Abstract
Background: Although the intake of high-fat and high-sugar food activates mesolimbic reward, gustatory, and oral somatosensory brain regions, contributing to overeating, few studies have examined the relative role of fat and sugar in the activation of these brain regions, which would inform policy, prevention, and treatment interventions designed to reduce obesity.
Objective: We evaluated the effect of a high-fat or high-sugar equicaloric chocolate milkshake and increasing fat or sugar milkshake content on the activation of these regions.
Design: Functional magnetic resonance imaging was used to assess the neural response to the intake of high-fat/high-sugar, high-fat/low-sugar, low-fat/high-sugar, and low-fat/low-sugar chocolate milkshakes and a tasteless solution in 106 lean adolescents (mean ± SD age = 15.00 ± 0.88 y). Analyses contrasted the activation to the various milkshakes.
Results: High-fat compared with high-sugar equicaloric milkshakes caused greater activation in the bilateral caudate, postcentral gyrus, hippocampus, and inferior frontal gyrus. High-sugar compared with high-fat equicaloric milkshakes caused greater activation in the bilateral insula extending into the putamen, the Rolandic operculum, and thalamus, which produced large activation regions. Increasing sugar in low-fat milkshakes caused greater activation in the bilateral insula and Rolandic operculum; increasing fat content did not elicit greater activation in any region.
Conclusions: Fat caused greater activation of the caudate and oral somatosensory regions than did sugar, sugar caused greater activation in the putamen and gustatory regions than did fat, increasing sugar caused greater activity in gustatory regions, and increasing fat did not affect the activation. Results imply that sugar more effectively recruits reward and gustatory regions, suggesting that policy, prevention, and treatment interventions should prioritize reductions in sugar intake. This trial was registered at clinicaltrials.gov as DK092468.
INTRODUCTION
The obesity epidemic has prompted a focus on the role of high-fat and high-sugar food consumption in overeating. High-fat/high-sugar food intake activates mesolimbic reward (midbrain and striatum) and gustatory regions (frontal operculum and insula) (1–4). The dopamine midbrain and striatum play a role in encoding the reward value of stimuli and reward learning (5), and striatal dopamine release correlates with ratings of meal pleasantness (6). The taste of fat in the mouth has been shown to activate the primary taste cortex as well as the orbitofrontal cortex and amygdala (7–10), and the texture of fat in the mouth has been shown to activate the taste and somatosensory insula, orbitofrontal cortex, and anterior cingulate cortex (11). Neural activity in the midorbitofrontal and anterior cingulate cortex has been correlated with the pleasantness of oral fat texture (12). The primary taste cortex (ie, the anterior insula and adjoining frontal operculum) appear to play a key role in the encoding of tastes [eg, sweet and bitter (8)], and the activation in the primary taste cortex had been correlated with the subjective reward from food (6, 13). The taste of glucose has also activated the amygdala (14), with perceived pleasantness correlated with the degree of activation (15). Rolls (8) argued that brain regions that represent the reward value of food are distinct from those that represent the viscosity of food (which reflects the fat content) and tastes such as sweet because eating to satiety reduces the activation of reward-valuation regions (eg, the caudate, amygdala, and orbitofrontal cortex), but not the activation in regions that represent fat and sweet tastes. Thus, data have suggested that high-fat and high-sugar food intake recruits brain reward regions, and the elevated perceived pleasantness of such food may contribute to overeating and consequent weight gain.
Although studies have advanced understanding of brain regions that represent the hedonic value of palatable foods, sweet tastes, and fat tastes, few studies have examined the relative role of sugar and fat-food contents in the activation of reward, gustatory, and oral somatosensory regions. Such an examination is important because knowing whether fat or sugar plays a more-potent role in the activation of these regions would inform policy interventions, such as whether to tax high-fat or high-sugar foods, or both, to reduce intakes of energy-dense foods and the prevalence of obesity. This information may also inform the design of more-effective obesity-prevention and -treatment interventions. Experiments with rodents have indicated that high-fat diets resulted in greater weight gain than did high-carbohydrate diets, in part because the rodents ate more of the high-fat than high-carbohydrate foods (16), which implied that high-fat foods might be more effective than high-sugar diets in the activation of reward, gustatory, and somatosensory regions. However, the finding that a regular intake of sugar but not fat is associated with naltrexone-precipitated signs of withdrawal (17) implies that sugar may more effectively activate these brain reward regions. The primary aim of the current study was to evaluate the effect of a high-fat or high-sugar equicaloric chocolate milkshake in the activation of these brain regions by using milkshakes that were equal in energy density and matched for flavor and palatability. The secondary aim was to test whether increasing fat or sugar milkshake content caused greater increases in the activation of these brain regions.
SUBJECTS AND METHODS
We used fMRI to examine BOLD activation in response to intakes of high-fat/high-sugar, high-fat/low-sugar, low-fat/high-sugar, and low-fat/low-sugar chocolate milkshakes and a calorie-free tasteless solution in 106 healthy-weight adolescents (Table 1). Participants reported the following racial and ethnic backgrounds: 7% of subjects were Asian, 6% of subjects were African American, 3% of subjects were American Indian or Alaskan Native, 71% of subjects were white, 9% of subjects were Hispanic, and 5% of subjects were multirace or other. Individuals who reported binge eating or compensatory behavior in the past 3 mo, weekly or more frequent use of psychotropic medications or illicit drugs, a head injury with a loss of consciousness, or an Axis I psychiatric disorder in the past year (including anorexia nervosa, bulimia nervosa, or binge-eating disorder) were excluded. Parents and adolescents provided informed written consent for the project. Oregon Research Institute's Institutional Review Board approved all methods.
