Abstract
Background
Only 4 prospective randomized phase III trials have been reported for anal cancer. Prognostic factor analysis for anal cancer from prospective database has been published from only one study (n=110). To confirm and uncover new prognostic factors, we analyzed the prospective database of intergroup RTOG 98-11.
Methods
Univariate and multivariate analyses of the baseline characteristics for 5-year overall survival (OS) and disease-free survival (DFS) were carried out. Various combinations of tumor diameter and clinically positive nodes (N+) were analyzed to identify subgroups.
Results
644 were assessable and analyzed. Tumor diameter >5 cm was associated with poorer 5-year DFS (p=0.0003) and poorer 5-year OS (p=0.0031) and N+ was associated with poorer 5-year DFS (p=<0.0001) and poorer 5-year OS (p=<0.0001) in the multivariate analysis. In stratified analyses, N+ had more adverse influence on DFS and OS than did tumor diameter. Patients with >5cm tumor and N+ had the worst DFS (only 30% at 3 years compared to 74% for the best group; <5 cm primary and N0) and OS (only 48% at 4 years compared to 81% for the best group; <5 cm primary and N0). Men had worst DFS (p=0.02) and OS (p=0.016). These factors maintained their influence in each treatment arm
Conclusions
This prospective prognostic factor analysis establishes tumor diameter as an independent prognosticator of poorer 5-year DFS and OS and confirms N+ and male gender as poor prognostic factors. This analysis also uncovers novel subgroups (derived from combining prognostic factors) with incremental worsening of DFS and OS.
Introduction
Anal carcinoma is a rare malignancy in the United States. Approximately 5,070 new cases of anal canal were projected for the year 2008.1 The chemoradiation strategy for anal carcinoma results in disease-free survival (DFS) in ~65% of cases.2–6 Prognosis of patients varies considerably and to some extent based on prognostic factors. Some prognostic factors (nodal status, skin ulceration, and gender) have been identified from one small prospective study5 but others (nodal status, gender, tumor diameter) have been identified by analyses of retrospective databases.7–16 Most published reports of predictive and prognostic variables for anal cancer include small number of patients, retrospective analyses of patients accrued over decades, and single institution experiences.8 Retrospective reviews are fraught with their inherent limitations; therefore, to establish reliable prognostic factors, evaluation in a large number of prospectively accrued patients is desirable. Knowledge of clinical prognostic variables is critical in developing novel therapeutic strategies and in communication with patients/relatives and colleagues. In the future, established clinical prognostic factors may be combined with biomarkers for even better understanding of clinical biology of anal cancer and its susceptibility to chemoradiation.
The European Organization for Research and Treatment of Cancer (EORTC) phase III study5 with 110 patients presented a formal prognostic factor analysis and reported that nodal involvement, skin ulceration, and male gender were associated with poorer OS and local control. However, this prospective study could not identify tumor diameter as a prognostic factor for DFS or OS. Two other prospective randomized control trials of 585 patients4 and 310 patients6 have not reported their data for prognostic factors.
The US GI Intergroup trial RTOG 98-11 recruited 682 patients between 1998 and 2005 with participation by several US cooperative groups.3 The study compared the previously established standard of concurrent 5-fluorouracil plus mitomycin-C and radiation (mitomycin-based therapy) to 5-fluorouracil plus cisplatin (induction and concurrent) and radiation (cisplatin-based therapy). A total of 644 assessable patients were available for the analysis of prognostic factors. Intergroup RTOG 98-11 represents the largest prospectively collected, multi-center database. For this study, patients from the two treatment arms were combined for a prognostic factor analysis to confirm previously reported prognostic factors and to uncover any new ones. We were particularly interested in combining confirmed prognostic factors to identify if novel subgroups would more specifically define the clinical biology of anal cancer with regards to 5-year DFS and OS.
Methods
Literature review
Literature review was conducted by searching for prospective randomize controlled phase III studies by using the terms “anal cancer”, “anal canal cancer”, anal canal carcinoma”, and “epidermoid cancer or carcinoma of the anal canal”. Medline, www.clinicaltrials.gov/, online databases of large societies (American Society of Clinical Oncolgy and European Society of Medical Oncology) were searched. In addition, cross-references were searched from the published prospective randomized phase III trials.
