Abstract
Background: Insulin resistance is a precursor of numerous chronic diseases, including cardiovascular disease (CVD). The fasting insulin concentration is considered a reasonable surrogate of insulin resistance, especially among nondiabetic individuals.
Objective: We aimed to quantitatively summarize the literature on the association of fasting insulin concentrations with risk of hypertension, stroke, and coronary heart disease (CHD) by conducting a meta-analysis of prospective cohort studies.
Design: Eligible studies were identified by searching PubMed and EMBASE through January 2013. Additional information was retrieved through Google Scholar or a hand review of the reference lists from relevant articles. Prospective cohort studies that reported RRs and corresponding 95% CIs for the association of interest were identified. Data were extracted independently by 2 investigators, and the weighted RRs and 95% CIs for the associations were obtained by using a random-effects model.
Results: Of the 22 identified studies, 10 reported results on hypertension (36,617 individuals and 4491 cases), 7 on stroke (27,887 individuals and 1550 cases), and 9 on CHD (22,379 individuals and 1986 cases). Comparison of the highest with the lowest quantile of fasting insulin concentrations showed a pooled RR (95% CI) of 1.63 (1.35, 1.97) for hypertension, 1.18 (0.87, 1.60) for stroke, and 1.50 (1.28, 1.77) for CHD. Each 50-pmol/L increment in fasting insulin was associated with a 25% increase in risk of hypertension [RR: 1.25 (1.14, 1.36)] and a 16% increase in risk of CHD [RR: 1.16 (1.10, 1.22)] but was not associated with risk of stroke [RR: 0.999 (0.99, 1.01)].
Conclusions: A higher fasting insulin concentration or hyperinsulinemia was significantly associated with an increased risk of hypertension and CHD but not stroke. This meta-analysis suggests that early fasting insulin ascertainment in the general population may help clinicians identify those who are potentially at high risk of CVD.
INTRODUCTION
Insulin resistance is either a precursor or a pivotal component of numerous chronic diseases (1–5) including cardiovascular disease (CVD)4 (4), which is the leading cause of morbidity and mortality and is responsible for >70% of total mortality among patients with type 2 diabetes (6). Hyperinsulinemia, as a surrogate or a compensatory reaction of insulin resistance, may play an important role in the pathogenesis of CVD. It has been hypothesized that hyperinsulinemia precedes type 2 diabetes, which is a major risk factor for developing macrovascular complications and then becomes associated with an adverse CVD risk profile, including hypertension (6). However, whether hyperinsulinemia per se is an independent risk factor for CVD remains controversial.
Although cross-sectional and longitudinal studies have explored whether an elevated insulin concentration or insulin resistance is associated with increased CVD risk (7–12), the literature is inconsistent regarding perspective relations of fasting insulin concentrations with subsequent risk of hypertension, stroke, and coronary heart disease (CHD). Many prospective cohort studies have thus far shown that fasting insulin concentrations may predict risk of hypertension (13–20), stroke (21), and CHD (21–23) independent of other known CVD risk factors, whereas others have not [hypertension (24, 25), stroke (22, 26–30), and CHD (28, 29, 31–34)]. Therefore, in this study, we aimed to quantitatively summarize the literature on the associations between fasting insulin concentrations and risk of hypertension, stroke, and CHD by conducting a meta-analysis of prospective cohort studies.
MATERIALS AND METHODS
Search strategy
The meta-analysis was performed based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (35). First, we conducted a systematic search of published studies in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) through January 2013 using the terms “insulin or hyperinsulinemia” and “hypertension or blood pressure or stroke or cerebrovascular accident or myocardial infarction or coronary heart disease” and “epidemiological studies” and “cohort/ prospective/follow-up/longitudinal studies” and “survival analysis or proportional hazard model or Cox or hazards ratio or risk.” Next, we reviewed EMBASE (http://www.elsevier.com/online-tools/embase), Google Scholar (http://scholar.google.com/), and the reference lists of the retrieved articles to identify any studies that were not identified from the preliminary literature searches. Third, to get additional data or de novo results for this meta-analysis, we contacted the authors of primary studies (19, 32).
