Abstract
Introduction
Women ≥45 years of age with persistent HPV infections have distinct peripheral circulating immune profiles. Few studies have comprehensively evaluated the cervical immunologic microenvironment in HPV-positive and HPV-negative perimenopausal women.
Methods
We collected cervical secretion specimens from 34 high risk HPV (HR-HPV) positive and 44 HR-HPV negative women enrolled in an ongoing prospective cohort assessing the natural history of HPV across the menopausal transition. We used these specimens to quantify concentrations of 27 different immune markers using multiplexed bead-based immunoassays.
Results
HR-HPV positive women had significantly higher median concentrations of IL-5 (0.11 ng/mgtotal protein vs. 0.08 ng/mgtotal protein), IL-9 (2.7 ng/mgtotal protein vs. 2.1 ng/mgtotal protein), IL-13 (2.1 ng/mgtotal protein vs. 0.9 ng/mgtotal protein), IL-17 (2.9 ng/mgtotal protein vs. 1.1 ng/mgtotal protein), EOTAXIN (4.1 ng/mgtotal protein vs. 1.1 ng/mgtotal protein), GM-CSF (4.3 ng/mgtotal protein vs. 3.3 ng/mgtotal protein), and MIP-1α (3.5 ng/mgtotal protein vs. 1.9 ng/mgtotal protein) compared to HR-HPV negative women. A shift in the correlation of T-cell and pro-inflammatory cytokines (IFN-γ, IL-5, IL-9, IL-10, IL-12, IL-13, IL-15, and TNF-α) from IL-2 to EOTAXIN was observed between HR-HPV negative and positive women.
Conclusions
Higher local concentrations of anti-inflammatory and allergy associated markers, with a shift in T-cell associated cytokine correlation from IL-2 to EOTAXIN, are associated with HPV infection among older women.
Keywords: Human papillomavirus, Interleukin-2, EOTAXIN, Menopause
1. Introduction
Anogenital human papillomavirus (HPV), the causative agent in cervical carcinoma, originates as a benign, localized infection of the cervical epithelium that is primarily transmitted through sexual contact [1]. The prevalence of HPV reaches a primary peak around the age of sexual debut. A second peak prevalence has been reported in certain geographic regions among women at or around the age of menopause [2]. This second peak in prevalence may be driven by a variety of factors such as birth cohort effects, increased sexual activity at older ages, and/or menopause-induced immunologic or physiological alterations leading to reactivation of latent HPV infection.
Some evidence has emerged to suggest that HPV-positive peri- and post-menopausal women may have altered immune responses. Resolution of an active HPV infection is mediated by a robust CD8+ cytotoxic T lymphocyte response that is characterized by elevated concentration of IL-2 and IFN-γ [3]. However, older women with persistent HPV infection were found to demonstrate reduced in vitro lymphoproliferative responses to common mitogens, as well as decreased release of pro-inflammatory markers such as IL-8 and TNF-α [4,5]. These same women had higher plasma concentrations of these and other pro-inflammatory cytokines such as IL-6, IL-1α and IL-1β as well as chemokines GM-CSF and MIP-1α. It is unclear, however, how representative these peripheral immune markers are to an epithelial localized HPV infection.
The few studies that compared local and peripheral measures of cervical cytokine and antibody concentration related to HPV infection have reported a wide variability in correlation [6,7]. A single study conducted among women >25 years of age enrolled in an on-going prospective study of the natural history of HPV reported no association between cervical IL-10 or IL-12 concentrations and HPV status [8]. Similar studies looking at mucosal immune markers related to HPV infection among older women have not been conducted.
The goal of this study is to describe local cervical concentrations of pro- and anti-inflammatory cytokine and chemokines in HR-HPV positive and negative women at or around the age of menopause.
2. Methods
2.1. Study population and specimen collection
Women included in this study are enrolled in an ongoing prospective cohort study assessing the natural history of HPV during the perimenopausal transition. Briefly, women aged 35–60 years, attending outpatient OBGYN clinics for routine examination at one of four clinic sites in Baltimore, MD, were approached for assessment of eligibility and recruitment. Women were eligible for enrollment if they were English speaking, had an intact uterus, were willing to provide primary locator information and able to provide informed consent. Women who were currently pregnant or planning to become pregnant during the 2 years of the study, and women with a history of organ transplant or known HIV infection were excluded from participation.
