Abstract
Background: Although estimation of percentage body fat with the Slaughter skinfold-thickness equations (PBFSlaughter) is widely used, the accuracy of this method has not been well studied.
Objective: The objective was to determine the accuracy of the Slaughter skinfold-thickness equations.
Design: We compared agreement between PBFSlaughter and estimations derived from dual-energy X-ray absorptiometry (PBFDXA) in 1169 children in the Pediatric Rosetta Body Composition Project and the relation to cardiovascular disease risk factors, as compared with body mass index (BMI), in 6725 children in the Bogalusa Heart Study.
Results: PBFSlaughter was highly correlated (r = 0.90) with PBFDXA, but it markedly overestimated levels of PBFDXA in children with large skinfold thicknesses. In the 65 boys with a sum of skinfold thicknesses (subscapular- plus triceps-skinfold thicknesses) ≥50 mm, PBFSlaughter overestimated PBFDXA by 12 percentage points. The comparable overestimation in girls with a high skinfold sum was 6 percentage points. We also found that, after adjustment for sex and age, BMI showed slightly stronger associations with lipid, lipoprotein, insulin, and blood pressure values than did PBFSlaughter.
Conclusions: These results indicate that PBFSlaughter, which was developed among a group of much thinner children and adolescents, is fairly accurate among nonobese children, but markedly overestimates the body fatness of children who have thick skinfold thicknesses. Furthermore, PBFSlaughter has no advantage over sex- and age-adjusted BMIs at identifying children who are at increased risk of cardiovascular disease based on lipid, lipoprotein, insulin, and blood pressure values.
INTRODUCTION
The BMI is widely used as a screening tool to identify children and adolescents who are obese, and a high BMI in childhood is associated with adverse levels of cardiovascular disease risk factors, the initial stages of atherosclerosis and adult obesity (1, 2). Children and adolescents with a high BMI also tend to have a high level of body fatness, but because BMI is based only on weight and height, BMI can be an inaccurate indicator of body fatness, particularly among those who have normal or relatively low levels of body fatness (3). An additional complication is that the relation of BMI to body fatness differs across race-ethnicity groups (4–6). At equivalent BMI levels, Asians generally have more body fat and blacks have less body fat than do whites.
Despite large measurement errors associated with skinfold thicknesses (7), they continue to be widely used among children and adolescents (8–11) because many investigators believe that they provide a more direct and accurate estimate of body fatness than does BMI. The stronger association of body fatness with skinfold thicknesses than with BMI (3, 12–14), however, does not necessarily indicate that skinfolds can more accurately identify children who have excess body fatness or adverse levels of cardiovascular disease (CVD)5 risk factors. We have found (15), for example, that the stronger association of skinfold thicknesses (compared with BMI) with body fatness largely results from the inability of BMI to accurately assess body fatness among children who have a BMI-for-age below the 85th percentile of the CDC growth charts. Furthermore, several studies, using various techniques to assess body fatness have found that adverse levels of CVD risk factors are related similarly to both BMI and body fatness (13, 16–20).
The objective of the current study was to determine the accuracy of the Slaughter skinfold thickness equations, which are based on sex, maturation, and skinfold thicknesses, at predicting levels of percentage body fat determined by using dual-energy X-ray absorptiometry (DXA; PBFDXA) among 1196 children and adolescents from the Pediatric Rosetta Body Composition Project. We also examined whether percentage body fat estimated by using the Slaughter skinfold-thickness equations (PBFSlaughter) was more strongly associated with levels of cardiovascular disease risk factors than was BMI in 6725 children and adolescents aged 5–17 y in the Bogalusa Heart Study. Previous studies (21–23) of the validity of the Slaughter equations have been based on smaller samples and have not examined associations with levels of cardiovascular disease risk factors.
SUBJECTS AND METHODS
Study populations
The current analyses are based on 2 data sets: the Pediatric Rosetta Body Composition Project (which includes PBFDXA) and the Bogalusa Heart Study (which includes various risk factors). The methods used in these studies was described previously (24, 25), and both studies included measures of weight, height, and skinfold (triceps and subscapular) thicknesses. The procedures followed in both studies were in accordance with the ethical standards of the institution, and approval was obtained from the relevant committees on human subjects.
Pediatric Rosetta Body Composition Project
Between 1995 and 2000, 1196 healthy volunteers aged 5–18 y were recruited in New York City through newspaper notices, announcements at schools and activity centers, and word of mouth. The Institutional Review Board of St Luke's–Roosevelt Hospital Center approved the study protocol, assent was obtained from each volunteer (when appropriate), and informed consent was obtained from each volunteer's parent or guardian. A questionnaire was used to establish race and ethnicity; the criterion was a consistent background (white, black, Hispanic, or Asian) for all 4 grandparents (6). Most Asian participants were of Chinese or Korean background, and most Hispanic participants were of Dominican or Puerto Rican origin. There were no height or weight restrictions to entry into the study, and normal heath was confirmed by a medical history from the parent or guardian and a physical examination.
Skinfold thicknesses were measured on the right side of the body to the nearest 1.0 mm with a Lange caliper by using procedures recommended by Lohman et al (26). The average of 2 readings was recorded, and subjects were measured by 1 of 2 examiners during the study period. Whole-body DXA scans were performed by using GE Lunar Corporation models DPX (with pediatric software version 3.8G) and DPX-L (with pediatric software 1.5G) (27). The scan mode was chosen according to the weight guidelines provided by the manufacturer, and each scan provided an estimate of PBFDXA. For quality control, an anthropomorphic spine phantom made up of calcium hydroxyapatite embedded in a Lucite block was scanned with both DXA instruments each morning before subject evaluation and immediately before and after all DXA maintenance visits. Bottles (8 L) of ethanol and water, simulating fat and fat-free soft tissues, respectively, were scanned monthly as quality-control markers.
Bogalusa Heart Study
The Bogalusa (Louisiana) Heart Study is a community-based (Ward 4 of Washington Parish) study of CVD risk factors in early life (24). Seven cross-sectional examinations of schoolchildren in this community were conducted between 1973 and 1994, with each consisting of ∼3500 children and adolescents. Assent was obtained from all participants, and informed consent was obtained from each volunteer's parent or guardian. All schoolchildren between the ages of 5 and 17 y in Ward 4 of Washington Parish were considered eligible. Because of the repeated cross-sectional design of the study, many subjects participated in 2 or more of the 7 examinations. A questionnaire, completed by the parents, was used to establish the race of the participants.