TABLE 1.
Subject characteristics and behavioral measures (n = 106)
| M (n = 47) | F (n = 59) | Full sample (n = 106) | |
| Age (y) | 14.83 ± 0.871 | 15.14 ± 0.88 | 15.0 ± 0.88 |
| BMI (kg/m2) | 20.93 ± 2.48 | 21.60 ± 2.32 | 21.3 ± 2.41 |
| Handedness (percentage of right-handed subjects) | 87.2 | 94.9 | 91.5 |
| Hunger2 | 0.49 ± 4.38 | 0.98 ± 4.28 | 0.76 ± 4.31 |
| Milkshake pleasantness (high fat/high sugar)3 | 15.15 ± 2.88 | 14.26 ± 3.41 | 14.66 ± 3.21a |
| Milkshake pleasantness (high fat/low sugar)3 | 12.84 ± 3.60 | 11.23 ± 3.98 | 11.94 ± 3.89b |
| Milkshake pleasantness (low fat/high sugar)3 | 13.75 ± 3.63 | 11.44 ± 5.02 | 12.46 ± 4.59b |
| Milkshake pleasantness (low fat/low sugar)3 | 10.59 ± 3.87 | 9.03 ± 4.26 | 9.72 ± 4.14c |
| Tasteless solution pleasantness3 | 10.61 ± 3.06 | 10.36 ± 3.72 | 10.47 ± 3.43c |
Mean ± SD (all such values).
Scale was from −10 (not at all hungry) to 10 (I have never been more hungry).
Scale was from 0 (most unpleasant sensation ever) to 20 (most pleasant sensation ever). Different superscript letters indicate significant differences in pleasantness ratings (P < 0.01) assessed by using within-subject t tests.
Sensory and hedonic measures
Participants were asked to consume their regular meals but to refrain from eating or drinking for 4 h immediately preceding their imaging session for standardization. On arrival to the session participants rated their hunger on a scale from 1 (not hungry at all) to 10 (extremely hungry); if a score ≥7 was indicated, subjects were offered a small snack to bring their hunger to a neutral state. After questionnaires were completed and paradigms explained, just before the scan, hunger and hedonic ratings of the tastants were assessed on 20-cm crossmodal visual analog scales (VASs). VAS ratings were anchored by −10 (not at all), 0 (neutral), and 10 (never been more hungry) for hunger. For hedonic ratings, participants sampled a small amount of each milkshake and the tasteless solution (order counterbalanced) and rated the pleasantness on a scale that ranged from 0 (most unpleasant sensation ever) to 20 (most pleasant sensation ever). The mean (±SD) hunger rated immediately before the scan on the VAS was neutral (0.8 ± 4.3; Table 1), which confirmed that participants were in a neutral hunger state during the scan.
fMRI milkshake receipt paradigm
We used a block version of our milkshake paradigm (4), which assessed BOLD activity in response to the receipt of milkshakes that varied in sugar and fat contents. Each milkshake included the same ice-cream base and chocolate syrup. No fat substitutes or thickeners (eg, olestra or guar gum) or artificial sweeteners (eg, aspartate or sucralose) were included. Fat contents of the milkshakes were manipulated by varying the type of milk (half and half compared with 2% milk). The sweetness was manipulated by varying the simple-syrup content. We investigated the response to the following milkshakes (16 fl oz is equivalent to a typical medium-sized, fast-food milkshake): a high-fat/high-sugar milkshake (804 kcal, 35.4 g fat, and 106.4 g sugar/16 fl oz; 170 kcal, 7.5g fat, and 23 g sugar/100 mL), a high-fat/low-sugar milkshake (605 kcal, 42.6 g fat, and 34.5g sugar/16 fl oz; 129 kcal, 9.0g fat, and 7.3g sugar/100 mL), a low-fat/high-sugar milkshake (587 kcal, 8.9 g fat, and 112.1 g sugar/16 fl oz; 124 kcal, 1.9 g fat, 23.7 g sugar/100 mL), and a low-fat/low-sugar milkshake (350 kcal, 11.4 g fat, 41.2 g sugar/16 fl oz; 74 kcal, 2.4g fat, and 8.7 g sugar/100 mL). Pilot testing showed these differences in fat and sugar contents were detectable without varying the flavor. In addition, the high-fat/low-sugar and low-fat/high-sugar milkshakes were designed such that they had similar energy densities (1.28 kcal/mL for the high-fat/low-sugar milkshake compared with 1.24 kcal/mL for the low-fat/high-sugar milkshake). We included a tasteless, odorless solution that contained the main ionic components of saliva (25 mmol/L KCl and 2.5 mmol/L NaHCO3 in distilled water) as a control contrast. The receipt of the tasteless solution compared with the baseline activity produced significant activation in brain regions previously associated with taste, including oral somatosensory regions [bilateral postcentral gyrus and Rolandic operculum (right: 54, −7, 25; k = 300; Z = 7.7; left: −54, −7, 25; k = 300; Z = 7.5) and bilateral putamen extending into the insula (right: 30, −10, −11; k = 37; Z = 5.8; left: −27, −7, −11; k = 33; Z = 6.1).