Women were enrolled at a baseline study visit, and were followed every six months for 2 years. Data reported here are from the baseline visit only. At this visit, an interviewer-administered questionnaire collected detailed information on demographics, reproductive and menstrual history, medication use, exogenous hormone use history, current and past sexual behaviors, general medical history, and tobacco and alcohol use. Weight, height, and waist circumference was measured. During a speculum-assisted cervical exam, the following specimens were collected (in order): cervical secretion specimens, liquid-based Pap smear, and cervical swab (Digene HPV sampler kit) for HPV testing. Cervical secretions were collected using a Merocel ophthalmic sponge (Medronix) which was placed at the 6 o'clock of the cervical os for 30 s. Afterwards, the sponge with the collected sample was placed in an empty cryovial and stored at 4 °C for <24 h before transfer to long term storage at −80 °C.
For this analysis, we selected all women who were HR-HPV positive at enrollment as of January 2010. A sample of HR-HPV negative frequency matched on menopausal stage was selected for comparison. Menopausal stage was determined based on day of last menstrual cycle and self-reported cycle length variability in the past 12 months. Women were classified as premenopausal if they had a menstrual period in the past 12 months and reported no menstrual cycle variability. Currently menstruating women who reported menstrual cycle variability were considered perimenopausal, and women without a menstrual cycle in the past 12 months were considered post-menopausal.
All study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
2.2. HPV detection and genotyping
All HPV DNA testing was performed on cervical cell samples stored in STM (Digene, Columbia, MD) at the Johns Hopkins University, Baltimore, MD. DNA was extracted using the QIAamp DNA Blood Kit (Qiagen, Courtaboeuf, France) according to manufacturer's instructions with modification. After extraction, 8 μl of DNA (4% of total volume of extracted DNA) was tested using the Roche HPV Linear Array© PCR assay (Roche Diagnostics, Indianapolis, IN). The HPV Linear Array© is based on the PGMY09/11 primer system that allows for high efficiency amplification of >40 types of HPV [9,10]. The quality and validity of the extracted DNA specimen was assessed by inclusion of β-globin gene-specific primers in the PCR reaction. Only specimens with detectable β-globin were used in this analysis.
HPV types considered to be high risk (HR-HPV) for this analysis included types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
2.3. Specimen processing and cytokine measurement
Protein for cytokine analysis was extracted from the ophthalmic sponges using previously described methods [11]. Briefly, sponges were thawed at room temperature for 10 min and then weighed. Sponges were then inserted into a microcentrifuge tube containing a 0.2 μm filter (SpinX, Costar), equilibrated by adding 300 μl of extraction buffer (10 mg/ml aprotinin in phosphate buffered saline with 10% sodium azide) and incubated for 30 min at 4 °C, followed by centrifugation at 4 °C for 30 min at 14,000 rpm. After centrifugation, specimens were stored at −20 °C until the time of immune marker measurement.
The following cytokines, chemokines, and growth factors were measured using a polystyrene non-magnetic bead-based multiplex assay according to the manufacturer's protocol (Bio-Rad, Hercules, CA): BASICFGF, EOTAXIN, granulocyte colony stimulating factor (GCSF), granulocyte macrophage colony stimulating factor (GMCSF), interferon-γ (IFN-γ), interleukin-1β (IL-1β), interleukin-1 receptor antagonist (IL-1ra), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-7 (IL-7), interleukin-8 (IL-8), interleukin-9 (IL-9), interleukin-10 (IL-10), interleukin-12p70 (IL-12p70), interleukin-13 (IL-13), interleukin-15 (IL-15), interleukin-17 (IL-17), inflammatory protein-10 (IP-10), monocyte chemotactic protein (MCP-1), macrophage inflammatory protein-1α (MIP-1α), macrophage inflammatory protein-1 β (MIP-1 β), platelet derived growth factor BB (PDGF-BB), regulated upon activation, normal T-cell expressed and secreted (RANTES), tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF). Plates were read using a Luminex instrument (Bio-Rad, Hercules, CA). An eight point standard curve was created and fit using a five parameter logistic model. All specimens were tested on a single plate to control for observed inter-assay variability. This assay has a reported lower limit of detection of 1–20 pg/ml depending on the cytokine target with an intra-assay coefficient of variation <20% for all markers measured [12].