RTOG 98-11 Infrastructure, Hypothesis and Objectives
RTOG 98-11 was a US GI Intergroup trial with participation by Eastern Cooperative Ooncology Group, Cancer and Leukemia Group B, North Central Cancer Treatment Group, Southwest Oncology Group, and RTOG (the coordinating group). The primary objective of the trial was to observe a DFS of 73% with cisplatin-based therapy compared to 63% with mitomycin-based therapy. In addition, the secondary objectives included time-to-colostomy (TTC), OS, and toxic effects. The protocol is available online for viewing (www.rtog.org/).
The objective of the current analysis was to confirm the prognostic factors established by one small prospective study,5 establish new prognostic factors, and examine if combination of prognostic factors will result in newly defined subgroups of anal carcinoma with differing clinical biology with regards to 5-year DFS and OS.
Patient Eligibility
All patients with histologically documented squamous, basaloid, or cloacogenic carcinoma of the anal canal were eligible provided they were more than 18 years of age, had Karnofsky performance status (KPS) ≥60%, had T 2–4 with any N cancer, had adequate organ function, and were willing to provide a written consent.
Patients were excluded if they had a T1 or M1 cancer, severe co-morbid conditions (including AIDS), or major malignancy treated in 5 years.
Evaluations
These have been previously described.3
Randomization, Stratification, and Therapy
Patients were randomized to 5-fluorouracil (5-FU) plus mitomycin-C and concurrent radiation or induction 5-FU plus cisplatin followed by concurrent 5-FU plus cisplatin and radiation. Patients were stratified according to gender, clinical nodal status (positive or negative), and the size of the primary (>2 cm to 5 cm or >5 cm).
The details of therapy have been previously described.3 Briefly, chemotherapy on Arm A included: mitomycin-C 10 mg/m2 iv bolus on days 1 and 29 and infusion 5-FU 1000 mg/m2 days 1–4 and 29–32. Chemotherapy on Arm B included: cisplatin 75 mg/m2 on days 1 and 29, and also repeated on days 57 and 85 and infusion 5-FU 1000 mg/m2 days 1–4 and 29–32, and also repeated on days 57–60 and 85–88 (days 57 and 85 should correspond to days 1 and 29 of radiotherapy).
All patients were to receive a minimum dose of 45 Gy in 25 fractions of 1.8 Gy over 5 weeks to the primary cancer with supervoltage radiation (photon energy of > 6 MV), using AP-PA or multi-field techniques.3 Un-involved nodal sites at risk received 30.6 to 36 Gy in 17 to 20 fractions of 1.8 Gy over 3.5 to 4 weeks. For patients with T3, T4, N+ disease or T2 patients with residual disease after 45 Gy, the intent was to deliver an additional boost of 10–14 Gy in 2 Gy fractions to the primary tumor/involved nodal disease (total dose of 55–59 Gy in 30–32 fractions over 5.5–6.5 weeks).
Statistical Methods
For the purpose of this report, univariate and multivariate analyses were carried out for the entire population and populations in each treatment group. The main focus was on the rates of DFS and OS. Failures for the efficacy endpoints were as follows: OS – death due to any cause and DFS – local, regional or distant failure, second primary or death due to any cause (local-regional failure – local or regional relapse, progression, or persistence; distant metastases – appearance of distant metastases). All efficacy endpoints were measured from the date of randomization to date of first failure for the given endpoint or date of last follow-up for patients that did not fail a given endpoint. OS and DFS were estimated univariately with the Kaplan-Meier method17 and comparisons were tested using the log-rank test.18 Time to local-regional failure, and distant metastases were estimated by the cumulative incidence method19 and comparisons were tested using Gray’s test.20 All reported p-values are 2-sided. Multivariate analyses were performed with Cox proportional hazard models to test for prognostic significance of treatment (Arm A vs. Arm B), gender (female vs. male), clinical nodal status (no vs. yes) and tumor diameter (>2 to ≤5 vs. >5 cm). All variables were coded such that a hazard ratio (HR) greater than 1 indicates an increased risk for the second level of the variable. For example, gender (female vs. male) was coded such that a HR >1 indicates an increased risk of failure for males. The prognostic factors were assessed in each treatment arm (as described above) and in a similar manner when patients in two arms were combined. Two permutations of tumor diameters (<5cm and >5cm) were combined with two permutations of nodal involvement (N0 and N1) to create 4 subgroups to test their effect on DFS and OS.