Subjects received the tastants through individual beverage tubes that were connected to a 5-channel gustometer that was anchored to the scanner bed and delivered the tastes in the same area of the mouth. Participants were cued with a picture (glass of milkshake or water). All milkshake variants were preceded by the same image of a milkshake to not confound the neural response to receipt with expectations (18). During milkshake and tasteless delivery, the cue (1 s) was presented followed by a fixation cross during delivery of the tastant. The delivery of the milkshake and tasteless solution occurred in variable-length blocks (1 block presented 4, 5, or 7 events in each of the 2 runs). An event was considered when a tastant was delivered (0.7 cc) over 5 s followed by 3 s to swallow. Four, 5, or 7 events in a row of the same tastant were considered a block. After a block was completed, subjects received a rinse of the tasteless solution followed by a swallow cue (0.5 s) and a jitter (9–11 s). The tasteless solution followed the same pattern without a rinse. The order of the presentation of blocks (ie, different tastants) was randomized. Two runs (13 min; 40 s/run) were performed. Each run presented 3 blocks of each of the 4 milkshake types and the tasteless solution in a randomized order. In total, there were 6 blocks (32 events) of each of the 5 tastants presented.
fMRI data acquisition and preprocessing
Scanning was performed by a Siemens Tim Trio 3 Tesla MRI scanner (Siemens). Functional scans used a T2*-weighted gradient single-shot echo planar imaging sequence (echo time: 30 ms; repetition time: 2000 ms; flip angle: 80°) with an in-plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192-mm2 field of view). Thirty-two 4-mm slices (interleaved acquisition, no skip) were acquired along the anterior-commissure–posterior-commissure transverse oblique plane, as determined by the midsagittal section. Rather than include motion regressors in analyses, a prospective acquisition correction was used to adjust the slice position and orientation as well as to regrid residual volume-to-volume motion in real time during data acquisition for the purpose of reducing motion-induced effects (19). Head motion >2 mm or degrees in any direction within each run was our a priori exclusion criterion, but no participants were excluded on this basis. A high-resolution inversion recovery T1-weighted sequence (magnetization-prepared rapid-acquisition gradient echo; field of view: 256 × 256 mm2, 256 × 256 matrix; thickness: 1.0 mm; slice number: ∼160) was also acquired.
Data were preprocessed and analyzed by using statistical parametric mapping (SPM8; Wellcome Department of Imaging Neuroscience) in MATLAB software (version 2011b for Macintosh OSX; Mathworks Inc). Images were manually reoriented to the anterior-commissure–posterior-commissure line and skull stripped. Functional images were realigned to the mean, and both anatomic and functional images were normalized to the standard Montreal Neurological Institute T1-template brain (ICBM 152). Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for high-resolution anatomic images. Functional images were smoothed with a 6-mm full-width at half-maximum isotropic Gaussian kernel. A 128-s high-pass filter removed low-frequency noise and signal drift.
To identify brain regions that showed increases in BOLD activation in response to the intake of each milkshake, we entered individual-level contrasts of (milkshake intake > tasteless solution intake) into the second level, 1-sample t test separately for each of the 4 milkshakes. To test whether the BOLD response to milkshakes varied according to fat and sugar contents, we compared individual data from milkshakes of interest in second level models by using within-subject t tests as implemented in SPM8 software (eg, high-fat/high-sugar milkshake intake compared with low-fat/high-sugar milkshake intake]). fMRI analyses were also performed with control for the menstrual phase in women (data not shown); no differences in results were observed when the menstrual phase was statistically controlled. We tested for differences between adolescent males and females in the presented contrasts by using between-group t tests in SPM8 software; no significant differences in the BOLD response in reward, gustatory, and oral somatosensory regions were observed.
Whole-brain analyses were used throughout; activity surviving a threshold of P < 0.001, with a cluster (k) ≥15 was considered significant for all analyses. This threshold had an overall significance level of P < 0.05 corrected for multiple comparisons across the whole brain. This significance level resulted from 10,000 Monte-Carlo simulations of random-noise distribution through an average gray-matter mask (3 mm3) by using the 3dClustSim module of the Analysis of Functional Neuroimages software (20). For use in the Monte Carlo simulations, the inherent smoothness was calculated from the residual mean squares for each of the main-effects analyses (receipt of 4 milkshakes compared with the tasteless solution) by using a 3d full-width at half-maximum module of the Analysis of Functional Neuroimages software (20) and then averaged. The mean gray-matter mask of the whole brain was derived from the sample by using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra segmentation in the SPM8 program according to standard methods in VBM8 (21). Once segmented, mean gray matter was resliced to 3 mm3 and binarized by using the image-calculator function in the SPM8 program at the level of i1 > 0.03. This mask was used in the Monte Carlo simulations and applied at the second level for all analyses to reduce the number of voxels in the white matter tested. Because the gray mater mask is an anatomic average and smoothed during binarization, it is possible that some masked activity extends into the boarder or across small sections of white matter. All stereotactic coordinates are presented in Montreal Neurological Institute space. Pearson's product-moment correlations (r) for fMRI data were calculated as
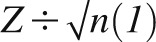 |
Tests of normality of distribution (skewness and kurtosis), descriptive statistics (means ± SDs), and testing for differences by sex or preferences of various tastants were performed with Statistical Package for the Social Sciences software (SPSS version 19 for Mac OS X, 2011; SPSS Inc).