Selection criteria
Studies were included in the meta-analysis if they met the following criteria: published in the English language, had a prospective cohort design, involved a general population, exposure was fasting insulin or hyperinsulinemia, and had an RR with 95% CI or these data could be derived from reported results.
Data extraction
Two investigators (PX and KH) assessed the eligibility of the literature independently, and any disagreements were resolved by consensus. From each retrieved article, we extracted the following data: specific outcome, name of the first author, year of publication, study name, country where the study was conducted, proportion of male sex, age at baseline, follow-up time, total number of individuals/person-years of follow-up, number of cases, exposure classification, outcome assessment, covariates that were adjusted in the analysis, and the RRs estimates with corresponding 95% CIs for corresponding categories and/or for continuous exposure.
RRs and 95% CIs transformed to their natural logarithms (ln) were used to compute the corresponding SEs. In studies in which RRs and 95% CIs were reported as per unit or per SD (either in pmol/L or μU/mL) increment in fasting insulin concentration, they were converted to a per 50-pmol/L increment consistently. If a study did not provide the linear association of fasting insulin with the outcome of interest, we estimated it by using Greenland and Longnecker's method if there were ≥3 categories for insulin concentrations (36), or we just calculated it under a linear assumption if there were only 2 categories. If the highest group of fasting insulin concentrations was an open range (eg, >73.27 pmol/L), then its upper limit was estimated by assuming its range as wide as the previous one.
In addition, in one study where only stratified RRs (95% CIs) for incident hypertension by status of family history of hypertension were reported (25), we pooled them with a random-effects model to get an overall estimate. Moreover, in another study in which the main effects of hyperinsulinemia (yes or no) and alcohol intake (yes or no) and their interaction on incidence of hypertension were reported, we recalculated the stratified results by alcohol status and pooled them with a random-effects model to get an overall estimate (24). Furthermore, in 2 other studies (19, 32), we contacted the authors and obtained de novo results for the meta-analysis.
Statistical analysis
We pooled RR estimates separately for each outcome using a random-effects model. We evaluated the statistical heterogeneity of the RRs by calculating the I2 statistic; low, moderate, and high degrees of heterogeneity corresponded to I2 values of 25%, 50%, and 75%, respectively. Publication bias was generally assessed by using Egger's (when the numbers of studies pooled was ≥3) or Begg's (when the numbers of studies pooled was <3) asymmetry test. If publication bias did exist, the Duval and Tweedie nonparametric “trim and fill” method was used to get the overall estimate (37). The meta-regression model was used to detect any potential modifiers.
The average of follow-up years was calculated as the sum of person-years divided by the total numbers of individuals. If unavailable, person-years were estimated by multiplying the number of individuals and the average (mean or median) of follow-up time. In stratified analyses, we examined subtypes of stroke (ischemic and hemorrhagic) and duration of follow-up (≥ compared with < the median). We also stratified the data by study region, if possible. Sensitivity analyses evaluated the effect of removing a single study from the analysis, and, if using a fixed-effects model, will substantially affect the results. All analyses were performed by using STATA statistical software (version 11.0; STATA Corporation LP).