The triceps and subscapular skinfold thicknesses were each measured 3 times to the nearest 1 mm by using Lange Skinfold Calipers. In each study, skinfold thicknesses measured by 2–3 trained observers, and the mean of the 3 measurements for each site was used in the analyses. Because our focus was on the relation of BMI and PBFSlaughter to levels of various risk factors, we restricted the analyses to 4 studies (1981–1982 through 1992–1994) that included levels of fasting insulin and both skinfold thicknesses. A total of 10,726 examinations were conducted among 7079 children and adolescents aged 5–17 y who reported that they were fasting; ∼87% of this group reported that they had fasted.
Of these 10,726 examinations, we deleted 1) 591 in which information was missing for any risk factor [triglycerides, LDL cholesterol, HDL cholesterol, insulin, systolic blood pressure (SBP) or diastolic blood pressure (DBP)]; 2) 29 in which data for weight, height, or a skinfold thickness measure was missing; 3) 11 in which a girl reported being pregnant; and 4) 7 in which a child's race was reported as other than white or black. These exclusions resulted in an analytic sample of 10,088 examinations among 6725 children. Of these children, 4166 participated in only 1 examination, 1853 participated in 2 examinations, and 706 participated in 3 or 4 examinations.
BMI and skinfold thicknesses
Body weight and height were measured in both studies by using standardized techniques, and BMI (in kg/m2) was calculated as a measure of relative weight. We calculated BMI-for-age z scores (SDs) and percentiles for each child based on the CDC growth charts (28), and these represent the BMIs of the examined children relative to their sex-age peers in the United States between 1963 and 1994. A child with a BMI-for-age ≥95th percentile of the CDC reference population is considered to be obese, and a child with a BMI ≥120% of the 95th percentile is considered to be extremely obese (29).
Because adjustment for sex and age through the use of the CDC BMI-for-age z scores may not be optimal for all analyses (30) and can be inaccurate at very high BMI levels (29), we also used the residuals of sex-specific regression models that predicted BMI from age with the use of restricted cubic splines. We refer to these residuals as a child's “adjusted BMI,” and in contrast with the CDC BMI-for-age z scores, these adjusted values are in the same units (kg/m2) as BMI.
The sum of subscapular and triceps skinfold thicknesses (SF sum) were used, in combination with sex, age, and race to estimate PBFSlaughter as described on page 719 of Slaughter et al (31). The Slaughter equations are available elsewhere (see Supplemental Table 1 under “Supplemental data” in the online issue) and are based on the sum of these 2 skinfold thicknesses, with the intercepts and regression coefficients varying by sex and SF sum and among boys by stage of maturation and race (white or black). We used the equations for white boys to estimate percentage body fat among Asian and Hispanic boys. As has been done in other investigations (8), we used the age of the child in these calculations: boys younger than 12 y were considered prepubescent, boys aged 12.0–13.9 y pubescent, and boys aged ≥14 y postpubescent. Additional analyses (data not shown) based on Tanner Stage (assessed by a physician or nurse, or by the child) indicated that PBFSlaughter based on age was highly correlated (eg, r = 0.984 in the Pediatric Rosetta Study) with PBFSlaughter based on maturation stage.
CVD risk factors
Serum concentrations of total cholesterol and triglycerides were measured, by using enzymatic procedures, in a centralized laboratory that met the requirements of the CDC Lipid Standardization Program. LDL and HDL cholesterol measurements were based on a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis (24). Plasma insulin was measured with a radioimmunoassay procedure (Phadebas Insulin Kit). Sitting SBP and DBP (4th Korotkoff sound) were measured on the right arm 6 times by trained observers with a mercury sphygmomanometer (Baumanometer); the mean of 6 measurements was used in all analyses (32).
Statistical analyses
All analyses were performed in R (33). Because the distributions of several characteristics were skewed, descriptive statistics included the median and IQR (75th percentile minus 25th percentile). The IQR is less strongly influenced by extreme values than is the SD.
For data from the Pediatric Rosetta Study, we examined levels of the PBFDXA and PBFSlaughter within categories of sex and the SF sum (4 categories, with sex-specific levels <33rd percentile, 33rd to 66th percentiles, 67th to 89th percentiles, and ≥90th percentile). We also examined plots of PBFDXA compared with PBFSlaughter, and we illustrated the smoothed association with Lowess (locally weighted scatter plot smoothing). Rather than assuming an overall function (eg, quadratic) for the association, each y value in Lowess is estimated from a weighted regression analysis based on surrounding points. The agreement between levels of PBFDXA and PBFSlaughter was also assessed by using Bland-Altman plots (34), in which the difference between the 2 variables (PBFSlaughter − PBFDXA) was plotted versus their mean. We also plotted the SF sum versus levels of PBFDXA and PBFSlaughter. At an SF sum >35 mm, the relation of skinfold thickness to PBFSlaughter is linear (31), with each 1-mm increase in the SF sum resulting in a 0.783 (boys) or 0.546 (girls) increase in percentage body fat.
For data from the Bogalusa Heart Study, we focused on differences in the relation of risk factor levels to adjusted BMI, BMI-for-age z score (CDC growth charts), and PBFSlaughter. Adjusted levels of BMI and the CVD risk factors were obtained from sex-specific regression models in which each characteristic was predicted by age (modeled by using restricted cubic splines). The residuals of these models, representing sex- and age-adjusted levels, were then used in the analysis of correlations. Principal components analysis (35) was used to derive an overall summary of the 6 risk factors. This technique converts a set of correlated variables into values (components) that are uncorrelated, and the first principal component, which has the largest variance of all components, was used as an overall risk factor summary. Correlations between the first component and levels of the 6 risk factors ranged from r = 0.39 (DBP) to r = 0.76 (triglycerides).