Results
Patient Characteristics
The study accrued 682 patients from October 1998 to June 2005. Pretreatment characteristics of 644 assessable patients were reported previously.3 Sixty-nine percent of patients were women, 27% had cancer size >5 cm in diameter (T3/T4), and 26% had clinically positive nodes. A total of 324 patients were randomized to mitomycin-based therapy and 320 were randomized to cisplatin-based therapy.
DFS and OS for the Entire Population
The median DFS and OS for the 644 assessable patients have not been reached at a median follow-up time of 2.21 years.
Prognostic factors for DFS and OS
Multivariate analysis is presented in the Table 1 with regard to Treatment (5FU/Mitomycin-C vs 5FU/Cisplatin), Gender (female vs male), Clinical nodal status (negative vs positive) and Tumor diameter (2–5cm vs >5 cm). This table was modified from a previous one21 to include data on OS. We have previously demonstrated that the tumor diameter ≥ 5cm correlates well with the increased likelihood of colostomy, irrespective of the type of cytotoxics received by the patients.21
Table 1.
Multivariate Analyses*; (n=644)
| Disease-free Survival | Overall Survival | |
|---|---|---|
|
| ||
| Adjustment Variables | Adjusted HR* 95% CI p-value |
Adjusted HR* 95% CI p-value |
|
Treatment (5FU/Mitomycin-C vs. 5-FU/Cisplatin) |
1.20 (0.93, 1.55) 0.17 |
1.28 (0.90, 1.84) 0.17 |
|
Gender (Female vs. Male) |
1.38 (1.05, 1.81) 0.02 |
1.57 (1.09, 2.27) 0.016 |
|
Clinical Nodal Status (Negative vs. Positive) |
2.66 (2.04, 3.46) <0.0001 |
2.09 (1.45, 3.01) <0.0001 |
|
Tumor Diameter (2–5cm vs. >5cm) |
1.5 (1.14, 1.97) 0.004 |
1.62 (1.12, 2.33) 0.01 |