RESULTS
Literature search
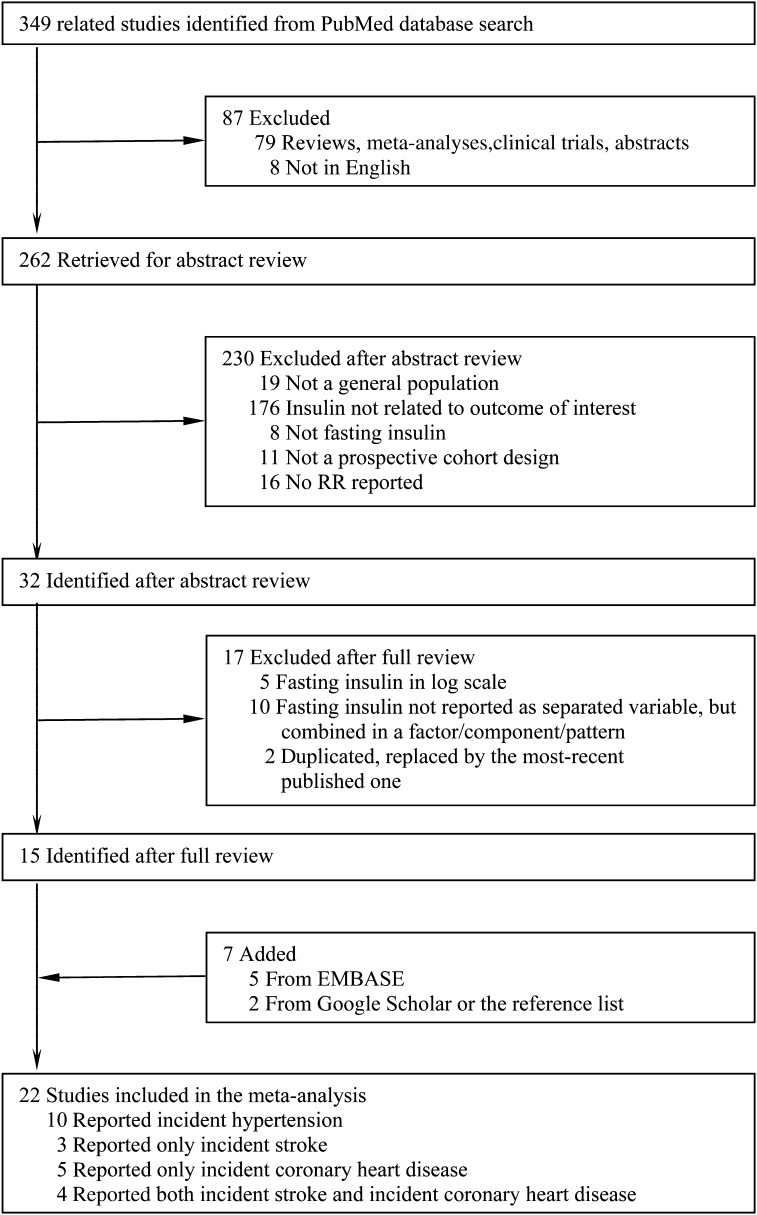
As shown in Figure 1, we retrieved 349 related articles from PubMed. Of these, 334 articles were excluded for one of the following reasons: 1) a review/meta-analysis, editorial, abstract, or letter to editor; 2) not published in English; 3) not conducted in a general population; 4) not relating fasting insulin to an outcome of interest; 5) fasting insulin was neither a categorical variable nor a continuous variable in original scale; 6) not a prospective cohort design; or 7) no RRs with 95% CIs were reported or such information could not be calculated. In addition, we identified 7 articles from EMBASE or by hand searching Google Scholar and the reference list. Therefore, 22 identified eligible studies were included in this meta-analysis.
FIGURE 1.
Study selection process. EMBASE, http://www.elsevier.com/online-tools/embase; Google Scholar, http://scholar.google.com/; PubMed, http://www.ncbi.nlm.nih.gov/pubmed.
Study characteristics
The information extracted from the 22 included independent studies, all of which were prospective cohort studies and had participants without prior diagnosed CVD at baseline, is shown in Table 1. For hypertension (10 studies), the total number of individuals were 36,617 with 5491 incident cases during an average 5.7 y of follow-up. For stroke (7 studies), the total number of individuals was 27,887 with 1550 incidence cases during an average of 12.3 y of follow-up. For CHD (9 studies), the total number of individuals was 22,379, with 1986 incidence cases during an average of 13.8 y of follow-up. The potential confounders included in the multivariable-adjusted model are listed in Table 1.