Because 61% of the children in the Bogalusa Heart Study participated in more than one examination, the observations are not independent. We therefore used the bootstrapping package in R (36) to calculate P values for the differences in the magnitudes of the correlations with BMI-for-age, adjusted BMI, and PBFSlaughter. These P values were based on the distributions of the observed differences between the observed correlations in 2500 replications in which the children were sampled with replacement. Children, rather than observations, were sampled with replacement to account for the within-child clustering.
RESULTS
Pediatric Rosetta Body Composition Study
Various characteristics of the sample in the Pediatric Rosetta Body Composition Project are shown among boys and girls in Table 1. As indicated by the median values of 0.54 (boys) and 0.52 (girls) for BMI-for-age, BMI levels in the Rosetta Body Composition Project were substantially higher than those in the CDC reference population, but there was little difference between boys and girls. About 15% of both boys and girls were obese, and 5% were extremely obese (BMI ≥120% of the CDC 95th percentile). In contrast, relative differences in median levels of the SF sum, PBFDXA, and PBFSlaughter between boys and girls ranged from 33% (subscapular skinfold thickness) to 56% (PBFDXA). As compared with the median level of percentage body fat based on DXA, the median level estimated by the Slaughter equation was higher among boys (18.1% compared with 16.5%) but was lower among girls (24.5% compared with 25.7%). Additional analyses indicated that PBFSlaughter and the SF sum were highly correlated (r = 0.98) and that both were strongly associated (r = 0.87–0.89) with PBFDXA. The correlation between PBFDXA and BMI-for-age (r ∼ 0.80) was significantly (P < 0.001) weaker than the PBFDXA compared with PBFSlaughter association.
TABLE 1.
Descriptive characteristics of 5- to 18-y-olds in the Pediatric Rosetta Body Composition Project1
| Characteristic | Boys (n = 626) | Girls (n = 570) |
| Age (y) | 11.9 ± 62 | 11.5 ± 6 |
| BMI (kg/m2) | 19.7 ± 6 | 19.8 ± 6 |
| BMI-for-age (SD units)3 | 0.54 ± 1.5 | 0.52 ± 1.5 |
| Obese (%)4 | 16 | 15 |
| Extremely obese (%)4 | 5 | 5 |
| White (%) | 26 | 25 |
| Black (%) | 21 | 25 |
| Hispanic (%) | 15 | 14 |
| Asian (%) | 31 | 29 |
| Skinfold thicknesses (mm) | ||
| Triceps | 12.0 ± 11 | 16.5 ± 12 |
| Subscapular | 9.0 ± 9 | 12.0 ± 12 |
| SF sum | 20.0 ± 18 | 28.0 ± 23 |
| PBFSlaughter (%) | 18.1 ± 15 | 24.5 ± 15 |
| PBFDXA (%) | 16.5 ± 15 | 25.7 ± 15 |
PBFDXA, percentage body fat derived by using dual-energy X-ray absorptiometry; PBFSlaughter, percentage body fat derived by using the Slaughter skinfold-thickness equations; SF sum, sum of subscapular and triceps skinfold thicknesses.
Medians ± IQRs (all such values).
z scores (SD scores) of children relative to the 2000 CDC growth charts.
Obesity is defined as a BMI-for-age ≥95th percentile of the CDC reference population or a BMI (in kg/m2) ≥30. Extreme obesity is a subset of the obese category and is defined as BMI ≥120% of the CDC 95th percentile.
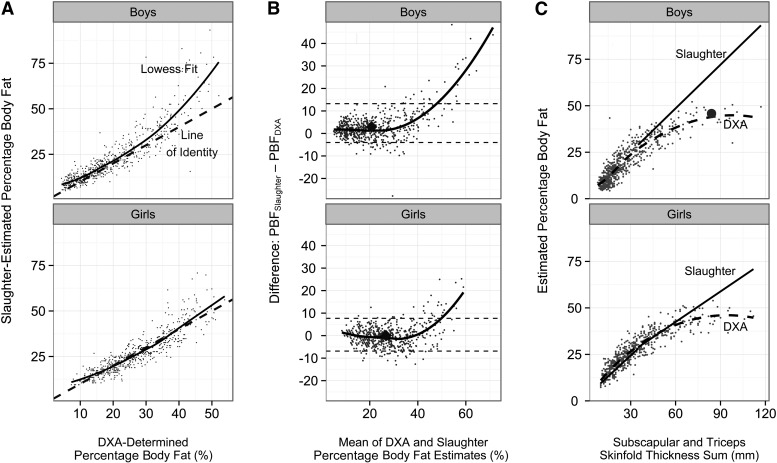
As seen in Figure 1A and Table 2, however, the agreement between PBFSlaughter and PBFDXA varied substantially by the level of body fatness. At low levels of the SF sum (<15 mm, boys; <20 mm, girls), estimates of PBFSlaughter and PBFDXA differed, on average, by <1 percentage point (final column of Table 2). At intermediate levels of SF sum among boys (15–49 mm), PBFSlaughter overestimated PBFDXA by 1.5–2.6 percentage points, whereas among girls with intermediate SF sum levels (20–59 mm), median levels of PBFSlaughter, and PBFDXA differed by <1 percentage point. Differences between PBFSlaughter and PBFDXA, however, were more evident at high levels of body fatness, particularly among boys. Among the 65 boys who had an SF sum ≥50 mm, the Slaughter equation overestimated PBFDXA, on average, by 12.0 percentage points. Among the 59 girls who had an SF sum ≥60 mm, PBFSlaughter overestimated PBFDXA by 5.8 percentage points.
FIGURE 1.
Values are based on the 626 boys and 570 girls in the Pediatric Rosetta Body Composition Project. A: Relation of DXA-determined PBF (PBFDXA) with PBF calculated from the Slaughter equations (PBFSlaughter). Data points represent the individual children, the dashed line is the line of identity (PBFDXA = PBFSlaughter), and the solid line is the smoothed (Lowess) curve. B: Bland-Altman plot for the agreement between PBFDXA and PBFSlaughter. Data points represent the individual children, and the black line is the Lowess curve. Overall medians are represented by the large diamonds, and the dashed lines represent the 95% CIs for the agreement between the 2 methods. C: Relation of SF sum to percentage body fat. Based on the Slaughter equations, all estimates of PBF for children with an SF sum ≥35 mm would fall on straight lines, with slopes of 0.783 (boys) and 0.546 (girls). The data points represent the DXA-calculated body fatness of each child, and the dashed lines represent the Lowess-estimated association between SF sum and PBFDXA. Among boys, the discrepancies between PBFDXA and PBFSlaughter increase substantially at higher SF sum levels. The large circle represents a boy with an SF sum of 84 mm (first row of Table 3). DXA, dual-energy X-ray absorptiometry; Lowess, locally weighted scatter plot smoothing; PBF, percentage body fat; PBFDXA, percentage body fat estimated by using dual-energy X-ray absorptiometry; PBFSlaughter, percentage body fat estimated by using the Slaughter skinfold-thickness equations; SF sum, sum of subscapular and triceps skinfold thicknesses.