Hazard Ratio: a hazard ratio of 1 indicates no difference between the two subgroups. HR > 1 indicates an increased risk of death for the second level of the variables listed.
p-value from Chi-square test using the Cox proportional hazards model.
Modified from Ajani J. et al.21
Gender
DFS and OS of women were better than those of men. In the multivariate analysis, male gender was significantly associated with poorer DFS (p=0.02) and OS (p=0.016).
Tumor diameter
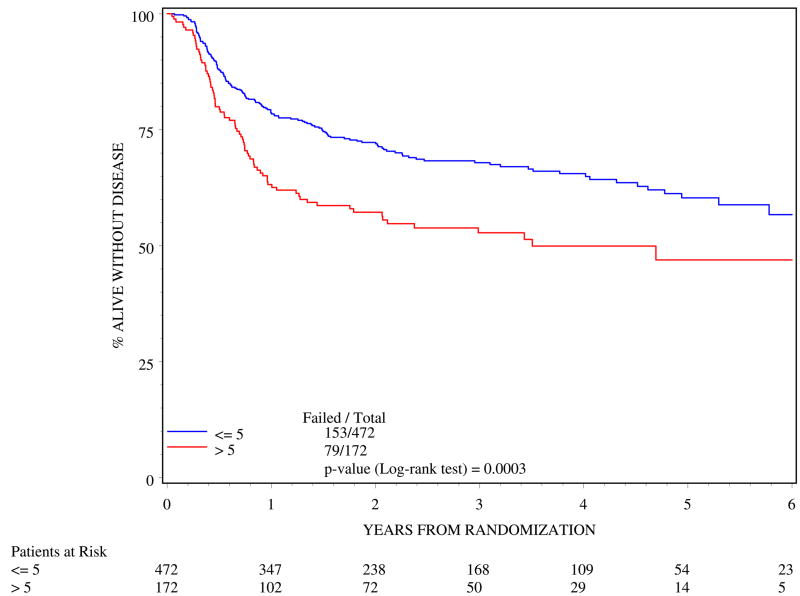
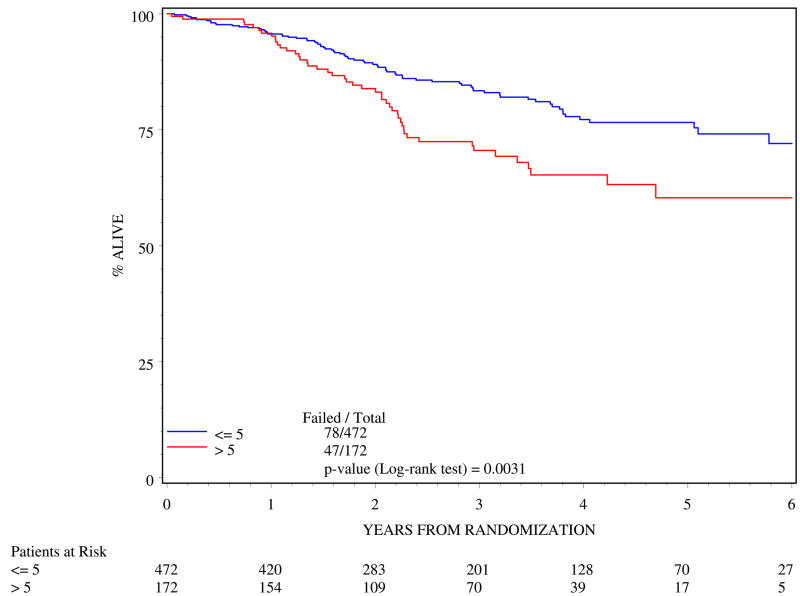
>5cm tumor diameter was associated with poorer outcome. In the multivariate analysis (Table 4), >5 cm tumor diameter resulted in poorer 5-year DFS (log rank p=0.0003; Figure 1) and poorer 5-year OS (log rank p=0.0031; Figure 2).
Table 4.
Randomized phase III trials for anal canal cancer and the extent of prognostic factor evaluation.
| No. of Patients | Prognostic Factor Evaluation | Prognostic factors | Comments | |
|---|---|---|---|---|
| UKCCCR4 | 585 | No | None listed | No follow-up analysis |
| EORTC5 | 110 | Yes | Gender, nodal status, and skin ulceration but not tumor diameter for DFS and OS | Small sample size |
| RTOG 87-046 | 310 | No | None listed | No follow-up analysis |
| RTOG 98-113 | 644 | Yes | Gender, Tumor diameter, and nodal status for DFS and OS | Four prognostic subgroups identified |
Figure 1.
Disease-free survival by tumor diameter (n=644)
Figure 2.
Overall survival by tumor diameter (n=644)
Clinical nodal status
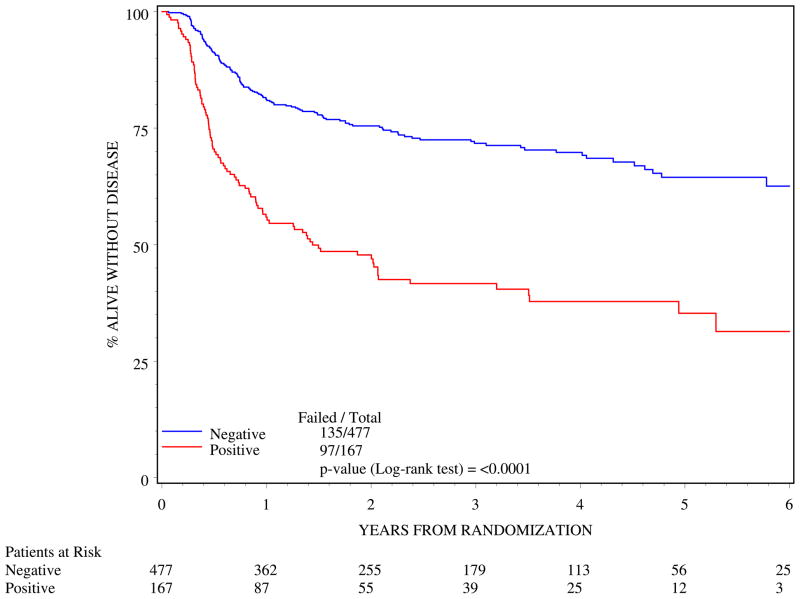
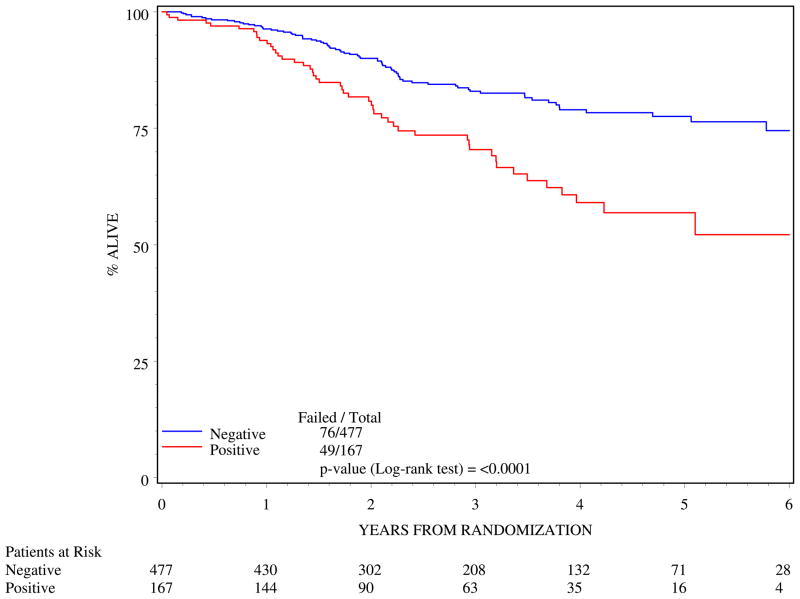
Clinically positive nodes were associated with poorer outcome. N1 status resulted in poorer 5-year DFS (log rank p=<0.0001; Figure 3) and poorer 5-year OS (log rank p=<0.0001; Figure 4).
