Abstract
Context
Inhalation of fine particulate matter (PM2.5) is associated with acute pulmonary inflammation and impairments in cardiovascular function. In many regions, PM2.5 is largely derived from diesel exhaust (DE), and these pathophysiological effects may be due in part to oxidative stress resulting from DE inhalation. The antioxidant glutathione (GSH) is important in limiting oxidative stress-induced vascular dysfunction. The rate-limiting enzyme in GSH synthesis is glutamate cysteine ligase and polymorphisms in its catalytic and modifier subunits (GCLC and GCLM) have been shown to influence vascular function and risk of myocardial infarction in humans.
Objective
We hypothesized that compromised de novo synthesis of GSH in Gclm−/+ mice would result in increased sensitivity to DE-induced lung inflammation and vascular effects.
Materials and methods
WT and Gclm−/+ mice were exposed to DE via inhalation (300 µg/m3) for 6 h. Neutrophil influx into the lungs, plasma GSH redox potential, vascular reactivity of aortic rings and aortic nitric oxide (NO•) were measured.
Results
DE inhalation resulted in mild bronchoalveolar neutrophil influx in both genotypes. DE-induced effects on plasma GSH oxidation and acetylcholine (ACh)-relaxation of aortic rings were only observed in Gclm−/+ mice. Contrary to our hypothesis, DE exposure enhanced ACh-induced relaxation of aortic rings in Gclm−/+ mice.
Discussion and conclusion
These data support the hypothesis that genetic determinants of antioxidant capacity influence the biological effects of acute inhalation of DE. However, the acute effects of DE on the vasculature may be dependent on the location and types of vessels involved. Polymorphisms in GSH synthesis genes are common in humans and further investigations into these potential gene-environment interactions are warranted.
Keywords: Diesel exhaust, glutathione, lung inflammation, nitric oxide, oxidative stress
Introduction
There is substantial evidence that exposure to fine ambient particulate matter (PM2.5) is associated with an increased risk of cardiopulmonary mortality (Dockery et al., 1993; Pope et al., 2002, 2004). From these initial observations, there have been further epidemiological investigations to understand the role of PM2.5 in eliciting acute myocardial events with both chronic exposures (Miller et al., 2007) and acute exposures resulting from transient excursions in ambient PM2.5 levels (Peters et al., 2001, 2000; Rich et al., 2005, 2006).
In many urban regions, ambient PM2.5 is largely derived from diesel exhaust (DE) emissions (Lewtas, 2007), thus many investigators have utilized controlled DE exposure facilities to investigate the pulmonary and cardiovascular effects of PM2.5 exposure from DE. Although the exact pathways by which PM2.5 and DE inhalation cause adverse effects are not fully elucidated, numerous studies investigating PM2.5 and DE toxicity have highlighted the role of oxidative stress in both the onset of inflammation and on bioavailable nitric oxide (NO•) within the vasculature, both of which are implicated as principle components in the observed impairments in vascular function and increased risk of acute myocardial infarction (Cherng et al., 2009, 2011; Kampfrath et al., 2011; Knuckles et al., 2008; Mudway et al., 2004; Pourazar et al., 2005; Weldy et al., 2011a,b). In addition, there is limited but supportive evidence to suggest that oxidative stress occurs in humans following inhalation of PM2.5 (Baccarelli et al., 2007; Bräuner et al., 2007; Chuang et al., 2007; Romieu et al., 2005, 2008; Sørensen et al., 2003; Vinzents et al., 2005). Together, evidence from in vitro, in vivo, and epidemiological studies support the notion that oxidative stress plays an important physiological role in the onset of cardiovascular toxicity following PM2.5 and DE inhalation, and thus the status of antioxidants is an important factor in mitigating these adverse effects.
We recently demonstrated that the de novo synthesis of the antioxidant glutathione (GSH) plays an important role in mediating pulmonary inflammation resulting from intranasal instillation of diesel exhaust particulate (DEP) (Weldy et al., 2011a). This finding was in support of our previous in vitro observation whereby increased GSH synthesis occurred in endothelial cells following both direct DEP exposure and following exposure to soluble factors released from DEPtreated macrophage cells (Weldy et al., 2011b). These findings suggest that the de novo synthesis of GSH plays a protective role in antioxidant defense and proinflammatory responses following DEP exposure. Taken together, these findings suggest that individuals who have compromised de novo GSH synthesis may be more sensitive to the adverse pulmonary and cardiovascular effects of PM2.5 inhalation.
GSH is a tripeptide thiol that is present in millimolar concentrations in certain cells such as hepatocytes. Due to its stereochemistry and bioavailable cysteine, GSH participates in redox cycling to maintain a reduced state in the cell through the action of GSH peroxidases (GPxs) and glutathione disulfide (GSSG) reductase (GRx) (Franklin et al., 2009). In many tissues GSH is the principal determinant of the intracellular redox state (Dalton et al., 2004), and numerous reports from Loscalzo, Stamler, and their colleagues have shown the clinical importance of GSH redox in vascular disease (Espinola-Klein et al., 2007; Jin et al., 2011; Leopold et al., 2007; Maron et al., 2009; Stamler et al., 1988; Weiss et al., 2001). GSH is synthesized in a two-step process; the first and rate-limiting step is carried out by the heterodimeric enzyme glutamate cysteine ligase (GCL), which is composed of catalytic (GCLC) and modifier (GCLM) subunits. Synthesis of GSH is determined by the total GCL activity within the cell, which is regulated by both GCL enzyme level, by GCLC and GCLM transcription, and by holoenzyme formation (Bea et al., 2003, 2009; Dickinson et al., 2004; Franklin et al., 2009; Krejsa et al., 2010; Lu, 2009; Shenvi et al., 2012; Wild & Mulcahy, 2000).
Because the activity of GCL and the promoters of both GCLC and GCLM will influence GSH content, and since we have previously demonstrated that GSH synthesis plays an important protective role in mediating DEP-induced inflammation, it is important to investigate the potential of genetic variations in GSH synthesis genes to influence cardiovascular response to DE inhalation. It has been previously demonstrated that single nucleotide polymorphisms in the 5′ promoter regions of both GCLC and GCLM lead to compromised promoter activity and are associated with an increased risk of myocardial infarction, impaired vasomotor function and dilated cardiomyopathy in a Japanese population (Koide et al., 2003; Nakamura et al., 2002, 2003; Watanabe et al., 2013). Although the effects of these polymorphisms are relatively small (one copy of the −588T GCLM allele results in ~2-fold increased risk of MI), their frequency (e.g. ~20% of the population have at least one GCLM −588T allele) highlights their importance to public health in the context of air pollution and cardiovascular disease.
Our previous studies have provided evidence that the Gclm−/+ mouse is a unique model for investigating the role of GSH and its de novo synthesis in mediating biological responses to toxicant exposures (McConnachie et al., 2007; Weldy et al., 2011a, 2012). As opposed to Gclm−/− mice, which can have GSH levels within tissues that range from 5% to 15% of wild type (WT) mice, Gclm−/+ mice typically have GSH levels that range from 70% to 90% of WT. Paradoxically, Gclm−/− mice appear to be protected from certain oxidative challenges, such as DEP (Weldy et al., 2011a) and ozone (Johansson et al., 2010). This lack of sensitivity of Gclm−/− mice appears to be due to dramatic compensatory responses that are still being investigated (Haque et al., 2010; Weldy et al., 2012). Alternatively, Gclm−/+ mice do not appear to upregulate the same compensatory response genes (or at least to the same magnitude as Gclm−/− mice), and are thus more sensitive to certain challenges such as DEP-induced lung inflammation (Weldy et al., 2011a). This heightened sensitivity is likely due to an inability to further upregulate Gclm and to engage in de novo GSH synthesis at a rate that is adequate to provide sufficient antioxidant protection. Importantly, we propose that Gclm−/+ mice are more similar to humans with GCL polymorphisms than Gclm−/− mice, as individuals with these polymorphisms have only modest reductions in GSH, as opposed to the dramatic loss of GSH that is present in Gclm−/− mice. Accordingly, investigating the effects of DE inhalation on Gclm−/+ mice provides a valuable opportunity to not only understand the mechanisms of DE-induced pathophysiology, but also provides insight into the potential susceptibility of humans with GCL polymorphisms to PM2.5 exposures.