TABLE 1.
Characteristics of the studies included in the meta-analysis1
| Source | Males | Age at baseline | Duration of follow-up2 | Total no. of individuals/person-years | No. of cases | Fasting insulin categories | Outcome assessment | Adjustment for confounders |
| % | y | y | ||||||
| Hypertension | ||||||||
| Lissner (15), 1992, Sweden | 0 | 50 ± 0 | 10.7 | 278/2982 | 70 | Quartiles (mU/L): ≤10, 11–13, 14–16, ≥17 | SBP/DBP ≥160/95 mm Hg; current, untreated hypertension as reported by the subject to the examining physician; any type of antihypertensive therapy at the time of the visit | BMI, WHR, and weight change between the first 2 examinations |
| Tsuruta (16), 1996, Japan | 48.9 | 50.0 ± 10.1 | 11 | 135/NA | 62 | Tertiles (pmol/L) | SBP/DBP ≥140/90 mm Hg or use of antihypertensive medication at the time of the follow-up examination | Age, sex, SBP, BMI, serum TG, serum creatinine, and alcohol consumption at baseline |
| Fagot-Campagna (25), 1997, The Paris Prospective Study, French | 100 | Median: 49.3 (43–54)† | 3.16 ± 1.35 | 4149/13,118 | 1035 | P90 compared with P10 (pmol/L) (136 compared with 29) | SBP/DBP ≥160/95 mm Hg or current use of antihypertensive medications | Age, excessive alcohol consumption, BMI, and iliac circumference by status for family history of hypertension |
| He (18), 1999, TOHP-1, USA | 56 | 43.9 ± 6.2 | 7.1 (6.3–7.9) | 377/NA | 96 | Continuous (pmol/L) | SBP/DBP ≥160/95 mm Hg or diagnosis by a physician | Age, sex, race, heart rate, and alcohol consumption at baseline and intervention assignment in the Trials of Hypertension Prevention, phase 1 (sodium reduction or weight loss compared with all others) |
| Arima (24), 2002, The Hisayma Study, Japan | 35.7 | 56 ± 10 (40–79) | 5 | 1133/NA | 186 | Tertiles (pmol/L) Men: <138, 138–234, and ≥235 Women: <180, 180–276, and ≥277 | SBP/DBP ≥160/ 95 mm Hg or current use of antihypertensive medication | Age, sex, alcohol consumption, BMI, fasting glucose, TC, HDL-C, TG, parental history of hypertension, smoking habits, physical activity, and intakes of total energy, saturated fatty acids, and sodium |
| Cheung (17), 2008, Hong Kong CRISPS2, China | 47 | 43.6 ± 11.2 | 6.4 (4–9) | 1602/NA | 258 | Continuous (mIU/L) | SBP/DBP ≥140/90 mm Hg or a previous diagnosis of hypertension and taking antihypertensive medications | Age and sex |
| Levin (14), 2010, The MESA study, USA | 49.0 | 59.1 ± 9.7 | 5 | 3513/14,846 | 965 | Quartiles (pmol/L): <3.3, 3.3–4.7, 4.8–7.3, >7.3 | SBP/DBP ≥140/90 mm Hg or use of an antihypertensive medication in combination with a self-report of hypertension | Age, sex, race-ethnicity, attained education, moderate/vigorous physical activity, smoking, alcohol use, BMI, and WC |
| Chul Sung (13), 2011, Korea | 68.9 | 40.5 ± 5.7 | 5 | 10,894/NA | 881 | Quartiles (mU/L): <5.35, 5.36–6.58, 6.59–8.45, ≥8.46 | SBP/DBP ≥140/90 mm Hg or history of hypertension during the follow-up period | Age, sex, smoking, alcohol use, exercise, education, SBP, DBP, fasting glucose, TG, HDL-C, uric acid, BMI, and BMI change |
| Xun (19), 2012, The CARDIA Study, USA | 47.4 | 25.0 ± 3.6 (18–30) | 20 | 3413/57,920 | 796 | Quartiles (mU/L): <9, 9–10.9, 11.0–14.2, ≥14.3 | SBP/DBP ≥140/90 mm Hg or taking antihypertensive medication at each examination | Age, sex, race, study center, BMI, physical activity, education, smoking, alcohol consumption, baseline SBP, family history of hypertension, glucose, and dietary intakes of sodium, potassium, and magnesium |