In order to control for inter-individual variability in immune marker measured due to differences in the amount of specimen collected, the total concentration of protein was measured in each specimen using a bicinchoninic acid (BCA) assay per manufacturer's protocol (Pierce, Rockford, IL). This assay has a dynamic range of 20–20,000 μg/ml and has a 14.7% mean coefficient of variance for repeat testing across 14 different human and non-human purified protein targets. Specimens were diluted 1:10 and 1:100 in PBS and run in duplicate. Colorimetric detection of test specimens was normalized to background specimens that contain extraction buffer only. Total protein concentration was estimated using an eight point standard curve and is expressed as μg/ml.
2.4. Statistical analysis
Immune marker values that were below the limit of detection and completely outside the dynamic range (therefore reported as undetectable by the Luminex instrument) were assigned ½ the lowest measured value in our sample [13]. Immune marker values that were below the manufacturer's published limit of detection, but a concentration was estimated by the instrument from the standard curve, were assigned that imputed value. The ratio of immune marker concentration to total protein for each specimen was created to minimize differences in collection across individuals and then this ratio was log-transformed to reduce the effect of outliers and normalize the distribution of values. This normalization procedure has been used in other settings involved in the measurement of local immune markers related to other sexually transmitted diseases such as HIV-1 [14,15], and minimizes the observed correlation of immune marker concentration and specimen weight (data not shown). Therefore, immune marker concentrations are expressed as [ng of immune marker]/[mg of total protein].
Differences in the median concentration of immune markers between HR-HPV positive and negative women were initially assessed using Wilcoxon rank-sum tests. Differences in the proportion of specimens that were determined to be completely undetectable by Luminex assay were compared between HR-HPV positive and negative women using Fisher's exact test. A p-value of <0.05 was considered statistically significant.
Spearman's rank correlation was estimated on each pair of immune markers in order to identify patterns of co-expression. A p-value of <0.01, after Sidak's correction for multiple comparisons, was considered statistically significant [16].
3. Results
3.1. Study population
Seventy-eight women were included in this study. Among this sample, 34 were determined to be HR-HPV positive. The median age of the sample was 45 (IQR: 39, 51); 27 (36.5%), 29 (39.2%), and 18 (24.3%) were identified as being pre-, peri-, and post-menopausal, respectively. There was no difference in age or menopausal stage by HR-HPV infection status.
3.2. HR-HPV positive women have higher concentrations of anti-inflammatory and allergy related immune markers
Compared to HR-HPV negative women, HR-HPV positive women had higher concentrations of IL-5 (p = 0.03), IL-9 (p = 0.04), IL-13 (p = 0.01), IL-17 (p = 0.003), EOTAXIN (p = 0.04), GM-CSF (p = 0.01), and MIP-1α (p = 0.005) (Table 1). Additionally, HR-HPV positive women had overall lower number of undetectable specimens for IL-17 (2.9% for HR-HPV positive vs. 25% for HR-HPV negative; p = 0.01) and EOTAXIN (32.4% for HR-HPV positive vs. 54.6% for HR-HPV negative; p = 0.04) (Table 1).