In this report, we investigated the effect of a 6 h DE inhalation (300 µg/m3) on neutrophilic airway inflammation, plasma GSH, GSSG and GSH reduction potential, aortic ring vascular reactivity by wire myography, as well as aortic NO• production by spin trap and electron spin resonance (ESR) in WT and Gclm−/+ mice. Since acute DE inhalation studies have been previously observed to cause pulmonary inflammation and impair vascular NO• production in a manner that is consistent with the generation of reactive oxygen species (ROS) and subsequently oxidative stress, we hypothesized that mice heterozygous for Gclm would be more sensitive to DE-induced effects due to a compromised ability to maintain sufficient GSH stores and thus be more sensitive to oxidative challenge, as measured by enhanced pulmonary inflammation, oxidation of the plasma GSH redox, and impaired aortic NO• generation and vascular reactivity.
Materials and methods
Mice and filtered air or diesel exhaust exposure
Gclm wild-type (WT) and Gclm−/+ mice were backcrossed for at least 10 generations onto the C57BL/6 background and were bred and housed in a modified specific pathogen-free (SPF) vivarium at the University of Washington. All animal experiments were approved by the University of Washington Institutional Animal Care and Use Committee. Male and female littermates were genotyped as previously described (McConnachie et al., 2007) and randomly assigned to either filtered air (FA) or diesel exhaust (DE) exposure treatments. For studies on lung inflammation, an equal number of male and female mice were used in both WT and Gclm−/+ genotypes, whereas in all additional studies on vascular reactivity, plasma GSH, and aortic NO•, only male mice were used. Mice were transferred to our diesel exposure facility where exposures to either FA or DE were conducted simultaneously in an Allentown caging system (Allentown, NJ) under SPF conditions. DE (300 µg/m3) was generated from a single cylinder Yanmar diesel engine (model YDG5500EV-6EI) operating on 75% load as previously described (Gould et al., 2008). A DE exposure concentration of 300 µg/m3 was chosen as this is a relevant exposure concentration in certain occupational settings such as mining (Janisko & Noll, 2010), and is consistent with prior studies conducted investigating the vascular effects of acute DE inhalation (Campen et al., 2010; Cherng et al., 2009, 2011; Knuckles et al., 2008).
Bronchial alveolar lavage, cell staining and flow cytometry
Mice were sacrificed by Isoflurane narcosis followed by cervical dislocation immediately after a 6-h exposure to either FA or DE. To perform bronchoalveolar lavage (BAL), the peritoneal, thoracic and cervical areas were carefully opened and the trachea was surgically isolated. A small incision was made in the trachea just below the larynx and an 18 G catheter attached to a 1 ml syringe was inserted to perform the lavage. One milliliter of phosphate-buffered saline (PBS) was slowly instilled into the lungs and subsequently withdrawn. This rinsing action was repeated 3× per wash, and three 1 ml washes were performed for each mouse. The lavage sample from the first wash was collected independently and placed into a 1.5 ml microcentrifuge tube, while the lavage from the second and third washes were combined. Cells in the lavage samples were then pelleted by centrifugation at 200 × G for 15 min at 4 °C. The supernatant from the first wash was collected for future analyses, whereas the supernatants from the 2nd and 3rd washes were discarded. Cells from all three washes were combined, treated with a red blood cell (RBC) lysis buffer (ammonium chloride lysing solution; 1.5 M NH4Cl, 10 mM NaHCO3, 1 mM disodium EDTA, in dH2O) at room temperature for 5 min, blocked for 30 min with 1% bovine serum albumin and 5% rat serum, and then subsequently stained for 15 min with primary antibodies directed against F4/80 antigen conjugated with Alexafluor 488 (eBioscience, San Diego, CA; Cat# 53-4801-80), phycoerythrin conjugated anti-mouse Ly-6 G/Ly6C (Gr1; BioLegend, San Diego, CA; Cat# 108404), and biotinylated anti-mouse CD11b. Subsequently, streptavidin Alexafluor 350 (Invitrogen, Carlsbad, CA; Cat# S11249) was added. Cells were analyzed on a Beckman-Coulter Altra fluorescence activated cell sorter (FACS) (Beckman-Coulter, Miami, FL), and 10 000 cells were examined for each animal. Neutrophils were identified as cells expressing low F4/80, high Gr1, and very high CD11b (F4/80lo/Gr1hi/CD11bvhi). A total of 48 mice were used in the assessment of neutrophil influx, 24 male and 24 female, equal numbers in each genotype (12 WT-FA, 12 WT-DE, 12 Gclm−/+-FA, 12 Gclm−/+-DE).
To determine if DE exposure resulted in uptake of DEP by alveolar macrophages, we performed BAL on male WT mice following a 6-h exposure to either FA or DE as described above. Fifty microliter samples of washed resuspended cells were further diluted with 450 µL PBS, placed into Shandon Cytospin cartridges (ThermoScientific, Waltham, MA), and the cartridges were centrifuged at 600 rpm for 10 min. Deposited cells were stained using a Diff-Quik Fixative (Siemens Healthcare Diagnostics, Newark, DE). Bright field images of representative alveolar macrophages were taken at 40× magnification using a Nikon Optiphot microscope (Nikon USA, Melville, NY).
Assessment of plasma GSH reduction potential by HPLC
Whole blood was collected directly into a heparin-coated plasma collection tube and inverted three times to prevent clotting. Blood was immediately centrifuged at 6000 rpm for 2 min in a microcentrifuge to separate out plasma from RBCs, and 100 µL of plasma was removed and diluted 1:1 with 10% 5-sulfosalicylic acid (SSA) to stabilize GSH and precipitate proteins. The plasma and acid mix was incubated on ice for 10 min, and then centrifuged at 15 600 × Gin a microcentrifuge for 2 min to obtain deproteinated supernatants. These were transferred to a cryotube, purged with argon and stored in liquid N2 for two weeks. Practice samples were measured immediately after collection and after two weeks of storage to ensure oxidation of plasma GSH did not take place. Concentrations of GSH and GSSG present in the original homogenate were determined by high-pressure liquid chromatography (HPLC) using a modification of previously described methods (Eaton & Hamel, 1994; Thompson et al., 1999). Briefly, for GSH measurements, deproteinized supernatant was mixed with monobromobimane (MBB) to derivatize GSH and measured by HPLC with fluorescence detection. For GSSG measurements, 2-vinylpiridine was added to a portion of the supernatant to react with GSH. Residual 2-vinylpiridine was then removed with chloroform extraction, and the remaining GSSG was reduced to GSH with tris(2-carboxyethyl)phosphine (TCEP; 10 µM), derivatized with MBB and measured by HPLC as above. Glutathione reduction potential (ΔEGSSG/2GSH) was calculated using the Nernst Equation, assuming a pH of 7.2 and 37 °C as previously described (Dalton et al., 2004). Twentyfour male mice were used for this experiment (6WT-FA, 6WT-DE, 6 Gclm−/+-FA, 6 Gclm−/+-DE).
Vascular reactivity
Following FA or DE exposures, aortas from male WT and Gclm−/+ mice were cut into 3-mm rings and transferred to an organ bath containing 6 ml of physiological saline solution (119 mM NaCl, 4.7 mM KCl, 2.4 mM MgSO4, 1.2 mM KH2PO4, 3.3 mM CaCl2, 25 mM NaHCO3, 30 µM EDTA, 6 mM dextrose), equilibrated with 95% O2 and 5% CO2. Buffer was maintained at 37 °C, pH 7.4. Aortic rings were hung with wire to a force transducer (Model 610 M, Danish Myo Technology, Aarhus, Denmark), and the transducer was interfaced to a Powerlab 8/26 recorder for measurement of isometric force. Rings were placed under an initial tension of 20 mN and equilibrated for 1 h. Ring contraction was measured using PE hydrochloride (Sigma-Aldrich, St. Louis, MO), and endothelium-dependent and -independent relaxations were measured using ACh and sodium nitroprusside, respectively. Relaxation curves were determined following ring pre-contraction with 3 × 10−7M PE. A total of 50 male mice were used for these vascular reactivity studies (n = 8 for both WT FA and WT DE; n = 17 for both Gclm−/+ FA and Gclm−/+ DE).