| Park (20), 2012, Korea | 65.6 | 39.4 ± 6.4 (20–65) | 4 | 11,123/NA | 1142 | Quartiles (pmol/L): ≤50.07, 50.14–60.42, 60.56–72.99, ≥73.27 | SBP/DBP ≥140/90 mm Hg or history of hypertension during the follow-up | Age, sex, BMI, percentage weight change, smoking status, alcohol consumption, regular exercise, SBP, and fasting glucose, TG, and HDL-C concentrations at baseline |
| Stroke | ||||||||
| Pyorala (29), 1998, The Helsinki Policemen Study, Finland | 100 | 47.4 ± 7.5 (34–64) | 22 | 970/NA | Total: 70 Ischemic: 55 Hemorrhagic: 7 | Hyperinsulinemia: AUCinsulin quintile 5 vs. quintiles 1–4 | WHO criterion (1981): a neurologic deficit observed by a physician for >24 h without other disease explaining the symptoms | Age, subscapular skinfold thickness, SBP, and smoking (yes or no) |
| Lakka (28), 2000, The KIHDRF Study, Finland | 100 | 42–60 | 9.4 (0.3–12.8) | 1521/14,297 | Total: 48 | Quartiles (pmol/L): <52, 52–66, 67–89, ≥90 | ICD-9 codes 430–436 | Age, examination year, smoking, blood leukocyte count, serum apolipoprotein B concentration, plasma fibrinogen concentration, maximal oxygen uptake |
| Lawlor (22), 2007, UK | 0 | 60–79 | Median: 4.6 | 3246/14,932 | Total: 52 | Continuous (mU/L) | Either stroke death (ICD-10 codes I60-I69, G45) or occurrence of a nonfatal stroke identified in the follow-up medical record reviews | Age, life course socioeconomic position, smoking, physical activity, BMI, WHR, HDL-C, TG, SBP, LDL-C, fasting glucose, and Hb A1c |
| Wiberg (30), 2009, The ULSAM Study, Sweden | 100 | 70 | Median: 8.8 (0.0–11.4) | 1151/9395 | Total: 150 | Continuous (pmol/L) | ICD-9 codes 430–436, ICD-10 codes I60-I64, G45 | Hypertension, diabetes, atrial fibrillation, ECG-LVH, serum TC, and smoking |
| Rasmussen-Torvik (21), 2010, The ARIC Study, USA | 43.2 | 53.8 (45–64) | 18 | 12,323/196,498 | Ischemic: 445 | Quintiles (mU/L): <6, 6–8, 8 to <11, 11 to <15, ≥15 | Validated definite or probable embolic or thrombotic brain infarctions | Age, sex, race, and study center |
| Thacker (27), 2011, The CHS Study, USA | 42 | 73 (≥65) | 12.4 | 3442/42,551 | Ischemic: 417 | Quintiles (pmol/L): 20.8–62.5, >62.5–83.3, >83.3–111.1, >111.1–722.3 | Ascertained from participant report, questions at annual visits, telephone contacts every 6 mo between annual visits and after annual visits ended, and screens of hospitalizations for key ICD-9 revision codes; confirmed with information from participant interviews, medical records, test results, and brain images | Age, sex, race, estimated GFR, CHD, congestive heart failure, atrial fibrillation, peripheral arterial disease, SBP, antihypertensive medication use, triglycerides, HDL-C, and LDL-C |
| Wieberdink (26), 2012, Rotterdam Study, Netherlands | 42.4 | Median: 67.9 (≥55) | Median: 8.6 | 5234/42,806 | Total: 366 | Quintiles (pmol/L): 10–46, 47–64, 65–90, 91–430 | Stroke was defined according to WHO criteria as a syndrome of rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 h or longer or leading to death, with no apparent cause other than of vascular origin | Age, sex, and a propensity score (current smoking, SBP, BP-lowering medication use, total cholesterol, HDL-C, triglycerides, lipid-lowering medication use, von Willebrand factor, BMI, and WHR) |