Table 1.
Differences in the median concentration and proportion of specimen outside the limit of detection of cytokine markers by HR-HPV status.
| Cytokine | Total (n = 78) | Median concentration (IQR)a | p-valueb | Outside the outer range of detection N (%) | p-valuec | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Median (IQR)a | OOR N (%) | HR-HPV −ve (n = 44) | HR-HPV +ve (n = 34) | HR-HPV −ve (n = 44) | HR-HPV +ve (n = 34) | |||
|
|
|
|||||||
| IL-1ra | 60,590 (19,781, 158,870) | 5 (6.4) | 60,591 (24,615, 147,800) | 46,999 (16,962, 198,572) | 0.57 | 4 (9.1) | 1 (2.9) | 0.27 |
| IL-1β | 32.8 (14.8, 122.9) | 1 (1.3) | 29.1 (10.6, 115.8) | 33.8 (18.1, 129.4) | 0.22 | 0 (0) | 1 (2.9) | 0.44 |
| IL-2 | 2.2(1.3, 3.1) | 0 (0) | 2.1 (1.2, 2.9) | 2.4 (1.6, 3.3) | 0.37 | 0 (0) | 0 (0) | – |
| IL-4 | 0.12 (0.01, 0.27) | 23 (29.5) | 0.11 (0.01, 0.26) | 0.16 (0.01, 0.30) | 0.49 | 12 (27.3) | 11 (32.4) | 0.80 |
| IL-5 | 0.10 (0.06, 0.13) | 0 (0) | 0.08 (0.05, 0.12) | 0.11 (0.07, 0.16) | 0.03 | 0 (0) | 0 (0) | – |
| IL-6 | 30.9 (9.6, 106.4) | 1 (1.3) | 24.3 (9.5, 96.7) | 35.6 (11.3, 117.7) | 0.56 | 0 (0) | 1 (2.9) | 0.44 |
| IL-7 | 0.93 (0.58, 1.57) | 0 (0) | 0.82 (0.57, 1.59) | 1.03 (0.58, 1.57) | 0.72 | 0 (0) | 0 (0) | – |
| IL-8 | 921963 (956, 3066168) | 42 (53.9) | 482709 (425, 2879034) | 1129549 (2296, 3244225) | 0.25 | 23 (52.3) | 19 (55.9) | 0.47 |
| IL-9 | 2.3 (1.4, 3.7) | 0 (0) | 2.1 (1.3, 3.3) | 2.7 (1.5, 4.5) | 0.04 | 0 (0) | 0 (0) | – |
| IL-10 | 10.2 (4.8, 15.2) | 0 (0) | 8.8 (4.6, 13.1) | 10.8 (4.8, 20.6) | 0.33 | 0 (0) | 0 (0) | – |
| IL-12p70 | 8.2 (4.2, 12.2) | 0 (0) | 7.5 (4.1, 10.9) | 8.4 (4.9, 15.7) | 0.26 | 0 (0) | 0 (0) | – |
| IL-13 | 1.3 (0.3, 2.5) | 23 (29.5) | 0.9 (0.2, 1.9) | 2.1 (0.9, 3.1) | 0.01 | 16 (36.4) | 7 (20.6) | 0.10 |
| IL-15 | 5.9 (3.9, 11.2) | 0 (0) | 5.8 (3.9, 10.9) | 6.5 (4.2, 11.6) | 0.69 | 0 (0) | 0 (0) | – |
| IL-17 | 2.6 (0.7, 3.5) | 12 (15.4) | 1.1 (0.01, 3.2) | 2.9 (1.6, 4.7) | 0.003 | 11 (25.0) | 1 (2.9) | 0.01 |
| EOTAXIN | 1.5 (0.6, 4.9) | 35 (44.9) | 1.1 (0.6, 2.9) | 4.1 (0.7, 6.3) | 0.04 | 24 (54.6) | 11 (32.4) | 0.04 |
| BASICFGF | 13.9 (6.5, 17.9) | 0 (0) | 12.1 (5.7, 17.5) | 16.1 (6.6, 22.5) | 0.18 | 0 (0) | 0 (0) | – |
| GCSF | 795.7 (138.9, 3548.8) | 0 (0) | 1144.6 (207.8, 4017.9) | 579.3 (106.5, 2843.9) | 0.42 | 0 (0) | 0 (0) | – |
| GMCSF | 3.7 (2.4, 5.6) | 0 (0) | 3.3 (2.1, 4.4) | 4.3 (2.9, 6.1) | 0.01 | 0 (0) | 0 (0) | – |
| IFN-γ | 23.2 (15.4, 31.7) | 0 (0) | 22.9 (13.7, 31.4) | 23.6 (15.9, 32.9) | 0.49 | 0 (0) | 0 (0) | – |
| IP-10 | 226.2 (92, 696.2) | 0 (0) | 260.4 (100.5, 644.6) | 210.4 (66.2, 772.1) | 0.41 | 0 (0) | 0 (0) | – |
| MCP-1 | 76.7 (21.8, 172.4) | 0 (0) | 78.7 (24.9, 178) | 51.3 (19.5, 172.4) | 0.78 | 0 (0) | 0 (0) | – |
| MIP-1α | 2.7 (1.4, 4.3) | 2 (2.6) | 1.9 (1.1, 3.6) | 3.5 (2.2, 4.4) | 0.005 | 2 (4.6) | 0 (0) | 0.32 |
| MIP-1β | 64.3 (30.3, 170.8) | 5 (6.4) | 62.2 (31.7, 155.3) | 73.8 (29.6, 213.6) | 0.79 | 5 (11.4) | 0 (0) | 0.07 |
| PDGBB | 22.5 (10.4, 33.0) | 0 (0) | 21.6 (10.2, 33.5) | 22.8 (10.4, 33.0) | 0.93 | 0 (0) | 0 (0) | – |
| RANTES | 12.5 (9.1, 22.0) | 0 (0) | 11.9 (8.2, 21.2) | 12.7 (9.8, 22.0) | 0.53 | 0 (0) | 0 (0) | – |
| TNF-α | 29.9 (18.7, 39.9) | 0 (0) | 29.9 (16.4, 38.8) | 29.1 (20.9, 41.7) | 0.46 | 0 (0) | 0 (0) | – |
| VEGF | 202.7 (106.7, 386.9) | 0 (0) | 188.3 (95.9, 371) | 232.8 (107.2, 415.5) | 0.35 | 0 (0) | 0 (0) | – |
ng immune marker/mg total protein.