Detection of aortic NO• production by Fe(DETC)2 spin trap and ESR
Aortic NO• production was detected in male WT and Gclm−/+ mice following 6-h FA or DE inhalation by methods previously described (Khoo et al., 2004). Aortas were quickly removed after sacrifice (as above), and the aortic vessel, along with the perivascular adipose tissue was incubated in a Krebs/HEPES buffer (99 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4; 1 mM KH2PO4, 1.9 mM CaCl2, 25 mM NaHCO3, 11.1 mM glucose, 20 mM HEPES) adjusted to pH 7.4. Colloidal iron diethyldithiocarbamate (Fe/DETC)2 spin trap was prepared by dissolving sodium DETC (3.6 mg) and FeSO4 7H2O (2.25 mg) under argon gas in 10 ml of ice cold Krebs-HEPES buffer. Aortas were then incubated at 37 °C in the (Fe/DETC)2 spin trap for 60 minutes. Immediately after incubation, three aortas of the same genotype and treatment were combined and placed into a 1 ml syringe (with the end cut off) and frozen in liquid nitrogen. The frozen pellet was then pressed out of the syringe and stored at −80 °C until NO• detection by electron spin resonance (ESR) spectroscopy. ESR studies were performed on a table-top x-band spectrometer Miniscope (Magnettech, Berlin, Germany). Measurements were taken on samples placed in a Dewar tube and kept in liquid nitrogen. Instrument settings were: biofield 3275, sweep 115 G, microwave frequency 9.78 GHz, microwave power 20 mW, and a kinetic time of 10 min. A total of 54 mice were used for this study (n = 3, 4, 5 and 6 for WT FA, WT DE, Gclm−/+ FA and Gclm−/+ DE, respectively, where each n represents aortic NO• measured from 3 mouse aortas of the same genotype and treatment).
Statistical analyses
Data were analyzed using Prism (Graphpad Software, La Jolla, CA). Differences were determined by ANOVA followed by a Dunnett’s post-hoc test. All error bars in figures represent standard error of the mean (SEM). *, **, *** = Significant difference from the matched control at p values of <0.05, 0.01 and 0.001, respectively. Vascular reactivity was analyzed by repeated-measurement 2-way ANOVA. Concentration-response curves were fitted with a nonlinear regression program (GraphPad Prism) to obtain effective concentration of 50% (EC50) and maximal effect values, which were compared by 1-way ANOVA.
Results
BAL, alveolar macrophage uptake of DEP, and neutrophilic lung inflammation
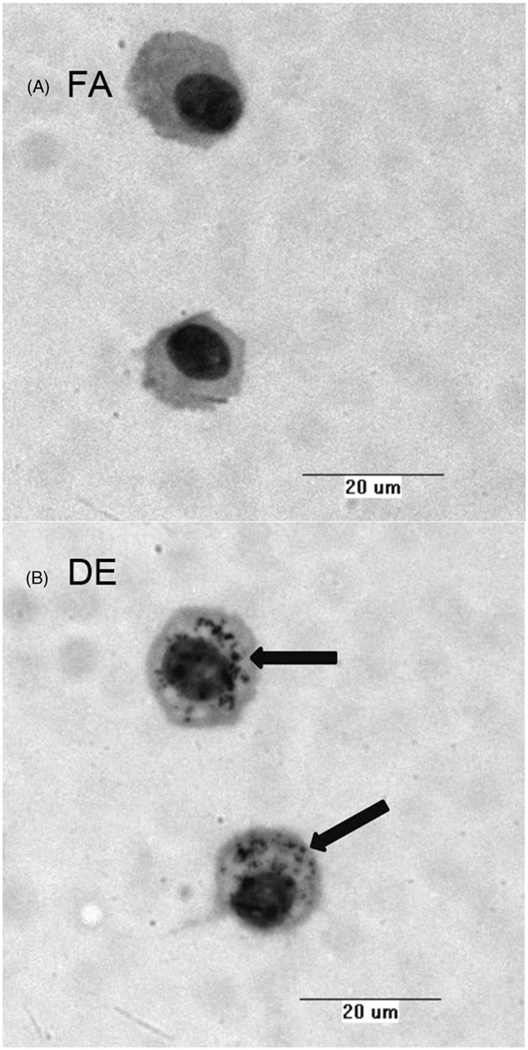
Images of alveolar macrophages isolated from BAL following acute exposure to DE (6 h, 300 µg/m3) show apparent uptake of DEP (Figure 1). After the washing steps taken during the preparation of cytospins, macrophages from DE exposed mice clearly show black DEP still associated with the cells, whereas such material was not observed in macrophages collected from FA-exposed mice.
Figure 1.