Figure 3.
Disease-free survival by clinical nodal status (n=644)
Figure 4.
Overall survival by clinical nodal status (n=644)
Subgroups derived from combining tumor diameter and nodal status
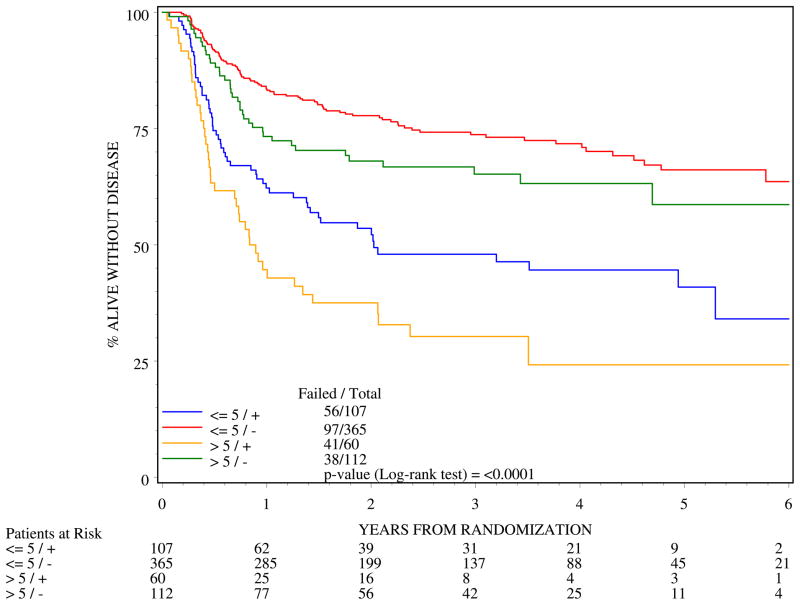
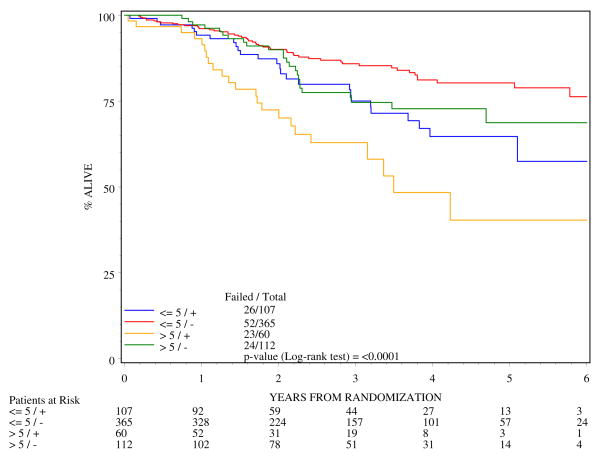
Four subgroups were analyzed for DFS and OS by combining the two permutations of tumor diameters (<5cm and >5cm) and two nodal designations (N0 and N1). Tables 2 and 3 show the DFS and OS rates from 0 to 5-years. The most favorable prognostic subgroup was patients with ≤ 5 cm diameter, clinically node negative tumors with 3-yr and 5-yr DFS of 74% and 66% respectively and 3-y and 5-y OS of 86% and 80%. The worst prognosis for DFS (Figure 5) and OS (Figure 6) were for patients whose cancer had >5cm diameter and clinically positive nodal status with 3-y DFS of only 30% and 4-y OS of 48%. Figures 5 and 6 and Tables 2 and 3 document a higher adverse effect of N+ status on all of the outcomes.