RESULTS
Pleasantness ratings varied significantly between the 4 milkshakes and tasteless solution (Table 1), with the exceptions of ratings of the high-fat/low-sugar milkshake compared with low-fat/high-sugar milkshake (P = 0.33) and low-fat/low-sugar milkshake compared with the tasteless solution (P = 0.15). Main effects of the 4 types of milkshakes compared with the tasteless solution on the BOLD response are shown in Table 2. High-fat/high-sugar milkshake (compared with the tasteless solution) intake elicited robust activity in the bilateral postcentral gyrus that extended into the insula and right Rolandic operculum. High-fat/low-sugar milkshake (compared with tasteless solution) intake also elicited significant activity in the bilateral postcentral gyrus and right anterior cingulate cortex. Low-fat/high-sugar milkshake (compared with tasteless solution) intake resulted in activity in the bilateral (mid)insula, extending into the postcentral gyrus, and right putamen as well as the thalamus, left caudate, and bilateral cingulate cortex. Low-fat/low-sugar milkshake (compared with the tasteless solution) intake resulted in activity in the right Rolandic operculum, bilateral postcentral gyrus, right thalamus, and right cingulate cortex.
TABLE 2.
Effects of BOLD responsivity to milkshake intake varied by macronutrient content
| Hemisphere of the brain | MNI1 coordinates x, y, z | k2 | Peak Z | r3 | Peak P | |
| High-fat/high-sugar milkshake intake (high-fat/high-sugar compared with tasteless solution intakes) | ||||||
| Postcentral gyrus | Right | 54, −7, 22 | 557 | 5.77 | 0.56 | 3.9 × 10−9 |
| Insula | Right | 36, −7, 7 | 5.62 | 0.55 | 9.3 × 10−9 | |
| Rolandic operculum | Right | 63, −13, 25 | 5.22 | 0.51 | — | |
| Postcentral gyrus | Left | −57, −7, 28 | 378 | 5.40 | 0.52 | 3.4 × 10−8 |
| Insula | Left | −36, −10, 10 | 4.39 | 0.43 | 5.7 × 10−6 | |
| Temporal operculum | Left | −45, −19, 7 | 4.01 | 0.39 | 2.9 × 10−5 | |
| Cerebellum | Right | 15, −61, −26 | 56 | 4.82 | 0.47 | 7.6 × 10−7 |
| Thalamus | Right | 9, −19, −2 | 99 | 4.62 | 0.45 | 1.9 × 10−6 |
| Thalamus | Left | −6, −22, −2 | 4.55 | 0.44 | 2.6 × 10−6 | |
| Thalamus | Right | 9, −7, 10 | 3.75 | 0.36 | 8.7 × 10−5 | |
| Cerebellum | Left | −15, −64, −26 | 40 | 4.41 | 0.43 | 3.1 × 10−6 |
| Anterior cingulate cortex | 0, 20, 19 | 43 | 3.97 | 0.39 | 3.5 × 10−5 | |
| 0, 29, 25 | 3.62 | 0.35 | — | |||
| High-fat/low-sugar milkshake intake (high-fat/low-sugar compared with tasteless solution intakes)4 | ||||||
| Postcentral gyrus | Right | 60, −7, 22 | 98 | 4.47 | 0.43 | 3.9 × 10−6 |
| 51, −10, 25 | 4.16 | 0.40 | — | |||
| 57, 5, 19 | 3.73 | 0.36 | — | |||
| Postcentral gyrus | Right | 54, −19, 34 | 20 | 4.01 | 0.39 | 3.1 × 10−5 |
| Postcentral gyrus | Left | −57, −10, 31 | 69 | 3.86 | 0.38 | 5.6 × 10−5 |
| −63, −10, 19 | 3.79 | 0.37 | — | |||
| −60, −19, 22 | 3.73 | 0.36 | — | |||
| Anterior cingulate cortex | Right | 3, 38, 19 | 17 | 3.73 | 0.36 | 9.7 × 10−5 |
| 3, 26, 19 | 3.22 | 0.31 | — | |||
| Low-fat/high-sugar milkshake intake (low-fat/high-sugar compared with tasteless solution intakes) | ||||||
| Insula (mid) | Right | 39, −7, 7 | 963 | 7.30 | 0.71 | 1.5 × 10−13 |
| Postcentral gyrus | Right | 60, −13, 22 | 6.73 | 0.65 | 8.7 × 10−12 | |
| Putamen | Right | 24, −4, 4 | 5.81 | 0.56 | 3.0 × 10−9 | |