Alveolar macrophages collected by BAL of mice treated with either filtered air (FA) or diesel exhaust (DE) for 6 h. Images reveal diesel exhaust particulate taken up into the cell in DE-exposed mice.
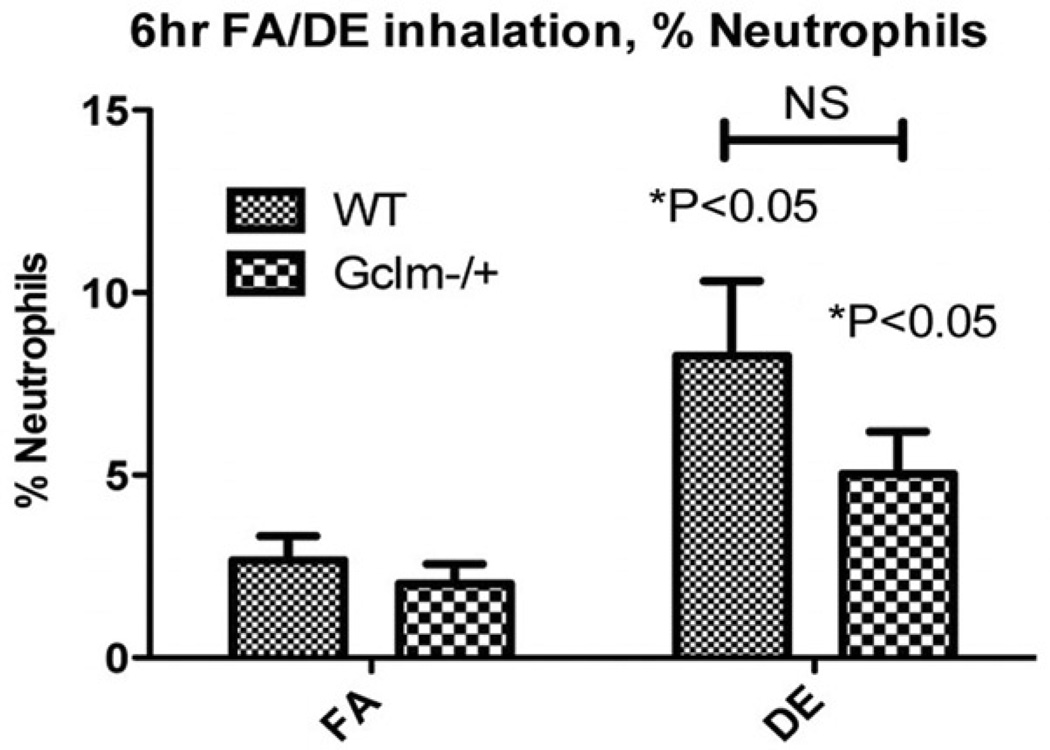
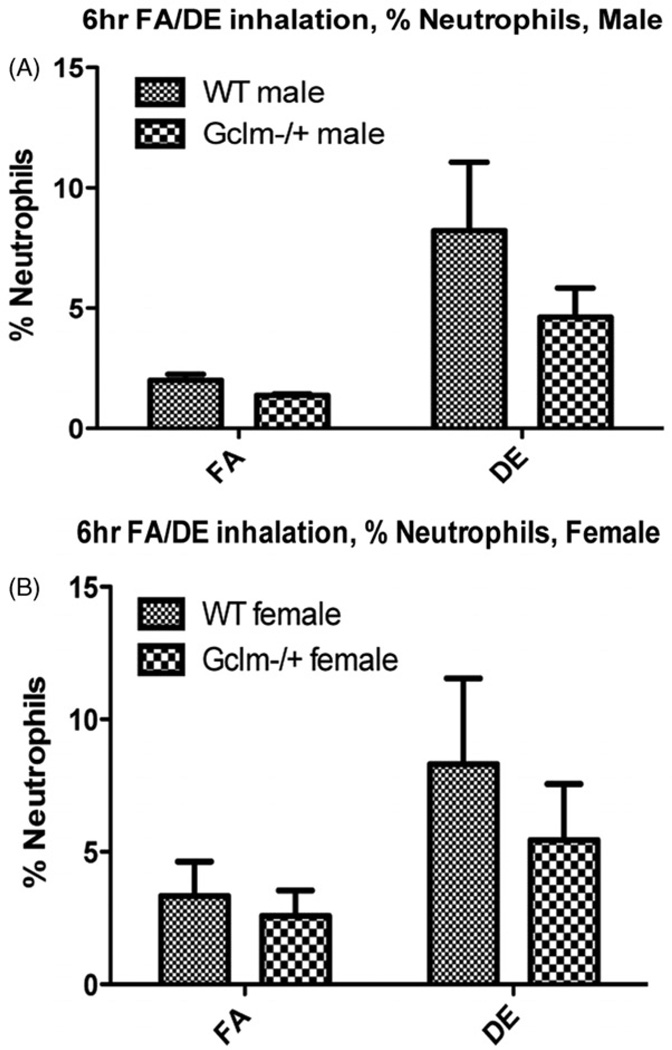
DE exposure produced a small but significant increase in neutrophils within the lungs of both WT and Gclm−/+ mice (Figure 2). There is a trend whereby Gclm−/+ mice had slightly less neutrophilic inflammation, but this did not reach statistical significance (p = 0.18). As we had performed this experiment with both male and female mice, we stratified our data to see if there was a sex-dependent response to DE exposure. No evidence for a differential effect in males versus females was found (Figure 3), and the trend of fewer BAL neutrophils in Gclm− + mice after DE inhalation, remained present for both sexes.
Figure 2.
Percent neutrophils (Gr1hi/F4/80lo/CD11bvhi) as measured by the FACS analysis of BAL cells collected from FA and DE-exposed male and female mice. N = 12 for each genotype and treatment with equal number of each sex in each group. *Significantly different (p < 0.05) compared to genotype specific FA control by ANOVA and Dunnett’s t-test.
Figure 3.
Stratification of percent neutrophils in BAL by sex. The data reveal nearly identical trends for each sex.
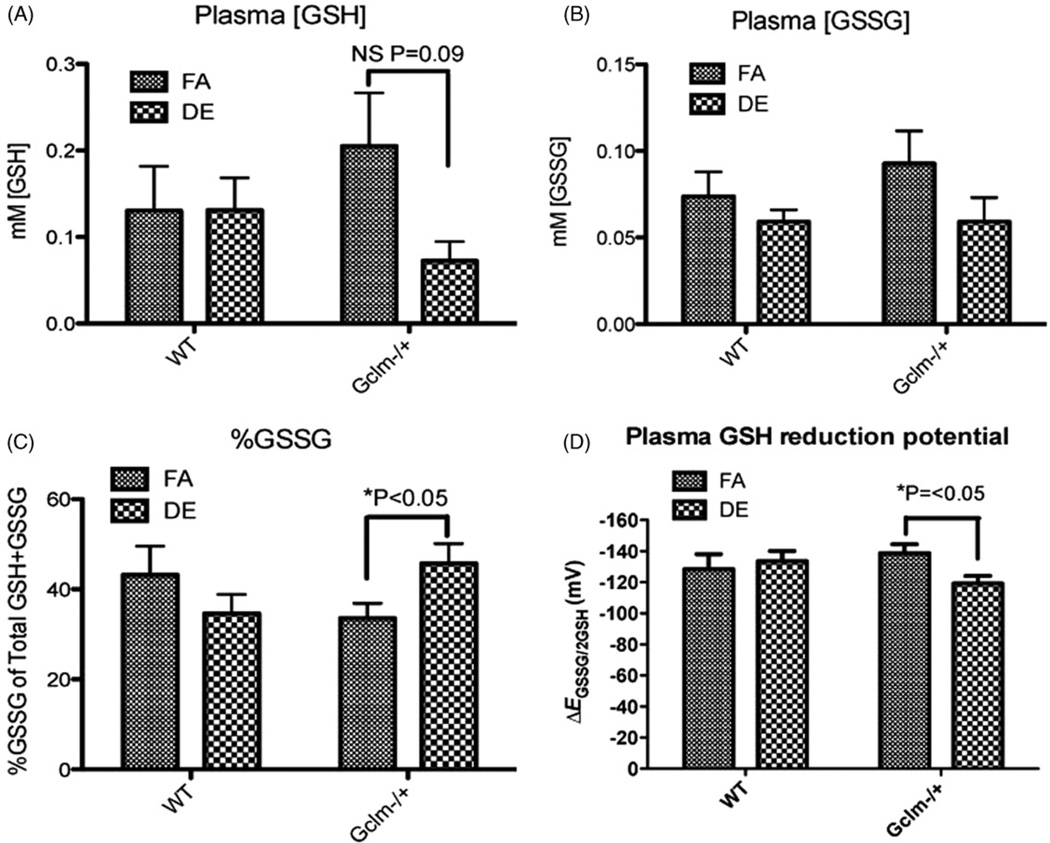
Plasma GSH, GSSG, %GSSG and GSH reduction potential (ΔEGSSG/2GSH)
To investigate the effects of DE on systemic oxidative stress, we measured the concentrations of reduced (GSH) and oxidized (GSSG) glutathione in the plasma of male WT and Gclm−/+ mice. DE exposure did not have any significant effect on plasma GSH in WT mice. Although there was an apparent decrease in plasma GSH in Gclm−/+ mice, statistical significance was not achieved (p = 0.09) (Figure 4A). Similarly, we observed slight trends for a DE-induced decrease in GSSG in both WT and Gclm−/+ mice, but again, this effect did not reach statistical significance (WT, p = 0.39; Gclm−/+, p = 0.19) (Figure 4B). Nonetheless, DE inhalation did significantly increase %GSSG, resulting in a more oxidized redox poise (i.e. a less negative ΔEGSSG/2GSH reduction potential) in the plasma of Gclm−/+ mice (Figure 4, panels C and D). We did not observe this effect in the plasma of WT mice. Interestingly, when comparing across FA control groups, ΔEGSSG/2GSH was not oxidized in the plasma of Gclm−/+ mice compared to WT mice (Figure 4, panel D).
Figure 4.
Plasma [GSH] (A), plasma [GSSG] (B), %GSSG of total glutathione (C), and GSH reduction potential (D) in male FA or DE exposed mice. N = 6 for each genotype and treatment. *Significantly different (p < 0.05) compared to genotype specific FA control by ANOVA and Dunnett’s t-test.