Wilcoxon Ranksum.
Fishers exact test.
3.3. Shifts in the correlation of cytokines with T-cell related markers suggest different regulatory networks in HPV positive vs. HPV negative older women
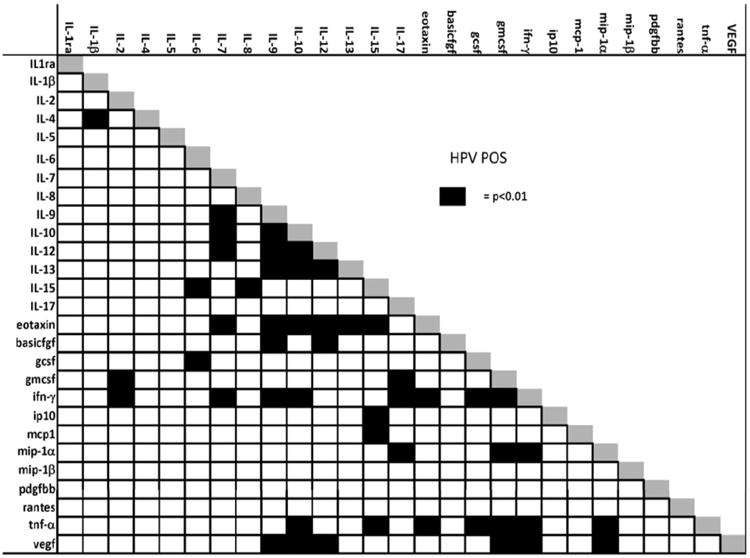
The correlation results are presented in Fig. 1a and 1b. Several notable differences between HR-HPV positive and negative women were observed. Among HR-HPV negative women, regulatory cytokines IL-5, IL-7, IL-9, IL-12, IL-15, IFN-γ, and TNF-α were significantly positively correlated with IL-2 (Table 2 and Fig. 1a). Furthermore, GM-CSF and PDGBB, which are related to monocyte function, were also significantly positively correlated with IL-2. Among HR-HPV positive women, IFN-γ, IL-7, IL-9, IL-12, IL-15, and TNF-α, were correlated with EOTAXIN, with only GM-CSF and IFN-γ remaining correlated with IL-2 (Table 2 and Fig. 1b). The immunoregulatory cytokines IL-10 and IL-13 were correlated with EOTAXIN, but not IL-2, in both HPV-negative and HPV-positive women. Furthermore, among HR-HPV negative women, IL-5 was significantly correlated with BASICFGF, GM-CSF, IFN-γ, IL-7, IL-9, IL-12 and TNF-α and GM-CSF was also correlated with IL-9, IL-12 and IL-15 (Table 2 and Fig. 1b).
Fig. 1.
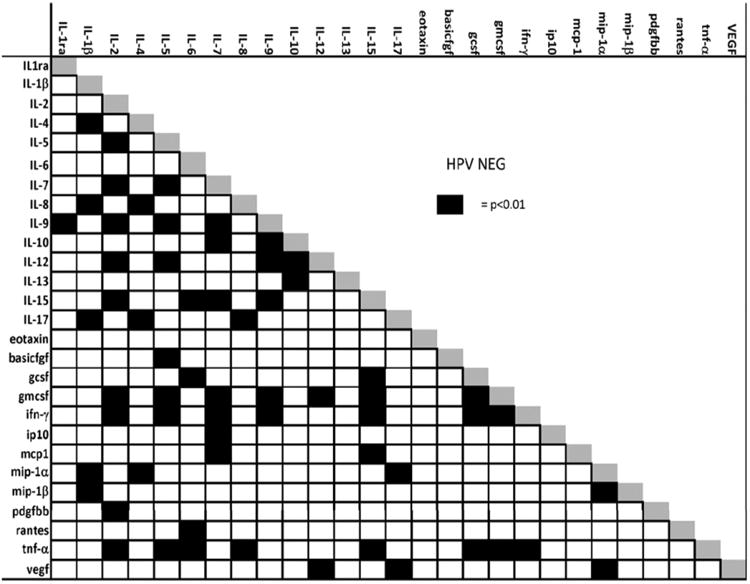
a. Correlation of immune markers measured from cervical secretion specimens among HPV negative women. Black squares indicate a p-value of <0.01 for the significance of the correlation using Spearman's rank correlation adjusting for multiple comparisons.