TABLE 2.
Levels of various characteristics within categories of body fatness in the Pediatric Rosetta Body Composition Project1
| Sex and SF sum category2 | Subjects | Age | Nonwhite | CDC BMI z score | Obese | SF sum | PBFSlaughter | PBFDXA | PBFSlaughter – PBFDXA |
| n | y | % | % | mm | % | % | % | ||
| Boys | |||||||||
| <15 mm | 143 | 9.8 ± 73 | 70 | −0.39 ± 1.1 | 0 | 12 ± 2 | 9.9 ± 3 | 9.6 ± 4 | 0.5 ± 3 |
| 15–24 mm | 240 | 11.9 ± 6 | 71 | 0.28 ± 0.9 | 2 | 18 ± 5 | 15.9 ± 5 | 14.5 ± 7 | 1.5 ± 5 |
| 25–49 mm | 178 | 12.4 ± 6 | 79 | 1.22 ± 0.7 | 22 | 33 ± 12 | 28.5 ± 9 | 25.7 ± 10 | 2.6 ± 6 |
| ≥50 mm | 65 | 12.2 ± 4 | 80 | 2.10 ± 0.5 | 89 | 61 ± 19 | 49.4 ± 15 | 38.9 ± 8 | 12.0 ± 10 |
| Girls | |||||||||
| <20 mm | 149 | 9.1 ± 4 | 70 | −0.26 ± 1.2 | 0 | 16 ± 5 | 15.5 ± 5 | 15.4 ± 6 | −0.6 ± 5 |
| 20–34 mm | 213 | 12.0 ± 6 | 71 | 0.32 ± 1.0 | 1 | 26 ± 7 | 23.3 ± 4 | 24.5 ± 7 | −0.9 ± 5 |
| 35–59 mm | 149 | 13.0 ± 5 | 84 | 1.19 ± 0.8 | 24 | 43 ± 10 | 33.2 ± 5 | 35.0 ± 7 | 0.2 ± 6 |
| ≥60 mm | 59 | 13.5 ± 5 | 80 | 2.06 ± 0.6 | 80 | 72 ± 17 | 49.0 ± 9 | 43.5 ± 7 | 5.8 ± 9 |
PBFDXA, percentage body fat estimated by using dual-energy X-ray absorptiometry; PBFSlaughter, percentage body fat estimated by using the Slaughter skinfold-thickness equations; SF sum, sum of subscapular and triceps skinfold thicknesses.
Cutoffs for the SF sum categories approximate the 33rd, 67th, and 90th percentiles within each sex; cutoffs were rounded to the nearest 5 mm.
Median ± IQR (all such values).
The overestimation of PBFDXA by the Slaughter equation is also evident in Bland-Altman plots of the agreement between PBFSlaughter and PBFDXA (Figure 1B). The 95% CIs of agreement were −4 and 13 among boys and −7 and 8 among girls, respectively. The extent of overestimation by PBFSlaughter increased greatly with the level of body fatness, and correlations between the average for the 2 estimates and their difference (Slaughter − DXA) were r = 0.54 (boys) and r = 0.26 (girls). Additional analyses indicated that PBFSlaughter overestimated PBFDXA by >20 percentage points among 14% of obese (BMI ≥ CDC 95th percentile) boys, but in only 1 of the 523 nonobese boys. The overestimation among obese girls was less marked, with only 5% having a PBFSlaughter value that overestimated PBFDXA by >20 percentage points.
Characteristics of the 12 boys who had an SF sum of >80 mm are shown in Table 3. Ten of these boys were extremely obese (BMI ≥120% of the CDC 95th percentile), with the 2 exceptions having BMIs of 29.4 and 31.2. PBFSlaughter overestimated PBFDXA by ≥20 percentage points for each of the 12 boys; for 3 boys, the overestimation was >40 percentage points.
TABLE 3.
Characteristics of the 12 boys who had an SF sum >80 mm in the Pediatric Rosetta Body Composition Project1
| Skinfold thickness |
|||||||||||
| Race or ethnicity | Age | Weight | Height | BMI | Percentage of 95th percentile2 | Triceps | Subscapular | Sum | PBFSlaughter | PBFDXA | PBFSlaughter – PBFDXA |
| y | kg | m | kg/m2 | % | mm | % | % | % | |||
| Hispanic | 12 | 58.3 | 1.41 | 29.4 | 119 | 44 | 40 | 84 | 67.4 | 45.8 | 21.6 |
| Asian | 12 | 72.0 | 1.53 | 30.7 | 127 | 40 | 48 | 88 | 70.5 | 41.0 | 29.5 |
| Black | 11 | 90.0 | 1.65 | 32.9 | 141 | 38 | 51 | 89 | 71.3 | 42.6 | 28.7 |
| Other | 9 | 66.3 | 1.46 | 31.1 | 142 | 40 | 50 | 90 | 72.1 | 41.1 | 31.0 |
| Hispanic | 12 | 73.4 | 1.51 | 32.0 | 131 | 52 | 41 | 93 | 74.4 | 48.4 | 26.0 |
| Other | 14 | 89.6 | 1.66 | 32.3 | 123 | 45 | 49 | 94 | 75.2 | 42.3 | 32.9 |
| Black | 14 | 107.3 | 1.70 | 37.0 | 140 | 54 | 42 | 96 | 76.8 | 43.2 | 33.6 |
| Asian | 15 | 93.7 | 1.73 | 31.2 | 115 | 44 | 54 | 98 | 78.3 | 30.0 | 48.3 |
| White | 15 | 128.4 | 1.82 | 38.7 | 143 | 48 | 50 | 98 | 78.3 | 50.5 | 27.8 |
| Black | 18 | 133.4 | 1.86 | 38.7 | 133 | 53 | 48 | 101 | 80.7 | 46.6 | 34.1 |
| Asian | 9 | 63.2 | 1.47 | 29.1 | 133 | 50 | 54 | 104 | 83.0 | 40.9 | 42.1 |
| Black | 11 | 105.0 | 1.61 | 40.3 | 168 | 52 | 65 | 117 | 93.2 | 49.4 | 43.8 |
Observations are sorted according to SF sum, which ranged from 84 to 117 mm in this subset. Of the 626 boys, these 12 boys had the highest SF sums. PBFDXA, percentage body fat estimated by using dual-energy X-ray absorptiometry; PBFSlaughter, percentage body fat estimated by using the Slaughter skinfold-thickness equations; SF sum, sum of subscapular and triceps skinfold thicknesses.