| Insula (mid) | Left | −36, −10, 10 | 729 | 6.41 | 0.62 | 7.4 × 10−11 |
| Postcentral gyrus | Left | −60, −7, 31 | 6.38 | 0.62 | 8.6 × 10−11 | |
| −48, −10, 28 | 5.93 | 0.58 | — | |||
| Thalamus | Right | 9, −19, −2 | 88 | 5.57 | 0.54 | 1.3 × 10−8 |
| Caudate | Right | 12, 8, 4 | 4.08 | 0.40 | 2.2 × 10−5 | |
| Thalamus | Right | 12, −1, 10 | 4.03 | 0.39 | 2.7 × 10−5 | |
| Thalamus | Left | −12, −22, 7 | 74 | 5.33 | 0.52 | 4.9 × 10−8 |
| Caudate | Left | −9, 5, 1 | 4.94 | 0.48 | 3.9 × 10−7 | |
| Thalamus | Left | −12, −1, 10 | 4.77 | 0.46 | 9.0 × 10−7 | |
| Cingulate cortex | 0, −22, 25 | 33 | 4.30 | 0.42 | 8.7 × 10−6 | |
| Right | 6, −31, 25 | 3.55 | 0.34 | — | ||
| Left | −3, −16, 34 | 3.44 | 0.33 | — | ||
| Cerebellum | Right | 15, −61, −26 | 23 | 4.13 | 0.40 | 1.8 × 10−5 |
| Cingulate cortex | 0, 20, 37 | 43 | 4.07 | 0.40 | 8.7 × 10−6 | |
| Left | −6, 11, 34 | 3.62 | 0.35 | — | ||
| Cerebellum | Left | −21, −67, −26 | 28 | 4.00 | 0.39 | 3.2 × 10−5 |
| Cingulate cortex | 0, 20, 22 | 40 | 3.97 | 0.39 | 3.6 × 10−5 | |
| Right | 3, 38, 19 | 3.67 | 0.36 | — | ||
| Low-fat/low-sugar milkshake intake (low-fat/low-sugar compared with tasteless solution intakes) | ||||||
| Rolandic operculum | Right | 60, −4, 13 | 179 | 4.90 | 0.48 | 4.8 × 10−7 |
| Postcentral gyrus | Right | 51, −10, 25 | 4.82 | 0.47 | 7.1 × 10−7 | |
| 60, −13, 4 | 4.12 | 0.40 | — | |||
| Cerebellum | Right | 21, −67 −23 | 82 | 4.55 | 0.44 | 2.7 × 10−6 |
| 12, −58, −17 | 3.42 | 0.33 | — | |||
| Postcentral gyrus | Left | −57, −7, 28 | 165 | 4.49 | 0.44 | 3.5 × 10−6 |
| −60, −7, 16 | 4.02 | 0.39 | — | |||
| Precentral gyrus | −57, 5, 25 | 3.93 | 0.38 | 4.3 × 10−5 | ||
| Lingual gyrus | Left | −21, −64, −14 | 108 | 4.38 | 0.43 | 5.9 × 10−6 |
| −12, −64, −14 | 3.52 | 0.34 | — | |||
| Temporal operculum | Left | −48, −19, 4 | 41 | 4.28 | 0.42 | 9.3 × 10−6 |
| −39, −28, 10 | 3.99 | 0.39 | — | |||
| Thalamus | Right | 6, −22, 1 | 33 | 3.99 | 0.39 | 3.2 × 10−5 |
| 12, −16, 7 | 3.87 | 0.38 | — | |||
| Cingulate cortex | Right | 3, 14, 46 | 30 | 3.86 | 0.38 | 5.8 × 10−5 |
| 3, 20, 34 | 3.71 | 0.36 | — |
MNI, Montreal Neurological Institute.
Cluster size. Clusters may contain more than one brain region as indicated by multiple names or P values under one cluster size.
Pearson's product-moment correlation (calculated as Z 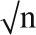 ).
).
Peaks were shown in the putamen (k = 7) and caudate (k = 11) for high-fat/low-sugar milkshake, but they fell under the minimum cluster size (k ≥ 15).
Direct comparison of equicaloric high-fat compared with high-sugar milkshakes on BOLD response
When we directly compared the BOLD response to the receipt of equicaloric high-fat compared with high-sugar milkshakes, we contrasted the high-fat/low-sugar compared with low-fat/high-sugar milkshakes, thereby controlling for energy density. High-fat compared with high-sugar equicaloric milkshakes caused greater activation in the bilateral hippocampus, caudate (Figure 1A), postcentral gyrus, left inferior frontal gyrus (Table 3; Figure 1A). The reverse contrast showed that the high-sugar compared with high-fat milkshakes caused greater activation in the bilateral insula (Figure 1B) extending into the putamen and the Rolandic operculum (Figure 1B) and left thalamus (Table 3).
FIGURE 1.