b. Correlation of immune markers measured from cervical secretion specimens among HPV positive women. Black squares indicate a p-value of <0.01 for the significance of the correlation between markers using Spearman's rank correlation adjusting for multiple comparisons.
Table 2.
Correlation of IL-2, EOTAXIN, GM-CSF, and IL-5 with select T-cell associated cytokines.
| IL-2 | EOTAXIN | GM-CSF | IL-5 | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| HPV negative | HPV positive | HPV negative | HPV positive | HPV negative | HPV positive | HPV negative | HPV positive | |
| IL5 | 0.7347*** | 0.3406 | 0.4020 | 0.2458 | 0.6934*** | 0.4848 | ||
| IL7 | 0.6991*** | 0.5829* | 0.3512 | 0.6837*** | 0.6354** | 0.6159* | 0.6771** | 0.1447 |
| IL9 | 0.7239*** | 0.4775 | 0.4507 | 0.7772*** | 0.7720*** | 0.5985 | 0.6037** | 0.2122 |
| IL10 | 0.4441 | 0.3867 | 0.5211* | 0.8451*** | 0.5401 | 0.5385 | 0.5415 | 0.2104 |
| IL12 | 0.5752*** | 0.4756 | 0.3398 | 0.7100*** | 0.6283** | 0.6024 | 0.6073** | 0.2697 |
| IL13 | −0.0016 | 0.2684 | 0.5092* | 0.7626*** | 0.0685 | 0.3892 | 0.3184 | 0.2541 |
| IL15 | 0.5963** | 0.4173 | 0.4478 | 0.6819*** | 0.6482** | 0.4732 | 0.4555 | 0.1804 |
| IFN-γ | 0.8125*** | 0.6639*** | 0.4610 | 0.7641*** | 0.9033** | 0.9025** | 0.7514*** | 0.4066 |
| TNF-α | 0.6451*** | 0.5013 | 0.5012* | 0.7629*** | 0.8750** | 0.813** | 0.6185** | 0.3311 |
| Basicfgf | 0.5044 | 0.4802 | 0.2771 | 0.4646 | 0.4826 | 0.5869 | 0.5891*** | 0.3565 |
| GM-CSF | 0.7700*** | 0.7525*** | 0.5081 | 0.5765 | 0.6934*** | 0.4848 | ||
p < 0.05.
p < 0.01.
p < 0.001.
4. Discussion
We show a significant difference in the immunologic micro-environment in HPV-positive vs. HPV-negative women aged 35–60 years. The immunoregulatory cytokine shift from IL-2 to EOTAXIN suggests a novel relationship of HPV with the host immune response in this group of women, with a possibly enhanced role of innate responses and a loss of regulation of antigen-specific adaptive responses.
Older HR-HPV positive women showed higher concentrations of cytokine markers that are prototypically associated with non-specific innate immunity seen in such instances as responses to environmental allergens. IL-5 and IL-13, which were elevated among the HR-HPV positive women, are considered cytokines responsible for mediating responses to airway pathogens through activation and control of eosinophils [17–19]. EOTAXIN is a chemokine principally responsible for recruitment and regulation of eosinophils and basophils. Eosinophils have also been explored as mediators of tumor immunity, and eosinophilia has been explored as a potential prognostic marker in cancers of the oral cavity [20]. Higher concentrations of IL-5-producing cells have also been detected in peripheral blood mononuclear cells of HPV positive women with cervical cancer [21].
Granulocyte macrophage colony stimulating factor (GM-CSF) and macrophage inflammatory protein-1 (MIP-1α) were also more highly expressed in the HR-HPV positive women. These are chemokines produced by epithelial cells and macrophages, respectively, and help recruit and stimulate granulocytes such as neutrophils, eosinophils and basophils [22,23]. Higher concentrations of plasma GM-CSF and MIP-1α have been previously associated with persistent HPV infection in older women, and GM-CSF has been tested as a potential therapeutic agent used to treat cervical low-grade squamous intraepithelial lesions [5,24].