Because the z score estimates of very high BMI levels is inaccurate in the CDC growth charts, it has been suggested that the BMIs of very obese children be expressed as a percentage of the 95th percentile (29); 120% of the 95th percentile has been used as an indicator of extreme obesity.
Because 11 of the 12 boys with the thickest SF sums were nonwhite, we performed additional analyses to assess the effect of race-ethnicity on the observed associations. These results indicated that there were relatively small, but statistically significant, differences in PBFDXA across race-ethnicity groups after levels of sex, age, and SF sum were controlled for. At equivalent levels of these covariates, the mean PBFDXA of Asian boys was 1 percentage point lower than that of white boys, and the mean PBFDXA of Asian girls was 2 percentage points lower than that of white girls.
Bogalusa Heart Study
Various characteristics among 6725 children who were examined in the Bogalusa Heart Study between 1981 and 1994 are shown in Table 4. As assessed by the median levels of BMI-for-age (∼0.27) and SF sum (19 mm, boys; 26 mm, girls), and the prevalence of obesity (12%), these children tended to be thinner than those in the Pediatric Rosetta Study. Levels of the 6 examined risk factors each differed significantly (P < 0.01) between boys and girls. As assessed in regression models that accounted for age and the within-child clustering, girls had higher triglyceride, LDL cholesterol, fasting insulin, and DBP values but slightly lower HDL cholesterol and SBP values than did boys.
TABLE 4.
Levels of various characteristics among children in the Bogalusa Heart Study1
| Boys | Girls | |
| Examinations (n) | 4985 | 5103 |
| Age (y) | 11.4 ± 52 | 11.3 ± 5 |
| Black (%) | 37 | 38 |
| BMI (kg/m2 ) | 18.2 ± 5.1 | 18.5 ± 5.3 |
| BMI-for-age (z score)3 | 0.27 ± 1.4 | 0.28 ± 1.6 |
| Obese (%)4 | 12 | 12 |
| Extremely obese (%)4 | 3 | 3 |
| Triceps skinfold thickness (mm) | 11.7 ± 9 | 16.0 ± 10 |
| Subscapular skinfold thickness (mm) | 7.0 ± 6 | 9.7 ± 9 |
| SF sum | 18.7 ± 13 | 26.0 ± 18 |
| PBFSlaughter (%) | 16.4 ± 11 | 23.3 ± 12 |
| Triglycerides (mg/dL) | 59 ± 34 | 64 ± 36 |
| LDL cholesterol (mg/dL) | 96 ± 33 | 100 ± 33 |
| HDL cholesterol (mg/dL) | 56 ± 21 | 55 ± 21 |
| Insulin (mU/L) | 7.8 ± 6 | 9.2 ± 6 |
| SBP (mm Hg) | 102 ± 15 | 102 ± 14 |
| DBP (mm Hg) | 61 ± 12 | 63 ± 12 |
Values are based on all examinations conducted among 3358 boys and 3367 girls. Most (61%) children participated in only 1 examination, but 1852 were examined 2 times, and 706 were examined ≥3 times. With the exception of BMI-for-age z score, all differences between boys and girls were statistically significant at the 0.01 level as assessed by ANCOVA that accounted for the within-child clustering. DBP, diastolic blood pressure; PBFSlaughter, percentage body fat derived by using the Slaughter skinfold-thickness equations; SBP, systolic blood pressure; SF sum, sum of subscapular and triceps skinfold thicknesses.
Median ± IQR (all such values).
z score (SD score) of children relative to the 2000 CDC growth charts.
Obesity is defined as a BMI-for-age ≥CDC 95th percentile. Extreme obesity is a BMI ≥120% of the CDC 95th percentile.
Correlations between the levels of the various risk factors (columns) with levels of adjusted BMI, BMI-for-age, and PBFSlaughter are shown in Table 5. (Adjusted BMI values are the residuals of a regression of BMI on age, modeled with cubic splines, within each sex.) Overall, as well as in sex-stratified analyses, adjusted BMI was more strongly related to the risk factor summary than was PBFSlaughter (P < 0.05 for each comparison); among all children, the observed correlations with the risk factor summary were r = 0.47 (adjusted BMI) and r = 0.42 (PBFSlaughter). The magnitudes of the relation of the risk factor summary to BMI-for-age were between those for adjusted BMI and PBFSlaughter in the overall sample and among girls; however, among boys, the risk factor summary was related similarly to levels of BMI-for-age and PBFSlaughter.
TABLE 5.