Vascular reactivity of aortic rings measured by wire myography
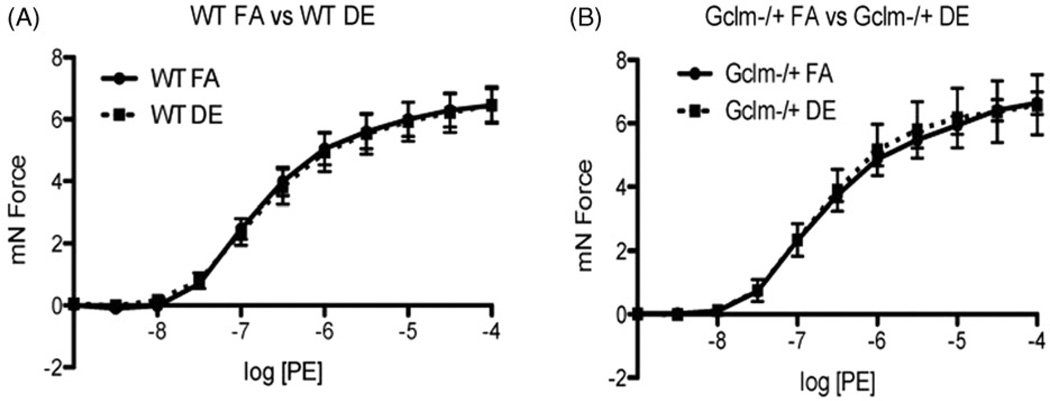
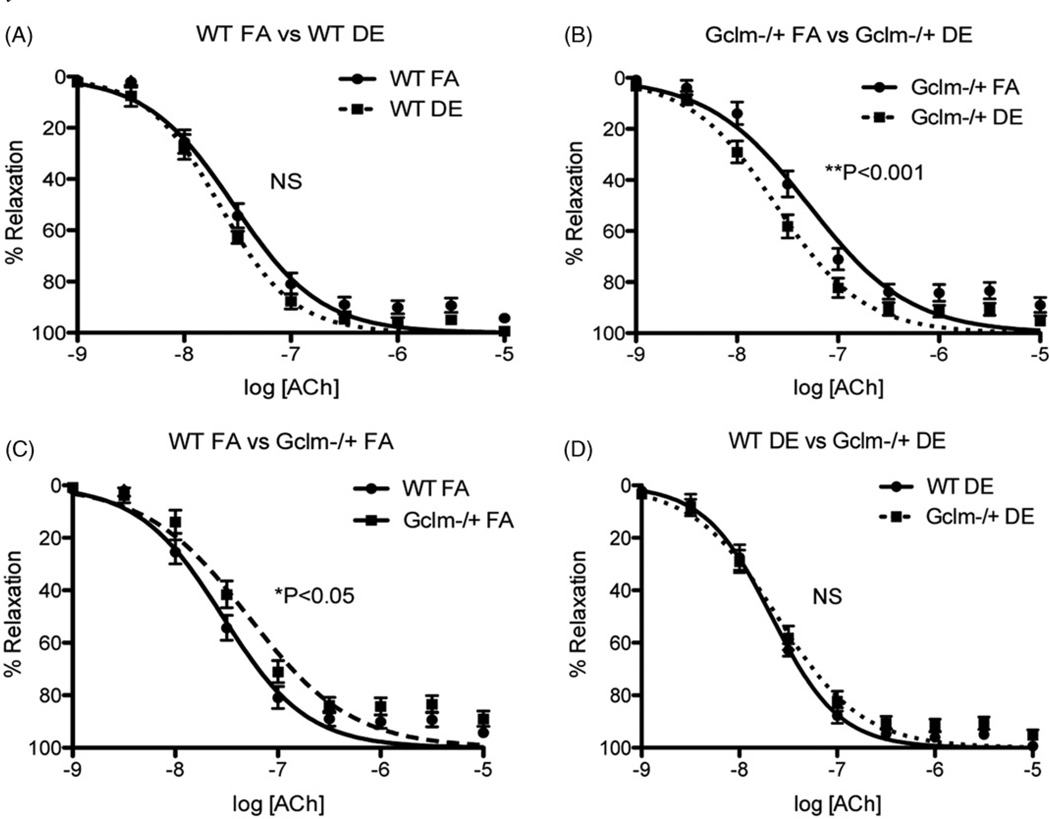
To investigate the effect of DE inhalation on aortic vascular reactivity, we exposed male WT and Gclm−/+ mice to either FA or DE and analyzed aortic ring vascular reactivity by wire myography. We exposed both WT and Gclm−/+ mice to either FA or DE for 6 h and assessed aortic ring vascular reactivity immediately thereafter. Stimulation with PE did not reveal any effect of DE inhalation or Gclm genotype on contractility (Figure 5). ACh-stimulated relaxation of aortic rings in WT mice showed that DE exposure caused no observable impairment in relaxation; however, there was a trend of increasing ACh-stimulated relaxation, but this trend did not reach significance (Figure 6, panel A). When assessing ACh-relaxation of aortic rings from Gclm−/+ mice, DE did not produce any impairment in relaxation, but, it did significantly increase ACh-stimulated relaxation (Figure 6, panel B) (nonlinear regression log EC50: Gclm−/+ FA = −7.287 (5.160 × 10−8M ACh) 95%CI −7.380 to −7.195; Gclm−/+ DE = −7.613 (2.438 × 10−8M ACh) 95%CI −7.683 to −7.543). This effect equates to DE producing a reduction in the ACh-EC50 by roughly 2-fold in the Gclm−/+ mouse. When comparing the response to ACh between the FA-exposed WT and Gclm−/+ mice, we observed that aortic rings from Gclm−/+ mice had a small but significant impairment in relaxation (Figure 6, panel C) (nonlinear regression log EC50: WT FA = −7.538 (2.896 × 10−8 M ACh) 95%CI −7.619 to −7.457; Gclm−/+ FA = −7.287 (5.160 × 10−8 M ACh) 95%CI −7.380 to −7.195). This observation is consistent with our previously reported findings (Weldy et al., 2012). When comparing aortic rings from WT and Gclm−/+ DE-exposed mice, we observed no difference in ACh-stimulated relaxation (Figure 6, panel D). Assessment of sodium nitroprusside (SNP)-stimulated aortic ring relaxation revealed no changes across genotype or treatment (data not shown), further suggesting that observations of enhanced or impaired ACh-relaxation are endothelium dependent.
Figure 5.
Measurement of phenylephrine (PE)-stimulated contraction of aortic rings from WT and Gclm−/+ mice following 6 h FA or DE inhalation. WT FA and DE: N = 8, Gclm−/+ FA and DE: N = 9.
Figure 6.
Measurement of acetylcholine (ACh)-stimulated relaxation of aortic rings from WT and Gclm−/+ mice following 6 h FA or DE inhalation. (A) WT FA versus WT DE, (B) Gclm−/+ FA versus Gclm−/+ DE, (C) WT FA versus Gclm−/+ FA, (D) WT DE versus Gclm−/+ DE. Curves represent nonlinear regression model used for EC50 and significance testing. Significance (*p < 0.05, **p < 0.001) determined by nonlinear regression fit and EC50 determination, as well as by two-way ANOVA. WT FA and DE: N = 8, Gclm−/+ FA and DE: N = 17.