Interleukin-9 is produced primarily by T-cells of a recently characterized lineage known as “Th9” that has been shown to mediate chronic allergic responses as well as protection against helminth parasite challenge [25]. More recently, IL-9, along with IL-4, IL-5, and IL-13, have been implicated as effector molecules of newly identified innate immune cells responsible for the initiation of type-2 responses against various infectious agents such as helminth parasites [26]. These same type-2 and anti-inflammatory T-cell responses are associated with an increased risk of HPV viral persistence and development of pre-cancerous lesions [27].
Interleukin-17, also increased in HR-HPV positive women, is a pro-inflammatory cytokine of a separate T-cell lineage known as Th17, which is associated with localized tissue inflammation that has been shown to play a role in response to bacterial and parasitic challenge. IL-17 has also recently been linked to asthma and heightened responses to airway allergens [28]. In addition to asthma, higher proportions of IL-17 producing CD4+ T-cells were observed in the peripheral blood of women with cervical intraepithelial neoplasia [29].
A majority of the markers measured in the current study have been shown to be either co-produced by the same immune cell subsets, or produced by cells that interact and regulate one another in a highly dynamic state to produce a specific immunologic phenotype. Correlation analysis offers a simple analytic technique to better explore the possible interactions between the immunologic markers [30]. A key observation in this study was the lack of correlation of IL-2, a key effector molecular of T-cell function, with other T-cell lineage specific effector molecules such as IL-5, IL-7, IL-9, IL-12, IL-15 and their pro-inflammatory downstream markers such as TNF-α in HPV positive women. Instead, these regulatory cytokines were increased in significance and strength of correlation with EOTAXIN in the HR-HPV positive women. It is tempting to speculate whether the shift from IL-2 to EOTAXIN is suggestive of the presence of a higher number of granulocytes, specifically cells such as eosinophils, basophils, mast cells, and neutrophils in the cervix of older, HR-HPV positive women, which may be mediating local T-cell responses. This hypothesis is supported in the data by the correlation of GM-CSF with IL-5, IL-7, IL-9, IL-12, and IL-15 in HPV negative women, which was not observed in HPV positive women. Experimental models might be useful to determine whether the shift in correlation of these T-cell markers with GM-CSF represents a loss of control in leukocyte production in favor of granulocytic cell types, which in turn induce non-specific inflammatory responses in the localized tissue.
Inferences regarding the temporal relationship of HPV and the host response are limited by the cross-sectional design of this study. It is difficult to discern whether the differences observed between HPV-negative and -positive women are caused by HPV infection, or represent an immunologic microenvironment that leads to enhanced detection of HPV (i.e., reactivation). In addition, we are unable to determine if the immunologic associations observed in the women in our study would be observed in younger women.
Some of the markers measured in this study were at the low end of the dynamic range of detectability for this assay and had individual values that were either completely undetectable or assigned by the luminex instrument. As a result, the assignment of ½ the lowest value and the inclusion of estimated marker concentrations based on extrapolation of the standard curve may inadvertently introduce bias leading to observed differences in median marker concentration and correlations by HPV infection status. We approached this limitation by first assessing differences in the proportion of completely undetectable marker values by HPV DNA status. Secondly, we performed a sensitivity analysis that excluded all values that were either completely undetectable or outside the dynamic range of the assay. We observed very little change in the magnitude of the difference in median marker concentration by HPV status after exclusion other than a loss of statistical significance due to reduced sample sizes. Furthermore, the observed differences in correlation of immune markers by HPV status remained the same. However, additional work is currently underway to validate these observations in a larger sample.
The higher cervical concentrations of specific immunologic markers in HR-HV positive women, combined with global shifts in the correlation structure towards a phenotype typically related to allergic inflammation, offer important new data relevant to our understanding of HPV infections at older ages. Specifically, these data support a hypothesis for a potential role of granulocyte populations and other non-antigen specific immune cells in mediating HPV host response in older women that can be further explored in both observational and experimental study designs.
Supplementary Material
Acknowledgments
We would like to thank Chris Thoburn for the collection of immune marker data as well as his feedback and comments regarding data collection and analysis.
Funding: This work was supported by the US National Cancer Institute R01 CA123467 and the Institutional Research Cancer Epidemiology Fellowship funded by the National Cancer Institute T32 CA0009314.
Footnotes
Conflict of interest: No author has a personal or financial conflict of interest in this work.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.cyto.2011.09.012.