Correlations of BMI and PBFSlaughter with age-adjusted levels of CVD risk factors in the Bogalusa Heart Study1
| Characteristic | Risk factor summary | Triglycerides2 | LDL cholesterol | HDL cholesterol | Insulin2 | SBP | DBP |
| Overall | |||||||
| Adjusted BMI | 0.47* | 0.32* | 0.17 | −0.22* | 0.46* | 0.22* | 0.14* |
| BMI-for-age | 0.44* | 0.28 | 0.15* | −0.20* | 0.42* | 0.24* | 0.16* |
| PBFSlaughter | 0.42 | 0.30 | 0.17 | −0.19 | 0.39 | 0.18 | 0.13 |
| Boys | |||||||
| Adjusted BMI | 0.48* | 0.33 | 0.18 | −0.24* | 0.46* | 0.21* | 0.13 |
| BMI-for-age | 0.44 | 0.29* | 0.16* | −0.22* | 0.41 | 0.22* | 0.13* |
| PBFSlaughter | 0.44 | 0.34 | 0.19 | −0.20 | 0.41 | 0.17 | 0.11 |
| Girls | |||||||
| Adjusted BMI | 0.47* | 0.30* | 0.16* | −0.20* | 0.46* | 0.24* | 0.16 |
| BMI-for-age | 0.45* | 0.28 | 0.14 | −0.19 | 0.43* | 0.27* | 0.18* |
| PBFSlaughter | 0.42 | 0.28 | 0.15 | −0.18 | 0.41 | 0.20 | 0.16 |
P values assess whether the correlation between the risk factor and either adjusted BMI or BMI-for-age is equal to the correlation between the risk factor and PBFSlaughter. Adjusted levels of BMI and risk factors were obtained from sex-specific regression models in which age was used to predict the level of each risk factor; residuals from these models represent sex- and age-adjusted levels. *P < 0.05 for H0: correlation with adjusted BMI or BMI-for-age is equal in magnitude to the correlation with PBFSlaughter. The P values were obtained through bootstrapping that accounted for the within-child clustering. For example, in the overall sample, the risk factor summary was more strongly associated with adjusted BMI (r = 0.47) and with BMI-for-age (r = 0.44) than with PBFSlaughter (r = 0.42); P < 0.05 for both comparisons. CVD, cardiovascular disease; DBP, diastolic blood pressure; PBFSlaughter, percentage body fat derived by using the Slaughter skinfold-thickness equations; SBP, systolic blood pressure.
Levels of triglycerides and insulin were log transformed.
Of the individual risk factors, all except LDL cholesterol were more strongly related to levels of adjusted BMI than to PBFSlaughter (P < 0.05 for each comparison) in the overall sample. Furthermore, BMI-for-age was also more strongly associated with levels of most of the risk factors than was PBFSlaughter. However, no statistically significant differences were found between the associations with triglyceride concentrations (r = 0.28 compared with 0.30), and LDL-cholesterol concentrations were more strongly associated with PBFSlaughter than with BMI-for-age (r = 0.15 compared with 0.17, P < 0.05). Differences were less consistent in sex-specific analyses, but there was no evidence that PBFSlaughter was more strongly associated with levels of any risk factor than was adjusted BMI.
DISCUSSION
Although BMI can be an inaccurate indicator of body fatness, particularly among children who are not overweight or obese (3), little evidence indicates that body fatness (as assessed by skinfold thicknesses or other methods) is more strongly associated with levels of CVD risk factors than is BMI (13, 16–20, 37). The results of the current study indicate that body fatness—as estimated from a prediction equation based on skinfold thicknesses, sex, maturation (age), and race (31)—was more strongly correlated with PBFDXA than was BMI (r ∼ 0.90 compared with 0.80). Although PBFSlaughter was a fairly accurate estimator of PBFDXA among nonobese children, it greatly overestimated the DXA-calculated body fatness of obese children. Among boys with an SF sum ≥50 mm, for example, the Slaughter equation estimates were, on average, 12 percentage points higher than those based on DXA. We also found that, after adjustment for sex and age, BMI was more strongly associated with levels of cardiovascular disease risk factors than was PBFSlaughter. Although some differences were not statistically significant, in no case were correlations with PBFSlaughter stronger than those with adjusted BMI.
In general, skinfold thicknesses (and estimates derived from them) are more strongly correlated with body fatness as measured by more accurate methods than is BMI, but some of the observed differences have been relatively small (3, 13, 38–40). For example, levels of PBFDXA among adolescents were almost as strongly correlated with levels of BMI (r = 0.87–0.89) as with skinfold thicknesses (r = 0.92–0.93) (13). Furthermore, the accuracy of skinfold thicknesses in estimating body fatness is likely to vary across sites and equations. Bray et al (3), for example, found that most skinfold thickness equations were better predictors of body fatness (determined from a 4-compartment model) than was BMI (R2 = 0.85 and 0.67), but that one skinfold equation was very inaccurate (R2 = 0.51). Previous studies of the validity of the Slaughter equations have emphasized the large random and/or systematic errors that can occur as compared with DXA (22, 23) and hydrodensitometry (21). Despite these errors, some investigators have concluded that the Slaughter equations could be useful in field or clinical studies (22).
Levels of body fatness differ substantially across race-ethnicity groups, and we previously reported that, at equivalent levels of BMI-for-age, black children have lower levels (mean: 3 percentage points) of PBFDXA than do white children (6). Several investigators have also found that the body fatness of Asians is generally greater than that of whites (41), but these differences appear to vary across Asian subpopulations (4). We found that, at equivalent levels of age and SF sum, PBFDXA varied (P < 0.01) across race-ethnicity groups, with the PBFDXA of Asian children being, on average, 1–2 percentage points lower than that of white children. Note that the Slaughter equations were developed in a sample of white and black children, but ∼50% of the children in the Pediatric Rosetta Study were Hispanic, Asian, or ”other.”
It is possible that much of the discrepancy between PBFSlaughter and PBFDXA at high SF sum levels results from the relatively low levels of body fatness of the sample (n = 242 children) in which the Slaughter equations were developed (31). Although BMI levels were not reported for these subjects, levels of weight and skinfold thicknesses differed substantially from those in the current analyses. For example, whereas the mean SF sum among postpubescent boys in Slaughter et al (31) was 18 mm (n = 58), mean levels among similarly aged boys were 26 mm (n = 210) in the Pediatric Rosetta Body Composition Project and 25 mm (n = 1299) in the Bogalusa Heart Study. For children with an SF sum below the 90th percentile (<50 mm, boys; <60 mm, girls), agreement was fairly good between PBFDXA and PBFSlaughter, with median differences of ≤3 percentage points.