Aortic NO• production as measured by Fe(DETC)2 spin trap and ESR
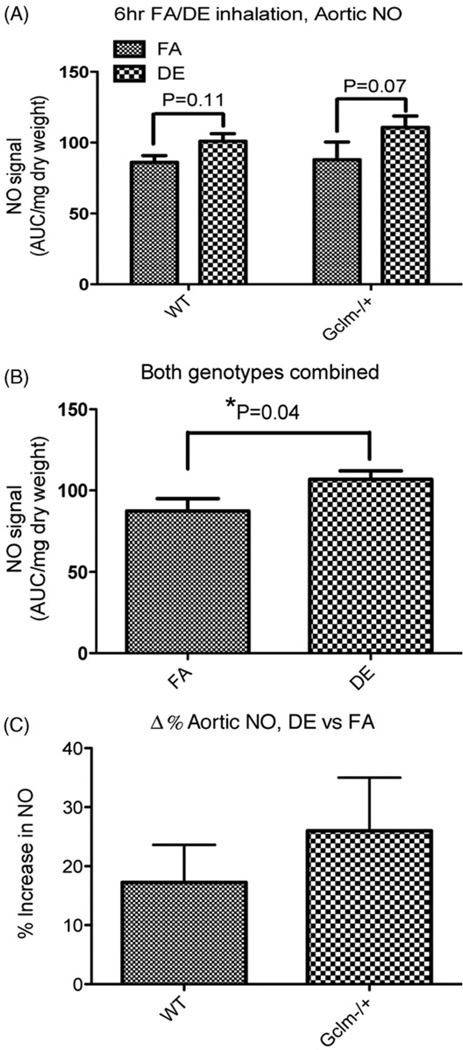
To determine if acute DE inhalation causes any effect on aortic NO• production or bioavailability, we measured aortic NO• by spin trap and ESR. We isolated the aortas of male WT and Gclm−/+ mice following either FA or DE inhalation, and measured the total NO-Fe(DETC)2 accumulated over 60 min. An acute 6-h exposure to DE in WT or Gclm−/+ mice did not cause any reduction in NO• production (Figure 7, panel A). However, there was a trend whereby DE appeared to increase aortic NO• production in both WT and Gclm−/+ mice compared to FA controls (Figure 7, panel A), with p values of 0.11 and 0.07 for WT and Gclm−/+ comparisons, respectively. When genotypes were combined, and the comparison was made between all FA and all DE-exposed mice, DE significantly increased aortic NO• production (Figure 7, panel B). Regarding the effect of DE on NO• produced across genotypes, we observed a trend for increased NO• in both WT mice (17% higher) and Gclm−/+ mice (26% higher) after DE exposures compared to FA controls (Figure 7, panel C). Although DE appeared to increase aortic NO• production more in Gclm−/+ mice than WT mice (Figure 7, panel C), this difference was not statistically significant.
Figure 7.
Measurement of aortic NO• production by Fe(DETC)2 spin trap and ESR. NO-Fe(DETC)2 signal relative to dry weight of aortic tissue (A) within genotypes, (B) with both genotypes combined, (C) delta of percentage increase in NO signal of DE versus FA in WT and Gclm−/+ mice. *Significantly different (p < 0.05) compared to genotype specific FA control by ANOVA and Dunnett’s t-test.
Discussion
We investigated the effects of acute DE inhalation and Gclm status on neutrophilic lung inflammation, plasma GSH redox status, aortic vascular reactivity and aortic NO• production in mice. The major observations of our studies were: (1) 6-h DE inhalation produced a measurable increase in neutrophilic lung inflammation, but this effect was not altered by genotype; (2) DE inhalation caused an oxidation of the plasma GSH reduction potential in mice heterozygous for Gclm but not in WT; (3) DE inhalation did not cause an impairment in ACh-stimulated relaxation or PE-stimulated contraction in aortic rings from either WT or Gclm−/+ mice; rather, there was an increase in ACh-stimulated relaxation in aortic rings from Gclm−/+ mice, (4) DE inhalation caused a significant increase in aortic NO• production, an effect that appeared to be enhanced in mice heterozygous for Gclm. These observations suggest that an acute 6-h DE (300 µg/m3) inhalation in WT C57BL/6 mice does not cause a direct impairment in aortic vascular reactivity, aortic NO• production or oxidation of plasma GSH redox status, but this acute exposure does produce a modest but measurable increase in neutrophilic airway inflammation. Alternatively, in this mouse model of compromised GSH synthesis, acute DE inhalation results in minimal lung inflammation that does not exceed the inflammation seen in WT mice, but does result in oxidation of plasma GSH redox status. Moreover, DE exposure enhanced ACh-stimulated relaxation of aortic rings, shifting the relaxation curve to the left of aortic rings from FA-exposed Gclm−/+ mice. This observation was in the opposite direction of what we had hypothesized, and the physiological relevance of this observation is currently unknown. However, these observations provide a platform for future investigations into the mechanisms of DE-induced changes in vascular function, and further support the hypothesis that Gclm genotype and de novo synthesis of GSH play important roles in mediating the effects of acute DE inhalation in mice.
Understanding the genetic determinants of DE-induced effects on cardiovascular function will be critically important to predict and reduce the adverse effects of PM2.5-inhalation within sensitive human populations. Previously published studies of controlled DE exposures have consistently shown DE to have adverse effects on vascular function, both in humans (Brook et al., 2002; Cosselman et al., 2012; Mills et al., 2005, 2007; Peretz et al., 2008) and animals (Campen et al., 2005; Cherng et al., 2009, 2011; Knuckles et al., 2008; Nurkiewicz et al., 2004). Although oxidative stress has been strongly suggested to play a key role in these observed effects, there have been relatively few investigations into genetic determinants of susceptibility due to compromised antioxidant synthesis. We have proposed that GSH and its de novo synthesis play an important role in modulating the adverse effects of DE inhalation. By investigating the effect of DE inhalation in mice with compromised GSH synthesis, our aim was to determine the role of Gclm genotype in DE-induced pulmonary inflammation and vascular dysfunction. If Gclm genotype and GSH synthesis were to play a key role, this would have implications for susceptible populations who may be especially sensitive to DE inhalation, i.e. those with functional genetic polymorphisms in GCLM and GCLC. We in fact did find that Gclm status in mice modifies certain responses to DE inhalation, but our observations further suggest that changes in vascular function may not be directly related to pulmonary inflammation, or be consistent within all systemic arteries.
The presence of neutrophils in BAL is taken as a measure of acute, innate immune response to DE inhalation, and we have previously demonstrated that neutrophilic airway inflammation in response to DEP is highly correlated to the production of proinflammatory cytokines IL6 and TNFα (Weldy et al., 2011a). Although we had previously shown neutrophilic lung inflammation to be enhanced in Gclm−/+ mice following intranasal instillation of DEP, we did not observe this effect in our current study. The percentage of neutrophils was significantly increased in both WT and Gclm−/+ mice following DE inhalation compared to FA controls, but this effect was not enhanced by Gclm heterozygosity. In fact, our results actually suggested a mild protective effect (although not statistically significant), which would be contrary to our hypothesis. Intranasal instillation of DEP, which is delivered as a single bolus treatment, is obviously a very different exposure scenario than whole DE inhalation. This suggests that in a single large exposure to DEP, the rapid de novo synthesis of GSH is necessary and requires two functional alleles of Gclm to maintain GSH content and limit DEP-induced inflammation. In the current inhalation study, DE exposure is over a 6-h time period, and the lack of enhanced inflammation suggests that this exposure scenario does not ‘max out’ the Gclm promoter capability and thus does not reveal any enhanced sensitivity to DE-induced lung inflammation. These observations may further highlight the complexities of extrapolating instillation studies to inhalation studies. But, our observation of mild, low-grade pulmonary inflammation following only a 6-h DE inhalation supports previous observations that DE inhalation results in neutrophilic airway inflammation (Salvi et al., 1999; Sunil et al., 2009).
Although we did not observe an effect of Gclm genotype on neutrophilic lung inflammation, DE did cause a significant oxidation of the plasma glutathione reduction potential in Gclm−/+ mice but not WT mice (Figure 4). By measuring this, we are able to quantitate the systemic oxidant/antioxidant balance following DE inhalation (Jones et al., 2000). In addition to a significantly oxidized GSH plasma reduction potential, measures of %GSSG were also significantly increased in the plasma of Gclm−/+ mice following DE inhalation. This effect seemed to be due to a loss of reduced GSH within the plasma, where we observed a strong trend of a lower level of GSH in the plasma following DE (p = 0.09). This observation would suggest that acute DE inhalation accelerates the oxidation of GSH to GSSG within the plasma, or it causes enhanced oxidation at various tissue locations, resulting in GSH being oxidized to GSSG within the cell and then exported to the plasma. Either would result in a subsequent drop in plasma GSH and increased plasma GSSG. As the Gclm−/+ mice do not appear to have increased plasma GSSG following DE inhalation, the drop in plasma GSH may have resulted from less export by tissues such as the liver, an enhanced breakdown by γ-glutamyltransferase in tissues, or participation in covalent modifications of electrophiles (such as reactive aldehydes) mediated by glutathione-S-transferase (GST) activity.