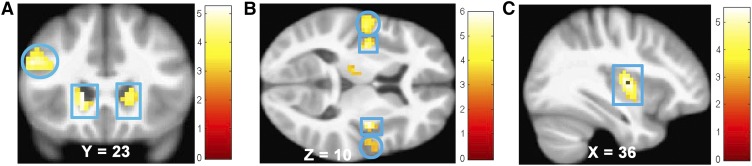
Activation in bilateral caudate [squares (MNI coordinates: left, −15, 23, 1, Z = 4.96, k = 42; right, 15, 23, 1, Z = 4.08, k = 25)] and left inferior frontal gyrus [circle (MNI coordinates: −48, 23, 25, Z = 4.79, k = 55)] in response to high-fat compared with high-sugar milkshakes (A), bilateral insula [squares (MNI coordinates: right, 36, −10, 10, Z = 5.73, k = 94; left, −33, −10, −7, Z = 5.11, k = 255)] extending into the Rolandic operculum [circles (MNI coordinates: right, 63, −13, 19, Z = 4.61, k = 70; left, −51, −10, 7, Z = 4.70)] in response to high-sugar compared with high-fat milkshakes (B), and right insula (MNI coordinates: 36, −7, 7, Z = 5.32, k = 94) in response to increasing sugar in low-fat milkshakes (C). MNI, Montreal Neurological Institute.
TABLE 3.
Direct comparison of equicaloric high-fat compared with high-sugar milkshakes on BOLD response
| Hemisphere of the brain | MNI1 coordinates x, y, z | k2 | Peak Z | r3 | Peak P | |
| High-fat compared with high-sugar milkshakes (high-fat/low-sugar compared with low-fat/high-sugar milkshakes) | ||||||
| Hippocampus | Left | −21, −37, 10 | 42 | 5.15 | 0.50 | 1.3 × 10−7 |
| Caudate | Left | −15, −23, 1 | 43 | 4.97 | 0.48 | 3.4 × 10−7 |
| Postcentral gyrus | Left | −21, −37, 70 | 34 | 4.46 | 0.43 | 4.2 × 10−6 |
| −15, −31, 73 | 3.86 | 0.37 | — | |||
| Inferior frontal gyrus | Left | −45, 23, 25 | 61 | 4.43 | 0.43 | 4.7 × 10−6 |
| Hippocampus | Right | 15, −34, 10 | 39 | 4.88 | 0.47 | 5.3 × 10−7 |
| 18, −25, 19 | 4.47 | 0.43 | — | |||
| Hippocampus | Right | 24,−37, 7 | 3.95 | 0.38 | 3.9 × 10−5 | |
| Cerebellum | Right | 15, −76, −32 | 37 | 4.19 | 0.41 | 1.4 × 10−5 |
| 9, −79, −41 | 3.77 | 0.36 | — | |||
| 27, −79, −41 | 3.76 | 0.36 | — | |||
| Caudate | Right | 18, 23, 1 | 27 | 4.06 | 0.39 | 2.4 × 10−5 |
| Postcentral gyrus | Right | 21, −37, 70 | 15 | 3.97 | 0.38 | 3.6 × 10−5 |
| Frontal pole | Left | −24, 59, 7 | 15 | 3.61 | 0.35 | 1.5 × 10−4 |
| −12, 62, 4 | 3.42 | 0.33 | — | |||
| High-sugar compared with high-fat milkshakes (low-fat/high-sugar compared with high-fat/low-sugar milkshakes) | ||||||
| Insula | Right | 36, −7, 7 | 78 | 5.32 | 0.52 | 5.1 × 10−8 |
| Putamen | Right | 30, −13, −5 | 3.70 | 0.36 | 1.8 × 10−4 | |
| Insula | Right | 42, 5, −5 | 3.51 | 0.34 | 2.2 × 10−4 | |
| Insula/putamen | Left | −36, −7, 7 | 154 | 4.59 | 0.45 | 2.2 × 10−7 |
| Rolandic operculum | Left | −51, 5, −2 | 4.41 | 0.42 | 5.2 × 10−6 | |
| Insula | Left | −36, −4, −2 | 4.22 | 0.41 | 1.2 × 10−5 | |
| Rolandic operculum | Right | 63, −13, 19 | 70 | 4.18 | 0.40 | 1.4 × 10−5 |
| Thalamus | Left | −15, −25, 7 | 15 | 3.68 | 0.36 | 1.2 × 10−4 |
| −9, −16, 7 | 3.34 | 0.32 | 4.2 × 10−4 |
MNI, Montreal Neurological Institute.
Cluster size. Clusters may contain more than one brain region as indicated by multiple names or P values under one cluster size.
Pearson's product-moment correlation (calculated as Z 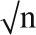 ).
).
Effect of increasing fat content in low- and high-sugar milkshakes on BOLD response
When we examined the effect of increasing fat content in low-sugar milkshakes (high-fat/low-sugar compared with low-fat/low-sugar milkshakes), we did not observe significant activation. Similarly, when we examined the effect of increasing fat in high-sugar milkshakes (high-fat/high-sugar compared with low-fat/high-sugar milkshakes), we did not observe significant activity.
Effects of increasing sugar content in low- and high-fat milkshakes on BOLD response
Increasing sugar in low-fat milkshakes (low-fat/high-sugar compared with low-fat/low-sugar milkshakes) resulted in increased activity in the bilateral insula (Figure 1C) and right Rolandic operculum (Table 4). When we examined the effect of increasing sugar content in high-fat milkshakes (high-fat/high-sugar compared with high-fat/low-sugar milkshakes), we did not observe significant activation.