However, equations based on a sample of relatively thin children who were examined in the 1980s are unlikely to accurately estimate the body fatness of much heavier children, and we observed the largest discrepancy (12 percentage points) between PBFSlaughter and PBFDXA among boys who had an SF sum ≥50 mm. Based on the Slaughter equations (31), percentage body fat increases by 0.783 percentage points among boys for each 1-mm increase in the SF sum >35 mm. Among girls with an SF sum >35 mm, the slope is 0.546. As seen in Table 3 and Figure 1C, this results in extremely high predicted levels of PBFSlaughter among children who have very thick skinfold thicknesses. In contrast with this linear association, the slope of the relation of the SF sum to PBFDXA decreases markedly at high levels of the SF sum (Figure 1C). This results in the Slaughter equations estimating implausible values of percentage body fat (eg, ≥67%) among very obese children.
Many investigators have also found that levels of various risk factors are related similarly to levels of BMI and to estimates of body fatness based on skinfold thicknesses (13, 42), air-displacement plethysmography (19), DXA (13, 18, 20, 43), and underwater weighing (44). The similarity of these associations may have been because associations with risk factor levels are largely influenced by levels among obese children, and the accuracy of BMI increases at higher levels of body fatness (3, 15).
Although it was not the primary focus, we also found that risk factor levels tended to show slightly stronger associations with levels of adjusted BMI, which is the difference (in kg/m2) between the child's actual and expected (based on sex and age) BMI, than with BMI-for-age (Table 5). The overall correlations, for example, with the risk factor summary, were r = 0.47 (adjusted BMI) and r = 0.44 (BMI-for-age z score); P < 0.05 for the difference between the 2 correlations. This relatively small difference may have been the result of the transformation used to normalize BMI values in the CDC growth charts. At very high BMI levels, the incremental increases in BMI-for-age become progressively smaller, and the upper limit of BMI-for-age in the CDC growth charts is ∼3.2 SDs (45). As was previously noted for longitudinal data (30), it may be preferable to use BMI in some analyses and control sex and age in regression models rather than with BMI-for-age z scores.
The current analyses had several limitations that should be considered. Large errors in the measurement of skinfold thicknesses are possible (7, 46). Although DXA estimates of body fatness are highly correlated with those from methods such as the 4-compartment model and neutron activation (47), large differences in the estimation of an individual's body fatness are possible. Furthermore, PBFDXA estimates may vary across machines, manufactures, and software versions. It is also possible that there are systematic differences in PBFDXA, with DXA underestimating the body fatness of leaner persons (48) and overestimating the body fatness of obese persons (49). If PBFDXA overestimated the true fat mass of obese children in the current study, the overestimation of body fatness by the Slaughter equations may be even more pronounced than what we observed.
Our results indicate that PBFSlaughter provides a fairly accurate estimate of body fatness among nonobese children, but can markedly overestimate the body fatness of obese children. Furthermore, PBFSlaughter is no more strongly associated with levels of various CVD risk factors than is BMI after adjustment for sex and age. These results indicate that PBFSlaughter, which was developed at a time when children were much thinner than today, has little advantage over BMI at identifying children and adolescents who are at increased risk of subsequent disease.
Supplementary Material
Acknowledgments
We thank James T Durant (Agency for Toxic Substance and Disease Registry) for carefully reading the manuscript and suggesting several improvements.
The authors’ responsibilities were as follows—DSF: data analyses, interpretation of the results, and writing of the manuscript; and MH and GSB (Principal Investigators): collection of the data, interpretation of the results, and editing of the manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DBP, diastolic blood pressure; DXA, dual-energy X-ray absorptiometry; Lowess, locally weighted scatter plot smoothing; PBFDXA, percentage body fat estimated by using dual-energy X-ray absorptiometry; PBFSlaughter, percentage body fat estimated by using the Slaughter skinfold-thickness equations; SBP, systolic blood pressure; SF sum, sum of subscapular and triceps skinfold thicknesses.
REFERENCES
- 1.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650–6. [DOI] [PubMed] [Google Scholar]
- 2.Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, Wathern AK, Kaye SJ, Eriksson JG, Osmond C, Cook DG. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes (Lond) 2009;33:866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray GA, DeLany JP, Volaufova J, Harsha DW, Champagne C. Prediction of body fat in 12-y-old African American and white children: evaluation of methods. Am J Clin Nutr 2002;76:980–90. [DOI] [PubMed] [Google Scholar]
- 4.Deurenberg P, Deurenberg-Yap M, Foo LF, Schmidt G, Wang J. Differences in body composition between Singapore Chinese, Beijing Chinese and Dutch children. Eur J Clin Nutr 2003;57:405–9. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, Borrud LG. High adiposity and high BMI-for-age in US children and adolescents by race-ethnic group. Am J Clin Nutr 2010;91:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman DS, Wang J, Thornton JC, Mei Z, Pierson RN, Jr, Dietz WH, Horlick M. Racial/ethnic differences in body fatness among children and adolescents. Obesity (Silver Spring) 2008;16:1105–11. [DOI] [PubMed] [Google Scholar]
- 7.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr 1999;82:165–77. [DOI] [PubMed] [Google Scholar]
- 8.Laurson KR, Eisenmann JC, Welk GJ. Development of youth percent body fat standards using receiver operating characteristic curves. Am J Prev Med 2011;41(suppl 2):S93–9. [DOI] [PubMed] [Google Scholar]
- 9.Leitão R, Rodrigues LP, Neves L, Carvalho GS. Changes in adiposity status from childhood to adolescence: a 6-year longitudinal study in Portuguese boys and girls. Ann Hum Biol 2011;38:520–8. [DOI] [PubMed] [Google Scholar]
- 10.Goacher PJ, Lambert R, Moffatt PG. Can weight-related health risk be more accurately assessed by BMI, or by gender specific calculations of Percentage Body Fatness? Med Hypotheses 2012;79:656–62. [DOI] [PubMed] [Google Scholar]
- 11.Going SB, Lohman TG, Cussler EC, Williams DP, Morrison JA, Horn PS. Percent body fat and chronic disease risk factors in U.S. children and youth. Am J Prev Med 2011;41(suppl 2):S77–86. [DOI] [PubMed] [Google Scholar]
