose, 0.70 Gy, range 0.2–1.8 (17), suggesting that further study of stomach cancer risk associated with this radiation modality is needed.
Conclusion
Our results demonstrated for the first time that radiation doses (≥4–5 Gy) to the anatomic site of the tumor increase the risk of stomach cancer. Although dose uncertainties associated with dose and location of the stomach precluded precise quantification of risk in the lower dose range, our results are consistent overall with a linear dose-response relationship for stomach cancer, as would be predicted from the atomic bomb survivor study. It is important to recognize that increased risks of radiation-related stomach cancer should be balanced against the benefit of the radiotherapy. Radiation continues to be an essential and successful component of cervical cancer treatment. In high-risk patients requiring extended field radiotherapy to achieve adequate tumor control, the reduction in risk of recurrence far outweighs the risk of second stomach cancer.
Our findings emphasize the importance of evaluating the risk of second cancers in gastrointestinal organs outside the primary radiation field. Of particular interest is the current use of conformal methods and the emerging use of intensity-modulated radiotherapy (IMRT) to treat more extensive cervical cancer (18). IMRT has been suggested to decrease the radiation dose to the normal tissue immediately surrounding the cancer site, yet possibly increase the dose to organs farther from the primary treatment field (19). In addition, modern treatment protocols for cervical cancer recommend combination radiotherapy with platinum-based chemotherapy (chemoradiation) for cervical cancer patients with stage ≥IB disease (18). Future studies will be needed to evaluate second cancer risk for organs at a distance from the cervix from these combined treatment approaches, quantify risk more precisely in the lower radiation dose range, and take into account dose-volume relationships and the dose to the stomach tumor location to the extent possible. Because the interval for radiation-related solid cancers is often 10 or more years after exposure, long-term follow-up studies will be needed for the large number of irradiated cervical cancer survivors treated in the past and to estimate risk among patients receiving current treatment approaches.
SUMMARY.
This international case-control study estimated radiation doses to the location of tumors within the stomach and showed for the first time a significant linear radiation dose-response relationship for risk of stomach cancer in long-term survivors of cervical cancer.
Acknowledgments
This research was supported in part by the intramural research program of the NIH and the National Cancer Institute, and with contracts from the National Cancer Institute to Cancer Care Ontario, Toronto, Canada (NO1-CP-31157); Danish Cancer Society, Copenhagen, Denmark (NO1-CP-31019); Finnish Cancer Registry, Helsinki, Finland (NO1-CP-31154); Information Management Services, Inc., Silver Spring, USA (N01-CP-31003); Karolinska Institute, Stockholm, Sweden (NO1-CP-31156); University of Iowa, Iowa City, USA (NO1-CP-31155); The University of Texas MD Anderson Cancer Center, Houston, USA (N02-CP-55503); and Westat, Inc., Rockville, USA (N02-CP-31136).
We thank Diane Fuchs, Janet Lawler-Heavner, and their staff at Westat, Inc. (Rockville, MD) for administrative assistance in conducting the field studies, and Jeremy Miller (Information Management Services, Silver Spring, MD) for computer programming support.
Abbreviations
- Gy
gray
- OR
odds ration
- CI
confidence interval
- EOR
excess odds ratio
- ant
anterior
- post
posterior
- IMRT
intensity modulated radiotherapy
Footnotes
Conflicts of Interest Notifications
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arbyn M, Castellsague X, de Sanjose S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncology. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: 2012. [Google Scholar]
- 3.Kleinerman RA, Boice JD, Jr, Storm HH, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995;76:442–452. doi: 10.1002/1097-0142(19950801)76:3<442::aid-cncr2820760315>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.New malignancies following cancer of the cervix uteri, vagina, and vulva. In: Kleinerman RA, Kosary CL, Hildesheim A, editors; Curtis RE, Freedman DM, Ron E, et al., editors. New malignancies among cancer survivors: SEER cancer registries. 1973-2000. Bethesda: National Institutes of Health; 2006. [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Gilbert ES, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007;99:1634–1643. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 6.Boice JD, Jr, Engholm G, Kleinerman RA, et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res. 1988;116:3–55. [PubMed] [Google Scholar]
- 7.Ohno T, Kato S, Sato S, et al. Long-term survival and risk of second cancers after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2007;69:740–745. doi: 10.1016/j.ijrobp.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Berrington A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatement in adults: a cohort study in the Us SEER cancer registries. Lancet. 2011;12:353–360. doi: 10.1016/S1470-2045(11)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dicker AP, Minsky BD. Stomach. In: Leibel SA, Phillips TL, editors. Textbook of Radiation Oncology. Philadelphia: WB Saunders; 2004. [Google Scholar]
- 10.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 11.Preston DL, Lubin JH, Pierce DA, et al. Epicure: User’s Guide. Seattle, WA: HiroSoft International Corporation; 1993. [Google Scholar]
- 12.van den Belt-Dusebout AW, Aleman BM, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420–1429. doi: 10.1016/j.ijrobp.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 13.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 14.Carr ZA, Kleinerman RA, Stovall M, et al. Malignant neoplasms after radiation therapy for peptic ulcer. Radiat Res. 2002;157:668–677. doi: 10.1667/0033-7587(2002)157[0668:mnartf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Weiss HA, Darby SC, Doll R. Cancer mortality following X-ray treatment for ankylosing spondylitis. Int J Cancer. 1994;59:327–338. doi: 10.1002/ijc.2910590307. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert ES. The impact of dosimetry uncertainties on dose-response analyses. Health Physics. 2009;97:487–492. doi: 10.1097/HP.0b013e3181adc3b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonn S, Gilbert ES, Ron E, et al. Comparison of second cancer risks from brachytherapy and external beam therapy after uterine corpus cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:464–474. doi: 10.1158/1055-9965.EPI-09-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer BE, Koh WJ, Abu-Rustum NR, et al. Cervical cancer. J Natl Compr Canc Netw. 2010;8:1388–1416. doi: 10.6004/jnccn.2010.0104. [DOI] [PubMed] [Google Scholar]
- 19.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]