TABLE 4.
Effect of increasing sugar on BOLD responsivity to low-fat milkshakes
| Hemisphere of the brain | MNI1 coordinates x, y, z | k2 | Peak Z | r3 | Peak P | |
| Increasing sugar in low-fat milkshakes (low-fat/high-sugar compared with low-fat/low-sugar milkshakes) | ||||||
| Insula | Right | 36, −7, 7 | 63 | 4.63 | 0.45 | 1.8 × 10−6 |
| Rolandic operculum/postcentral gyrus | Right | 60, −16, 22 | 23 | 4.05 | 0.39 | 2.5 × 10−5 |
| Insula | Left | −36,−4, 4 | 30 | 3.81 | 0.37 | 7.0 × 10−5 |
| −36, −10, 10 | 3.72 | 0.36 | 1.0 × 10−4 |
MNI, Montreal Neurological Institute.
Cluster size. Clusters may contain more than one brain region as indicated by multiple names or P values under one cluster size.
Pearson's product-moment correlation (calculated as Z 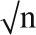 ).
).
DISCUSSION
Main-effects analyses indicated that the various milkshakes activated reward (putamen, caudate), gustatory (insula), and oral somatosensory regions (Rolandic operculum) as well as attention regions (anterior cingulate cortex), which converged with findings from previous sensory neuroscience studies (1, 2, 7–11, 22, 23). The low-fat/high-sugar milkshake tended to activate more classic reward regions (eg, putamen and caudate) than did the high-fat/high-sugar and other milkshakes. It was also noteworthy that the low-fat/low-sugar milkshake activated gustatory and somatosensory but not traditional reward regions. Thus, main-effects analyses provide evidence for the validity of our fMRI food-receipt paradigm.
The first aim of the current study was to evaluate the effect of a high-fat or high-sugar chocolate milkshake in the activation of these brain regions by using equicaloric milkshakes matched for flavor. Results indicated that high-fat compared with high-sugar milkshake receipt elicited greater activation in the bilateral caudate, postcentral gyrus, hippocampus, and left inferior frontal gyrus. In contrast, the high-sugar compared with high-fat milkshakes elicited greater activation in the bilateral insula extending into the putamen and the Rolandic operculum and left thalamus. This pattern of findings suggested that tastes of high-fat compared with high-sugar milkshakes prompted greater activation in regions involved in associative learning processes (caudate and hippocampus) and somatosensory regions (postcentral gyrus), whereas tastes of high-sugar compared with high-fat milkshakes prompted greater activation in regions associated with reward and motivation (insula and putamen), oral somatosensation (Rolandic operculum), and gustatory stimulation (thalamus) (24–28). To our knowledge, these findings make a novel contribution to the literature in that it appears that no previous study has compared the neural response to foods that are high in fat compared with high in sugar, with control for energy density and flavor.
The second aim was to evaluate the effects of increasing the fat or sugar milkshake content on the neural response in reward, gustatory, and oral somatosensory regions. Analyses revealed that increasing the fat contents of both low-sugar and high-sugar milkshakes did not lead to changes in the responsivity of brain regions implicated in these processes. In juxtaposition, the increased sugar content of low-fat milkshakes caused greater activation in the bilateral insula and right Rolandic operculum. Thus, increasing sugar content of a low-fat milkshake resulted in greater activity in gustatory regions, but increasing fat content did not affect the neural response. It is possible that this pattern of findings emerged because sweet is a primary reward, and it has been subsequently theorized that humans are predisposed with a sweet preference, whereas fat is viewed as a texture, and preferences are acquired through conditioning at an early age (29, 30). It is also conceivable that gustatory regions that encode tastes project more directly to reward-valuation regions than oral somatosensory regions that encode viscosity and, therefore, the fat content of foods. Collectively, results from the current study supported the notion that increasing the sugar content of food results in a greater neural response than increasing the fat content. Specifically, in every contrast that examined a relative increase in sugar, we observed increased activation in the insula and consistently observed greater activity in the Rolandic operculum and regions along typical dopaminergic pathways (ie, caudate, putamen, and anterior cingulate cortex). In addition, when we examined the degree of activation caused by individual milkshakes compared with the tasteless solution, the low-fat/high-sugar milkshake elicited the most robust activity in regions previously associated with rewarding food intake (striatum, insula, and oral somatosensory regions) relative to the other types of milkshakes.
Note that the pattern of results from the main-effects analyses did not suggest any type of interaction between fat and sugar contents (ie, high-fat/high-sugar did not consistently activate regions more than high-fat/low-sugar or low-fat/high-sugar). Thus, results implied that fat and sugar contents did not interact in the degree to which these macronutrients activated reward, gustatory, and oral somatosensory regions. This null finding is particularly interesting because of how fat and sugar are often paired in energy-dense foods in the food environment (31).
Collectively, the current results supported the notion that increases in the sugar content of food results in a greater neural response relative to changes in fat. Particularly, fat manipulation had less of an impact on the neural response particularly in hypothesized reward regions. However, results tended to show that increases in fat resulted in activity in oral somatosensory regions in most contrasts, dovetailing previous evidence (11). The less-consistent findings regarding fat manipulation could have been because the low-fat milkshake contained too-little fat (a floor effect), the high-fat milkshake contained too much fat (a ceiling effect), or the ability to detect differences in the fat content on a neural level was weaker relative to the ability to detect changes in sugar. However, the milkshakes were specifically designed for the current study and were done so in the most generalizable and controlled fashion possible by maintaining flavor and palatability without the use of artificial sweeteners or texture manipulation and presenting detectable differences in fat and sugar contents.