- 12.Sardinha LB, Going SB, Teixeira PJ, Lohman TG. Receiver operating characteristic analysis of body mass index, triceps skinfold thickness, and arm girth for obesity screening in children and adolescents. Am J Clin Nutr 1999;70:1090–5. [DOI] [PubMed] [Google Scholar]
- 13.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong CP, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 2005;29:1346–52. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Wang J, Ogden CL, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. The prediction of body fatness by BMI and skinfold thicknesses among children and adolescents. Ann Hum Biol 2007;34:183–94. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DS, Ogden CL, Blanck HM, Borrud LG, Dietz WH. The abilities of body mass index and skinfold thicknesses to identify children with low or elevated levels of dual-energy X-Ray absorptiometry−determined body fatness. J Pediatr 2013;163:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Shaper AG, Morris RW, Whincup PH. Measures of adiposity in the identification of metabolic abnormalities in elderly men. Am J Clin Nutr 2005;81:1313–21. [DOI] [PubMed] [Google Scholar]
- 17.Willett K, Jiang R, Lenart E, Spiegelman D, Willett W. Comparison of bioelectrical impedance and BMI in predicting obesity-related medical conditions. Obesity (Silver Spring) 2006;14:480–90. [DOI] [PubMed] [Google Scholar]
- 18.Lee K, Song Y-M, Sung J. Which obesity indicators are better predictors of metabolic risk?: healthy twin study. Obesity (Silver Spring) 2008;16:834–40. [DOI] [PubMed] [Google Scholar]
- 19.Bosy-Westphal A, Geisler C, Onur S, Korth O, Selberg O, Schrezenmeir J, Müller MJ. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes (Lond) 2006;30:475–83. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, Van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB, van Dam RM. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol 2010;172:1442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly JJ, Wilson J, Durnin JV. Determination of body composition from skinfold thickness: a validation study. Arch Dis Child 1995;73:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez G, Moreno LA, Blay MG, Blay VA, Fleta J, Sarria A, Bueno M, Sarrı A. Group AV-ZS. Body fat measurement in adolescents: comparison of skinfold thickness equations with dual-energy X-ray absorptiometry. Eur J Clin Nutr 2005;59:1158–66. [DOI] [PubMed] [Google Scholar]
- 23.Yeung DC, Hui S. Validity and reliability of skinfold measurement in assessing body fatness of Chinese children. Asia Pac J Clin Nutr 2010;19:350–7. [PubMed] [Google Scholar]
- 24.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC, Foster TA, Blonde CV. Cardiovascular risk factors in children: the early natural history of atherosclerosis and essential hypertension. New York, NY: Oxford University Press, 1980.
- 25.Freedman DS, Wang J, Thornton JC, Mei Z, Sopher AB, Pierson RN, Dietz WH, Horlick M. Classification of body fatness by body mass index-for-age categories among children. Arch Pediatr Adolesc Med 2009;163:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books, 1988.
- 27.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51:1106–12. [DOI] [PubMed] [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics. Series 11. 2002;11:1–190. [PubMed] [Google Scholar]
- 29.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr 2009;90:1314–20. [DOI] [PubMed] [Google Scholar]
- 30.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI%, BMI z-score or BMI centile? Eur J Clin Nutr 2005;59:419–25. [DOI] [PubMed] [Google Scholar]
- 31.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol 1988;60:709–23. [PubMed] [Google Scholar]
- 32.Berenson GS, Cresanta JL, Webber LS. High blood pressure in the young. Annu Rev Med 1984;35:535–60. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. 2013. Available from: http://www.r-project.org (cited 28 July 2013).
- 34.Bland JM, Altman DG. Difference versus mean plots. Ann Clin Biochem 1997;34:570–1. [DOI] [PubMed] [Google Scholar]
- 35.Everitt B. An R and S-PLUS companion to multivariate analysis. London, United Kingdom: Springer, 2005. [Google Scholar]
- 36.Canty A, Ripley B. boot: Bootstrap R (S-Plus) functions. R package version 1.3-9. 2013. Available from: http://cran.r-project.org/web/packages/boot/boot.pdf (cited 28 July 2013).
- 37.Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and skinfold thicknesses to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Clin Nutr 2009;90:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Himes JH, Bouchard C. Validity of anthropometry in classifying youths as obese. Int J Obes 1989;13:183–93. [PubMed] [Google Scholar]
- 39.Moreno LA, Fleta J, Sarria A, Rodriguez G, Bueno M. Secular increases in body fat percentage in male children of Zaragoza, Spain, 1980-1995. Prev Med 2001;33:357–63. [DOI] [PubMed] [Google Scholar]
- 40.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 1994;60:23–8. [DOI] [PubMed] [Google Scholar]
- 42.Geiss HC, Parhofer KG, Schwandt P. Parameters of childhood obesity and their relationship to cardiovascular risk factors in healthy prepubescent children. Int J Obes Relat Metab Disord 2001;25:830–7. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay RS, Hanson RL, Roumain J, Ravussin E, Knowler WC, Tataranni PA. Body mass index as a measure of adiposity in children and adolescents: relationship to adiposity by dual energy x-ray absorptiometry and to cardiovascular risk factors. J Clin Endocrinol Metab 2001;86:4061–7. [DOI] [PubMed] [Google Scholar]
- 44.Spiegelman D, Israel RG, Bouchard C, Willett WC. Absolute fat mass, percent body fat, and body-fat distribution: which is the real determinant of blood pressure and serum glucose? Am J Clin Nutr 1992;55:1033–44. [DOI] [PubMed] [Google Scholar]
- 45.CDC. Cut-offs to define outliers in the 2000 CDC growth charts. Available from: http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/BIV-cutoffs.pdf (cited 28 July 2013).
- 46.WHO Multicentre Growth Reference Study Group. Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl 2006;450:38–46. [DOI] [PubMed] [Google Scholar]
- 47.Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring) 2012;20:30–9. [DOI] [PubMed] [Google Scholar]
- 48.Van Der Ploeg GE, Withers RT, Laforgia J. Percent body fat via DEXA: comparison with a four-compartment model. J Appl Physiol 2003;94:499–506. [DOI] [PubMed] [Google Scholar]
- 49.Wells JC, Haroun D, Williams JE, Wilson C, Darch T, Viner RM, Eaton S, Fewtrell MS. Evaluation of DXA against the four-component model of body composition in obese children and adolescents aged 5-21 years. Int J Obes (Lond) 2010;34:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.