| Coronary heart disease | ||||||||
| Welin (31), 1992, Sweden | 100 | 67 ± 0 | 8 | 595/NA | 66 | Quintiles (mU/L): <6.3, 6.3–9.3, 9.4–12.9, 13.0–17.9, ≥18 | Fatal or nonfatal MI or death from CHD | Serum TC and TG |
| Moller (23), 1995, Denmark | 47.9 | 40 ± 0 | 17 | 1052/NA | 54 | Continuous (pmol/L) | ICD-8 codes 410–414 | Sex, BMI, physical activity, tobacco consumption, alcohol consumption, SBP, TC, TG, and HDL-C |
| Pyorala (29), 1998, The Helsinki Policemen Study, Finland | 100 | 47.4 ± 7.5 (34–64) | 22 | 970/NA | 164 | Hyperinsulinemia: AUCinsulin quintile 5 compared with quintiles 1–4 | ICD-8 codes 410–413, ICD-9 codes 410–414 | Age, BMI, subscapular skinfold thickness, cholesterol, triglycerides, SBP, smoking, physical activity, and AUC glucose |
| Lakka (28), 2000 The KIHDRF Study, Finland | 100 | 42–60 | 9.3 (0.1–12.8) | 1521/14,145 | 110 | Quartiles (pmol/L): <52, 52–66, 67–89, ≥90 | ICD-9 codes 410–414 | Age, examination year, smoking, blood leukocyte count, serum apolipoprotein B concentration, plasma fibrinogen concentration, and maximal oxygen uptake |
| Yudkin (32), 2002, The Caerphilly Study, UK | 100 | 50–64 | 10–14 | 983/NA | 113 | Quartiles (pmol/L): <18.4, 18.4–26.4, 26.5–39.3, ≥39.4 | CHD death (ICD-9 codes 410–414), together with similarly classified hospital admissions and subjects who had developed new MI on ECG | Age and BMI |
| Zethelius (33), 2002, Sweden | 100 | 50 | 26.7 | 874/NA | 219 | Continuous (pmol/L) | ICD-9 codes 410–414 | Age at entry, BMI, SBP at office, smoking fasting glucose, serum TC, and LDL-C/HDL-C ratio |
| Zethelius (34), 2005, Sweden | 100 | 70 | Median: 7.9 | 815/5792 | 126 | Continuous (pmol/L) | ICD-9 codes 410–414, ICD-10 codes 120–25 | Serum TC, SBP, fasting plasma glucose, BMI, and smoking |
| Lawlor (22), 2007, UK | 0 | 60–79 | 4.6 | 3246/14,932 | 174 | Continuous (mU/L) | CHD death (ICD-10 codes I20-I25, I51.6) or a nonfatal myocardial infarction, angina diagnosis or coronary artery bypass, or angioplasty identified in the follow-up medical record reviews | Age, life-course socioeconomic position, smoking, physical activity, BMI, WHR, HDL-C, TG, SBP, LDL-C, fasting glucose, and Hb A1c |
| Rasmussen-Torvik (21), 2010, The ARIC Study, USA | 43.2 | 53.8 (45–64) | 18 | 12,323/193,069 | 960 | Quintiles (mU/L): <6, 6–8, 8 to <11, 11 to <15, ≥15 | Definite, probable, or silent MI or definite CHD death | Age, sex, race, and study center |
ARIC, Atherosclerosis Risk in Communities; CARDIA, Coronary Artery Risk Development in Young Adults; CHD, coronary heart disease; CHS, Cardiovascular Health Study; DBP, diastolic blood pressure; ECG, electrocardiography; GFR, glomerular filtration rate; Hb A1c, hemoglobin A1c; HDL-C, HDL cholesterol; ICD, International Classification of Diseases; KIHDRF, Kuopio Ischemic Heart Disease Risk Factor; LDL-C, LDL cholesterol; LVH, left ventricular hypertrophy; MESA, Multi-Ethnic Study of Atherosclerosis; MI, myocardial infarction; NA, not available; TC, total cholesterol; TG, triglycerides; TOHP-1, Trials of Hypertension Prevention, phase 1; SBP, systolic blood pressure; ULSAM, Uppsala Longitudinal Study of Adult Men; WC, waist circumference; WHR, waist-hip ratio.