Table 2.
Disease-free survival for all patients (n=644)
| Tumor Diameter | Positive Nodes | Tumor Diameter by Nodes | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 5 | > 5 | Negative | Positive | ≤ 5,− | > 5,− | ≤ 5,+ | > 5,+ | |
| Years | (n=472) | (n=172) | (n=477) | (n=167) | (n=365) | (n=112) | (n=107) | (n=60) |
| 0 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| 1 | 79% | 63% | 81% | 56% | 84% | 73% | 62% | 45% |
| 2 | 72% | 57% | 76% | 48% | 78% | 68% | 53% | 38% |
| 3 | 68% | 53% | 72% | 42% | 74% | 65% | 48% | 30% |
| 4 | 66% | 50% | 70% | 38% | 72% | 63% | 45% | N/A* |
| 5 | 60% | 47% | 64% | 35% | 66% | 59% | 41% | N/A* |
| Total Failed | 153(32.4) | 79(45.9) | 135(28.3) | 97(58.1) | 97(26.6) | 38(33.9) | 56(52.3) | 41(68.3) |
| No./% | ||||||||
| Median (years) | Not reached | 3.5 | Not reached | 1.4 | Not reached | Not reached | 2.0 | 0.9 |
| 95% CI | (1.8, ∞) | (1.0, 2.4) | (1.4, 5.3) | (0.5, 1.4) | ||||
Too few patients at risk, estimate unstable.
Table 3.
Overall Survival for All Patients (n=644)
| Tumor Diameter | Positive Nodes | Tumor Diameter by Nodes | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 5 | > 5 | Negative | Positive | ≤ 5,− | > 5,− | ≤ 5,+ | > 5,+ | |
| Years | (n=472) | (n=172) | (n=477) | (n=167) | (n=365) | (n=112) | (n=107) | (n=60) |
| 0 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| 1 | 96% | 96% | 96% | 94% | 96% | 97% | 94% | 93% |
| 2 | 89% | 84% | 90% | 81% | 90% | 90% | 86% | 72% |
| 3 | 83% | 71% | 83% | 70% | 86% | 75% | 75% | 63% |
| 4 | 77% | 65% | 79% | 59% | 81% | 73% | 65% | 48% |
| 5 | 77% | 60% | 78% | 57% | 80% | 69% | 65% | N/A* |
| Total Deaths | 78(14.8) | 47(27.3) | 76(15.9) | 49(29.3) | 52(14.2) | 24(21.4) | 26(24.3) | 23(38.3) |
| No./% | ||||||||
| Median (years) | Not reached | Not reached | Not reached | Not reached | Not reached | Not reached | Not reached | 3.5 |
| 95% CI | (2.4, ∞) | |||||||
Too few patients at risk, estimate unstable.
Figure 5.
Disease-free survival by four subgroups of patients created by combining tumor diameter and nodal designation (n=644)
Figure 6.
Overall survival by four subgroups of patients created by combining tumor diameter and nodal designation (n=644)
Discussion
While reviewing the results of the literature search, we limited our focus to prospectively conducted phase III studies. Only 4 interventional anal cancer studies have been performed to date.3–6 Table 4 summarizes the prognostic factors established thus far by prospective analyses. The British study4 (n=585) and an RTOG study6 (n=310) have not reported a prognostic factor evaluation, however, the EORTC study5 (n=110) is only one that has reported a formal prognostic factor analysis. The EORTC found that nodal status (local control, p=0.003 and OS, p=0.0003), gender (male with worse outcome; DFS, p=0.01, and OS, p=0.011), and skin ulceration (DFS, p=0.0033 and OS, p=0.005) were prognostic but tumor size was not. We now report our analysis from the second RTOG study3 (n=644). For the RTOG 98-11 study, data for skin ulceration were not available.