Overall, our observation of enhanced sensitivity to DE-induced oxidation of plasma GSH in Gclm−/+ mice suggests that Gclm−/+ mice are unable to appropriately balance oxidant generation with antioxidants following DE inhalation. Although not necessarily causative, this observation further suggests that the de novo synthesis of GSH plays a key role in balancing this oxidative effect. This observation suggests that individuals with GCLM polymorphisms may also have increased sensitivity to DE-induced effects on systemic oxidant/antioxidant balance.
There is an extensive body of evidence indicating a strong role for vascular oxidative stress in endothelial dysfunction where the generation of excessive ROS in addition to loss of antioxidant capacity results in the inactivation of NO• and oxidation of tetrahydrobiopterin (BH4) a vital cofactor necessary for proper eNOS function (Brocq et al., 2008). It is therefore our hypothesis that in a model of compromised antioxidant synthesis, i.e. the Gclm−/+ mouse, the ability of DE to increase oxidative stress should be exacerbated, thus resulting in impaired endothelial function and NO• generation. However, DE did not cause any impairment in ACh-stimulated vessel relaxation or PE-stimulated vessel contraction in aortic rings from either WT or Gclm−/+ mice (Figures 5 and 6). Rather, there was an unexpected effect whereby DE inhalation increased ACh-stimulated vessel relaxation in the aortic rings from Gclm−/+ mice (Figure 6, panel B). A trend for this effect was also observed in WT mice but this did not reach statistical significance. When comparing the ACh-stimulated relaxation across genotype in the FA controls only, we observe a slight but significant impairment in ACh-response in aortic rings from the Gclm−/+ mice compared to WT (Figure 7, panel C). This observation is consistent with our previous report, where we observed this effect in combination with increased aortic 3-nitrotyrosine protein modifications and compromised NO• generation (Weldy et al., 2012), suggesting that these Gclm−/+ mice do have a mild phenotype of impaired aortic ring relaxation in response to ACh. Thus, our observation that DE did not exacerbate this impairment, but rather produced an increase in relaxation, is counterintuitive.
We anticipated that DE exposure would be associated with a reduction in aortic NO• in either WT or Gclm−/+ mice. Contrary to our expectations, DE inhalation tended to produce an increase in aortic NO• production in both WT and Gclm−/+ mice (Figure 7, panel A). While these were only trends that approached statistical significance, when the data from both genotypes are combined, we did observe DE exposure to produce a significant increase in NO• production (Figure 7, panel B).
Although measuring aortic ring function can provide valuable insight into vascular reactivity in mice, our observations suggest that acute DE inhalation produces no effect in aortic ring vascular reactivity in WT mice; and in Gclm−/+ mice an effect opposite to what had been previously observed in either coronary arteries or mesenteric arteries in other studies of rodents exposed to DE. For example, Cherng and colleagues measured the acute (6 h exposure) effects of DE-inhalation on vascular reactivity of coronary arteries in rats (Cherng et al., 2009, 2011). They observed enhanced contraction from ET-1 and impaired ACh-stimulated relaxation, and these effects were attributed to uncoupling of eNOS and loss of bioavailable NO• due to oxidation of its cofactor BH4. In a study of chronic exposure to PM2.5 in rats, Sun and colleagues (Sun et al., 2008) demonstrated similar effects in the aorta, whereby impaired ACh-stimulated relaxation and enhanced PE-stimulated contraction was attributed to vascular oxidative stress, loss of bioavailable NO• and activation of the Rho/ROCK pathway. Although we hypothesized a similar effect would occur in the aorta following acute exposures in mice, our observations might highlight the dramatic differences in physiological function between the coronary arteries and the aorta, as well as the vascular response to PM following acute as opposed to chronic exposures. Nurkiewicz and colleagues examined the effects of intratracheal instillation of PM on microvascular function and observed that PM caused marked effects on microvascular dilation (Nurkiewicz et al., 2004, 2006). This observation highlights a potentially crucial part of the mechanism of PM2.5-induced effects on cardiovascular function whereby acute effects may be limited to the microvasculature, and large conductance vessels such as the aorta may show opposite effects following acute exposures.
The observation that aortic rings from Gclm−/+ mice have enhanced ACh-mediated relaxation was unexpected. However, because Gclm−/+ mice have elevated aortic oxidative stress and impaired NO• synthesis at baseline (Weldy et al., 2012), an increased relaxation following DE inhalation suggests either activation of antioxidant response pathways (thus preventing oxidative stress and increasing bioavailable NO•), or increased NOS activity by either upregulating eNOS/iNOS expression or post-translational modifications (i.e. phosphorylation or glutathionylation of eNOS). Using whole mRNA transcriptome analysis, we previously observed the Nrf2-antioxidant response pathway to be slightly increased in the aortas of Gclm−/+ mice (Weldy et al., 2012). If antioxidant response pathways within the aorta were increased following acute DE inhalation, we would expect NO• bioavailability to return to that of WT-FA controls, as the antioxidant capacity would reduce ROS and prevent the loss of bioavailable NO•, but not necessarily increase it beyond that present in WT mice. This appeared to be the case, as aortic rings from Gclm−/+-DE mice responded to ACh in a manner similar to that of WT-FA mice. This observation suggests that compensatory antioxidant responses following DE inhalation in the Gclm−/+ mice may explain the enhanced ACh relaxation. If enhanced antioxidant protection did explain this observation, then we would also expect baseline aortic NO• (as measured by ESR) to be similar to that of WT-FA. Since aortic NO• was greater in the aortas of DE-exposed Gclm−/+ mice than that of WT-FA, it seems likely that acute DE inhalation causes a modest increase in NO• formation possibly due to increased expression or activity of NOS isoforms, and this will be pursued in future studies.
Conclusions
In the studies reported here, we examined the effects of acute DE inhalation on neutrophilic airway and lung inflammation, plasma GSH, GSSG, %GSSG, and GSH reduction potential, aortic ring vascular reactivity and aortic NO• production in both WT and Gclm−/+ mouse models. Polymorphisms in GCLM are highly frequent in humans (~20% of the public) and have been demonstrated to influence clinical outcomes of cardiovascular disease. The observations reported here indicate that Gclm genotype in mice influences their biological response to acute DE inhalation, but our observations of enhanced ACh-stimulated vessel relaxation of aortic rings suggest that previously observed impairments in ACh-relaxation and NO• generation are likely limited to coronary arteries, the mesenteric arteries and the microvasculature. The observations reported here provide the impetus to further investigate genetic determinants of antioxidant capacity as factors influencing sensitivity to PM2.5 inhalation on vascular function at the microvascular level.