References
- 1.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. HPV in the etiology of human cancer. Vaccine. 2006;24S3:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. Chapter 1. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 3.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(1):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Pineres AJ, Hildesheim A, Herrero R, Trivett M, Williams M, Atmetlla I, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66:11070–6. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 5.Kemp TJ, Hildesheim A, Garcia-Pineres A, Williams MC, Shearer GM, Rodriguez AC, et al. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2010;19:1954–9. doi: 10.1158/1055-9965.EPI-10-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castle PE, Hildesheim A, Bowman FP, Strickler HD, Walker JL, Pustilnik T, et al. Cervical concentrations of interleukin-10 and interleukin-12 do not correlate with plasma levels. J Clin Immunol. 2002;22:23–7. doi: 10.1023/a:1014252402630. [DOI] [PubMed] [Google Scholar]
- 7.Kemp TJ, Garcia-Pineres A, Falk RT, Poncelet S, Dessy F, Giannini SL, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26:3608–16. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravitt PE, Hildesheim A, Herrero R, Schiffman M, Sherman ME, Bratti MC, et al. Correlates of IL-10 and IL-12 concentrations in cervical secretions. J Clin Immunol. 2003;23:175–83. doi: 10.1023/a:1023305827971. [DOI] [PubMed] [Google Scholar]
- 9.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutlee F, Gravitt P, Kornegay J, Hankins C, Richardson H, Lapointe N, et al. Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol. 2002;40:902–7. doi: 10.1128/JCM.40.3.902-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, et al. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95:1128–37. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 12.Bio-Rad Laboratories I. Bio-Plex suspension array system tech note. Hercules, CA: 2005. [Google Scholar]
- 13.Bio-Rad Laboratories I. Bio-Plex pro human cytokine, chemokine, and growth factor assays technical bulletin. 2009:1–4. [Google Scholar]
- 14.Jespers V, Francis SC, van de Wijgert J, Crucitti T. Methodological issues in sampling the local immune system of the female genital tract in the context of HIV prevention trials. Am J Reprod Immunol. 2011;65:368–76. doi: 10.1111/j.1600-0897.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 15.Jespers V, Harandi AM, Hinkula J, Medaglini D, Le Grand R, Stahl-Hennig C, et al. Assessment of mucosal immunity to HIV-1. Expert Rev Vaccine. 2010;9:381–94. doi: 10.1586/erv.10.21. [DOI] [PubMed] [Google Scholar]
- 16.UCLA: Academic technology services SCG. [accessed 17 July 2011];Sidak-Holm: adjusted p-values. Available from: http://www.ats.ucla.edu/stat/stata/code/sidak_holm.htm.
- 17.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immun. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 18.Rothenberg ME, Zimmermann N, Mishra A, Brandt E, Birkenberger LA, Hogan SP, et al. Chemokines and chemokine receptors: their role in allergic airway disease. J Clin Immunol. 1999;19:250–65. doi: 10.1023/a:1020531322556. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immun. 2003;111:227–42. doi: 10.1067/mai.2003.139. quiz 243. [DOI] [PubMed] [Google Scholar]
- 20.Martinelli-Klay CP, Mendis BR, Lombardi T. Eosinophils and oral squamous cell carcinoma: a short review. J Oncol. 2009:310132. doi: 10.1155/2009/310132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–55. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–8. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 23.Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–604. [PubMed] [Google Scholar]
- 24.Hubert P, Doyen J, Capelle X, Arafa M, Renoux V, Bisig B, et al. Am J Reprod Immunol. Vol. 64. New York, NY: 2010. Local applications of GM-CSF induce the recruitment of immune cells in cervical low-grade squamous intraepithelial lesions; pp. 126–36. [DOI] [PubMed] [Google Scholar]
- 25.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–8. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neill DR, McKenzie AN. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol. 2011;27:214–21. doi: 10.1016/j.pt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109:S15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–98. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Ma D, Tian Y, Wang X, Qiao Y, Cui B. The imbalance of Th17/Treg in patients with uterine cervical cancer. Clin Chim Acta. 2011;412:894–900. doi: 10.1016/j.cca.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Ryckman KK, Simhan HN, Krohn MA, Williams SM. Cervical cytokine network patterns during pregnancy: the role of bacterial vaginosis and geographic ancestry. J Reprod Immunol. 2009;79:174–82. doi: 10.1016/j.jri.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