Of all 4 reported randomized prospective phase III trials, the US GI Intergroup anal canal cancer trial RTOG 98-11 has the highest number of patients (n=682; 644 analyzed) studied to date,3 and the current analysis for prognostic factors provides additional understanding of the clinical biology of anal carcinoma. Data in this analysis establishes that tumor diameter is an independent pretreatment variable that predicts 5-year DFS and OS. The data also confirm two previously reported prognostic factors (nodal status and gender). In addition, the current analysis documents that anal cancer exhibits four types of clinical biology based on four subgroups of prognostic factor combinations. Greater understanding of clinical factors that drive clinical biology would reduce uncertainties that prevail today when treating patients with anal cancer. Knowledge that patients with >5cm tumor and N1 status has only 30% chance of being disease-free at 3 years allows one to more effectively communicate with our colleagues, patients, and their relatives. Such knowledge would also lead to newer therapeutic algorithms. In addition, identification of poorly performing groups that are likely to have treatment failure early could allow one to test novel therapy quickly because the endpoints are reached sooner in this group that they do in more favorable groups.
Reliable clinical prognostic parameters still do not account for inherent heterogeneity in outcome of individual patient. These unexpected patient outcomes, when patients are treated similarly, are multifactorial and can include type of therapy, toxicity, treatment completion but most likely include the degree of sensitivity to chemoradiation and to some extent patient’s genetic makeup. In the future, it would be important to combine clinical, epigenetic, genetic,22 and germ-line variables to develop a model that predicts response to therapy and prognosticates outcome. The first step in developing such sophisticated models could be the establishment of reliable prognostic factors and their interaction and the next step would be conduct biomarker studies from tissue repositories that might be available.
Approximately 25% of newly diagnosed patients fall into the worst prognostic category (>5cm tumor and N1 status). The current analysis does not provide any clue as to whether this group should be treated differently than other three groups with lower risk of experiencing a DFS or OS event. Since anal cancer is rare, many trials cannot be proposed. However, newer tools such as positron emission tomography23–25 could be incorporated for early assessment of response in a poor prognostic group. If validated, it would help avoid the morbidity of chemoradiation in some patients who can be offered surgery. We acknowledge that there is no data to support this notion and perhaps a combination of clinical and biology-based tools will provide some guidance in the future. It would appear that this group of patients is likely to end up with colostomy anyway even after the completion of chemoradiation.21 On the otherhand, one could also argue that patients with all the poor prognostic factors could benefit from more aggressive therapy.
In conclusion, our analysis of prospectively collected data establishes that tumor diameter is an independent prognosticator of DFS and OS. The analysis confirms that nodal status and gender are also independently prognostic. In addition, the analysis uncovers four subgroups, derived from combining prognostic factors, of anal cancers with differing clinical biology. Further understanding of the clinical biology of anal cancer could emerge from studying molecular biology and patient genetic along with clinical prognostic variables and imaging.
Acknowledgments
Supported by RTOG U10 CA21661, CCOP U10 CA37422, Stat U10 CA32115 from the National Cancer Institute, Bethesda, MD
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18287387. [DOI] [PubMed] [Google Scholar]
- 2.Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51(10):1826–9. doi: 10.1002/1097-0142(19830515)51:10<1826::aid-cncr2820511012>3.0.co;2-l. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6831348. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. Jama. 2008;299(16):1914–21. doi: 10.1001/jama.299.16.1914. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18430910. [DOI] [PubMed] [Google Scholar]
- 4.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348(9034):1049–54. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8874455. [PubMed] [Google Scholar]
- 5.Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15(5):2040–9. doi: 10.1200/JCO.1997.15.5.2040. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9164216. [DOI] [PubMed] [Google Scholar]
- 6.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14(9):2527–39. doi: 10.1200/JCO.1996.14.9.2527. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8823332. [DOI] [PubMed] [Google Scholar]