Acknowledgments
This study was supported by NIH grants P50ES015915, P30ES007033, T32ES007032, T32HL007312, U24DK076126 and R01DK073878.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Baccarelli A, Zanobetti A, Martinelli I, et al. Air pollution, smoking, and plasma homocysteine. Environ Health Perspect. 2007;115:176–181. doi: 10.1289/ehp.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bea F, Hudson FN, Chait A, et al. Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circulation Res. 2003;92:386–393. doi: 10.1161/01.RES.0000059561.65545.16. [DOI] [PubMed] [Google Scholar]
- Bea F, Hudson FN, Neff-Laford H, et al. Homocysteine stimulates antioxidant response element-mediated expression of glutamate-cysteine ligase in mouse macrophages. Atherosclerosis. 2009;203:105–111. doi: 10.1016/j.atherosclerosis.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuner EV, Forchhammer L, Møller P, et al. Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environ Health Perspect. 2007;115:1177–1182. doi: 10.1289/ehp.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocq ML, Leslie SJ, Miliken P, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1673. doi: 10.1089/ars.2007.2013. [DOI] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, et al. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Babu NS, Helms GA, et al. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE−/− mice. Toxicol Sci. 2005;88:95–102. doi: 10.1093/toxsci/kfi283. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Knuckles TL, et al. Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE−/− mice. Toxicol Appl Pharmacol. 2010;242:310–317. doi: 10.1016/j.taap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng TW, Campen MJ, Knuckles TL, et al. Impairment of coronary endothelial cell ETB receptor function after short-term inhalation exposure to whole diesel emissions. Am J Physiol Regul Integr Comp Physiol. 2009;297:R640–R647. doi: 10.1152/ajpregu.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng TW, Paffett ML, Jackson-Weaver O, et al. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ Health Perspect. 2011;119:98–103. doi: 10.1289/ehp.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang K-J, Chan C-C, Su T-C, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Cosselman KE, Krishnan MR, Oron AP, et al. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension. 2012;59:943–948. doi: 10.1161/HYPERTENSIONAHA.111.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, et al. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Levonen A-L, Moellering DR, et al. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radical Biol Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Hamel DM. Increase in gamma-glutamylcysteine synthetase activity as a mechanism for butylated hydroxyanisole-mediated elevation of hepatic glutathione. Toxicol Appl Pharmacol. 1994;126:145–149. doi: 10.1006/taap.1994.1100. [DOI] [PubMed] [Google Scholar]
- Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am J Cardiol. 2007;99:808–812. doi: 10.1016/j.amjcard.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, Mohar I, et al. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould T, Larson T, Stewart J, et al. A controlled inhalation diesel exhaust exposure facility with dynamic feedback control of PM concentration. Inhalation Toxicol. 2008;20:49–52. doi: 10.1080/08958370701758478. [DOI] [PubMed] [Google Scholar]
- Haque JA, Mcmahan RS, Campbell JS, et al. Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice. Lab Invest. 2010;90:1704–1717. doi: 10.1038/labinvest.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janisko S, Noll JD. Field evaluation of diesel particulate matter using portable elemental carbon monitors. In: Hardcastle S, McKinnon DL, editors. Sudbury, Ontario, Canada: Proceedings of the 13th U.S./North American Mine Ventilation Symposium; 2010. [Last accessed: 3 Jun 2013]. 4752. MIRARCO Mining Innovation [Online]. Available from: http://www.cdc.gov/niosh/mining/UserFiles/works/pdfs/feodp.pdf. [Google Scholar]
- Jin RC, Mahoney CE, Coleman Anderson L, et al. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation. 2011;123:1963–1973. doi: 10.1161/CIRCULATIONAHA.110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E, Wesselkamper SC, Shertzer HG, et al. Glutathione deficient C57BL/6J mice are not sensitized to ozone-induced lung injury. Biochem Biophys Res Commun. 2010;396:407–412. doi: 10.1016/j.bbrc.2010.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Mody VC, et al. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- Kampfrath T, Maiseyeu A, Ying Z, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circulation Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo JP, Alp NJ, Bendall JK, et al. EPR quantification of vascular nitric oxide production in genetically modified mouse models. Nitric Oxide. 2004;10:156–161. doi: 10.1016/j.niox.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol. 2008;230:346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Koide S-I, Kugiyama K, Sugiyama S, et al. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasomotor dysfunction and myocardial infarction. J Am Coll Cardiol. 2003;41:539–545. doi: 10.1016/s0735-1097(02)02866-8. [DOI] [PubMed] [Google Scholar]
- Krejsa CM, Franklin CC, White CC, et al. Rapid activation of glutamate cysteine ligase following oxidative stress. J Biol Chem. 2010;285:16116–16124. doi: 10.1074/jbc.M110.116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold JA, Dam A, Maron BA, et al. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res. 2007;636:95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Molec Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron BA, Zhang Y-Y, Handy DE, et al. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem. 2009;284:7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnachie LA, Mohar I, Hudson FN, et al. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol Sci. 2007;99:628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tärnqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- Mills NL, Törnqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Mudway IS, Stenfors N, Duggan ST, et al. An in vitro and in vivo investigation of the effects of diesel exhaust on human airway lining fluid antioxidants. Arch Biochem Biophys. 2004;423:200–212. doi: 10.1016/j.abb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Nakamura S-I, Kugiyama K, Sugiyama S, et al. Polymorphism in the 5′-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- Nakamura S-I, Sugiyama S, Fujioka D, et al. Polymorphism in glutamate-cysteine ligase modifier subunit gene is associated with impairment of nitric oxide-mediated coronary vasomotor function. Circulation. 2003;108:1425–1427. doi: 10.1161/01.CIR.0000091255.63645.98. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, et al. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, et al. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Sullivan JH, Leotta DF, et al. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–942. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pourazar J, Mudway IS, Samet JM, et al. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol. 2005;289:L724–L730. doi: 10.1152/ajplung.00055.2005. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, et al. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006;114:120–123. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, et al. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Romieu I, Garcia-Esteban R, Sunyer J, et al. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5) Environ Health Perspect. 2008;116:1237–1242. doi: 10.1289/ehp.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Téllez-Rojo MM, Lazo M, et al. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005;172:1534–1540. doi: 10.1164/rccm.200503-372OC. [DOI] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respiratory Crit Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Shenvi SV, Smith E, Hagen TM. Identification of age-specific Nrf2 binding to a novel antioxidant response element locus in the Gclc promoter: a compensatory means for the loss of glutathione synthetic capacity in the aging rat liver? Aging Cell. 2012;11:297–304. doi: 10.1111/j.1474-9726.2011.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen M, Daneshvar B, Hansen M, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. 2003;111:161–166. doi: 10.1289/ehp.111-1241344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J, Cunningham M, Loscalzo J. Reduced thiols and the effect of intravenous nitroglycerin on platelet aggregation. Am J Cardiol. 1988;62:377–380. doi: 10.1016/0002-9149(88)90962-9. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Ying Z, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil VR, Patel KJ, Mainelis G, et al. Pulmonary effects of inhaled diesel exhaust in aged mice. Toxicol Appl Pharmacol. 2009;241:283–293. doi: 10.1016/j.taap.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, White CC, Krejsa CM, et al. Induction of glutamate-cysteine ligase (gamma-glutamylcysteine synthetase) in the brains of adult female mice subchronically exposed to methylmercury. Toxicol Lett. 1999;110:1–9. doi: 10.1016/s0378-4274(99)00133-2. [DOI] [PubMed] [Google Scholar]
- Vinzents PS, Møller P, Sørensen M, et al. Personal exposure to ultrafine particles and oxidative DNA damage. Environ Health Perspect. 2005;113:1485–1490. doi: 10.1289/ehp.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Watanabe K, Kobayashi T, et al. Chronic depletion of glutathione exacerbates ventricular remodeling and dysfunction in the pressure-overloaded heart. Cardiovasc Res. 2013;97:282–292. doi: 10.1093/cvr/cvs333. [DOI] [PubMed] [Google Scholar]
- Weiss N, Zhang YY, Heydrick S, et al. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci USA. 2001;98:12503–12508. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldy CS, Luttrell IP, White CC, et al. Glutathione (GSH) and the GSH synthesis gene Gclm modulate vascular reactivity in mice. Free Radic Biol Med. 2012;53:1264–1278. doi: 10.1016/j.freeradbiomed.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldy CS, White CC, Wilkerson H-W, et al. Heterozygosity in the glutathione synthesis gene Gclm increases sensitivity to diesel exhaust particulate induced lung inflammation in mice. Inhal Toxicol. 2011a;23:724–735. doi: 10.3109/08958378.2011.608095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldy CS, Wilkerson H-W, Larson TV, et al. DIESEL particulate exposed macrophages alter endothelial cell expression of eNOS, iNOS, MCP1, and glutathione synthesis genes. Toxicol In Vitro. 2011b;25:2064–2073. doi: 10.1016/j.tiv.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild AC, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radical Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]