In conclusion, the current study showed neural differences of a generalizable food item that varied the fat and sugar contents without changes of flavor. The current results indicate that the high-sugar milkshake more-effectively recruited reward regions than did the equicaloric high-fat milkshake, and in addition, increasing the sugar content compared with increasing the fat content of milkshakes caused greater activity in brain regions previously associated with the intake of rewarding foods. Last, fat and sugar contents of the milkshakes did not appear to operate interactively to activate reward regions, which may highlight the need for examining individual differences surrounding the relative preference, taste sensitivity, and habitual intake of these macronutrients.
Acknowledgments
We thank the Lewis Center for Neuroimaging at the University of Oregon for their assistance in data collection for these projects.
The authors’ responsibilities were as follows—ES, KSB, and SY: designed and conducted the research and wrote the manuscript; KSB and SY: performed statistical analyses; ES: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Kringelbach ML, O'Doherty JJ, Rolls ET, Andrews CC. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 2003;13:1064–71. [DOI] [PubMed] [Google Scholar]
- 2.Small DM, Veldhuizen M, Felsted J, Mak Y, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron 2008;57:786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010;30:13105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stice E, Yokum S, Burger K, Epstein L, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci 2012;32:10093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 2006;57:87–115. [DOI] [PubMed] [Google Scholar]
- 6.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in the dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003;19:1709–15. [DOI] [PubMed] [Google Scholar]
- 7.Kadohisa M, Rolls E, Verhagen J. The primate amygdala: neuronal representations of the viscosity, fat texture, temperature, grittiness, and taste of foods. Neuroscience 2005;132:33–48. [DOI] [PubMed] [Google Scholar]
- 8.Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes (Lond) 2011;35:550–61. [DOI] [PubMed] [Google Scholar]
- 9.Verhagen JV, Rolls E, Kadohisa M. Neurons in the primate orbitofrontal cortex respond to fat texture independently of viscosity. J Neurophysiol 2003;90:1514–25. [DOI] [PubMed] [Google Scholar]
- 10.Verhagen JV, Kadohisa M, Rolls E. The primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness, temperature and taste of foods. J Neurophysiol 2004;92:1685–99. [DOI] [PubMed] [Google Scholar]
- 11.De Araujo IE, Rolls E. The representation in the human brain of food texture and oral fat. J Neurosci 2004;24:3086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabenhorst F, Rolls E, Parris B, D'Souza A. How the brain represents the reward value of fat in the mouth. Cereb Cortex 2010;20:1082–91. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol 2006;69:243–55. [DOI] [PubMed] [Google Scholar]
- 14.Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 1999;10:453–9. [DOI] [PubMed] [Google Scholar]
- 15.O'Doherty J, Rolls E, Francis S, Bowtell R, McGlone F. The representation of pleasant and aversive taste in the human brain. J Neurophysiol 2001;85:1315–21. [DOI] [PubMed] [Google Scholar]
- 16.Warwick ZS, Schiffman SS. Role of dietary fat in calorie intake and weight gain. Neurosci Biobehav Rev 1992;16:585–96. [DOI] [PubMed] [Google Scholar]
- 17.Avena NM, Bocarsly ME, Hoebel BG. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol 2012;829:351–65. [DOI] [PubMed] [Google Scholar]
- 18.Ng J, Stice E, Yokum S, Bohon C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 2011;57:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med 2000;44:457–65. [DOI] [PubMed] [Google Scholar]
- 20.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 22.Small DM. Taste representation in the human insula. Brain Struct Funct 2010;214:551–61. [DOI] [PubMed] [Google Scholar]
- 23.Small DM, DiLeone RJ. An introduction to the special issue. Biol Psychiatry 2013;73(9):779–801. [Google Scholar]
- 24.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8:1481–9. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Takeda M, Hattori N, Fukunaga M, Sasabe T, Inoue N, Ngai Y, Sawada T, Sadato N, Watanabe Y. Functional imaging of gustatory perception and imagery: “top-down” processing of gustatory signals. Neuroimage 2004;23:1271–82. [DOI] [PubMed] [Google Scholar]
- 26.Nolan-Poupart S, Veldhuizen MG, Geha P, Small DM. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite 2013;60:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of reward from food intake and anticipated intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117:924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004;5:483–94. [DOI] [PubMed] [Google Scholar]
- 29.Birch LL. Development of food preferences. Annu Rev Nutr 1999;19:41–62. [DOI] [PubMed] [Google Scholar]
- 30.Drewnowski A. Taste preferences and food intake. Annu Rev Nutr 1997;17:237–53. [DOI] [PubMed] [Google Scholar]
- 31.Drewnowski A. Obesity and the food environment: dietary energy density and diet costs. Am J Prev Med 2004;27:154–62. [DOI] [PubMed] [Google Scholar]