- 7.Boman BM, Moertel CG, O’Connell MJ, Scott M, Weiland LH, Beart RW, et al. Carcinoma of the anal canal. A clinical and pathologic study of 188 cases. Cancer. 1984;54(1):114–25. doi: 10.1002/1097-0142(19840701)54:1<114::aid-cncr2820540124>3.0.co;2-p. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6326995. [DOI] [PubMed] [Google Scholar]
- 8.Das P, Crane CH, Ajani JA. Current treatment for localized anal carcinoma. Curr Opin Oncol. 2007;19(4):396–400. doi: 10.1097/CCO.0b013e32816f76de. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17545807. [DOI] [PubMed] [Google Scholar]
- 9.Das P, Bhatia S, Eng C, Ajani JA, Skibber JM, Rodriguez-Bigas MA, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):794–800. doi: 10.1016/j.ijrobp.2006.12.052. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17379452. [DOI] [PubMed] [Google Scholar]
- 10.Greenall MJ, Quan SH, Urmacher C, DeCosse JJ. Treatment of epidermoid carcinoma of the anal canal. Surg Gynecol Obstet. 1985;161(6):509–17. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3934775. [PubMed] [Google Scholar]
- 11.Sato H, Koh PK, Bartolo DC. Management of anal canal cancer. Dis Colon Rectum. 2005;48(6):1301–15. doi: 10.1007/s10350-004-0934-z. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15793642. [DOI] [PubMed] [Google Scholar]
- 12.Peiffert D, Bey P, Pernot M, Guillemin F, Luporsi E, Hoffstetter S, et al. Conservative treatment by irradiation of epidermoid cancers of the anal canal: prognostic factors of tumoral control and complications. Int J Radiat Oncol Biol Phys. 1997;37(2):313–24. doi: 10.1016/s0360-3016(96)00493-2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9069302. [DOI] [PubMed] [Google Scholar]
- 13.Longo WE, Vernava AM, 3rd, Wade TP, Coplin MA, Virgo KS, Johnson FE. Recurrent squamous cell carcinoma of the anal canal. Predictors of initial treatment failure and results of salvage therapy. Ann Surg. 1994;220(1):40–9. doi: 10.1097/00000658-199407000-00007. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8024357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sischy B, Doggett RL, Krall JM, Taylor DG, Sause WT, Lipsett JA, et al. Definitive irradiation and chemotherapy for radiosensitization in management of anal carcinoma: interim report on Radiation Therapy Oncology Group study no. 8314. J Natl Cancer Inst. 1989;81(11):850–6. doi: 10.1093/jnci/81.11.850. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2724350. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson PJ, Svensson C, Goldman S, Ljungqvist O, Glimelius B. Epidermoid anal cancer: a review of a population-based series of 308 consecutive patients treated according to prospective protocols. Int J Radiat Oncol Biol Phys. 2005;61(1):92–102. doi: 10.1016/j.ijrobp.2004.03.034. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15629599. [DOI] [PubMed] [Google Scholar]
- 16.Svensson C, Goldman S, Friberg B. Radiation treatment of epidermoid cancer of the anus. Int J Radiat Oncol Biol Phys. 1993;27(1):67–73. doi: 10.1016/0360-3016(93)90422-r. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7690019. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Stat Assoc. 1958;53:457–81. [Google Scholar]
- 18.Mantel N. Evaluation of survival data and two new rank order statistics arising in ints consideration. Cancer Chemother Reports. 1996;60:163–70. [PubMed] [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980. [Google Scholar]
- 20.Gray RJ. A class of K-samples test for comparing the cumulative incidence of a competing risk. Ann Statistics. 1988;16:1141–54. [Google Scholar]
- 21.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, et al. US Intergroup Anal Carcinoma Trial: Tumor Diameter Predicts for Colostomy. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.19.6857. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19139424. [DOI] [PMC free article] [PubMed]
- 22.Bruland O, Fluge O, Immervoll H, Balteskard L, Myklebust M, Skarstein A, et al. Gene expression reveals two distinct groups of anal carcinomas with clinical implications. Br J Cancer. 2008;98(7):1264–73. doi: 10.1038/sj.bjc.6604285. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18349847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz JK, Siegel BA, Dehdashti F, Myerson RJ, Fleshman JW, Grigsby PW. Tumor response and survival predicted by post-therapy FDG-PET/CT in anal cancer. Int J Radiat Oncol Biol Phys. 2008;71(1):180–6. doi: 10.1016/j.ijrobp.2007.09.005. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17996387. [DOI] [PubMed] [Google Scholar]
- 24.Piperkova E, Raphael B, Altinyay M, Castellon I, Libes R, Sandella N, et al. Impact of PET/CT on initial staging, restaging and treatment management of anal cancer: a clinical case with literature review. J Buon. 2006;11(4):523–7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17309188. [PubMed] [Google Scholar]
- 25.Nguyen BT, Joon DL, Khoo V, Quong G, Chao M, Wada M, et al. Assessing the impact of FDG-PET in the management of anal cancer. Radiother Oncol. 2008 doi: 10.1016/j.radonc.2008.04.003. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18453023. [DOI] [PubMed]