The durations of follow-up periods were reported or calculated based on the reported person-years in the primary studies.
Fasting insulin concentrations and risk of hypertension
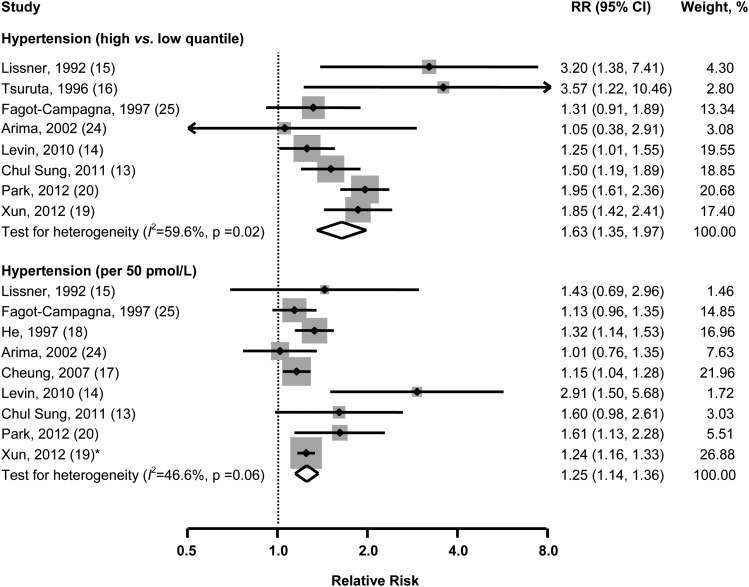
The result from the random-effects meta-analysis of the relation between fasting insulin concentrations and incidence of hypertension are shown in Figure 2. Eight studies reported RRs (95% CIs) for the highest compared with the lowest quantile. As compared with those in the lowest quantile, the pooled RR for individuals in the highest quantile was 1.63 (95% CI: 1.35, 1.97) for hypertension. There was moderate heterogeneity among studies (I2 = 59.6%, P = 0.02). No statistically significant evidence of publication bias was observed (Egger's test, P = 0.64).
FIGURE 2.
Multivariable-adjusted RRs and 95% CIs (horizontal lines) for incident hypertension. The pooled estimates (diamond data markers) were obtained by using a random-effects model. The dots indicate the adjusted RRs by comparing high with low quantiles or per 50-pmol/L increase in fasting insulin concentrations. The size of the shaded square is proportional to the percentage weight of each study. *De novo data for the meta-analysis.
The linear trend analysis involved 9 studies. The combined RR was 1.25 (1.14, 1.36) for an increment of 50 pmol/L insulin concentrations. Modest heterogeneity of effect estimates was observed (I2 = 46.6%, P = 0.06), and Egg's test for publication bias showed no significant difference (P = 0.22). Neither study region (Eastern compared with Western countries) nor duration of follow-up modified the observed associations. Because only 2 studies reported results separately by sex, we could not assess the potential effect modification by sex.
Fasting insulin concentrations and risk of stroke
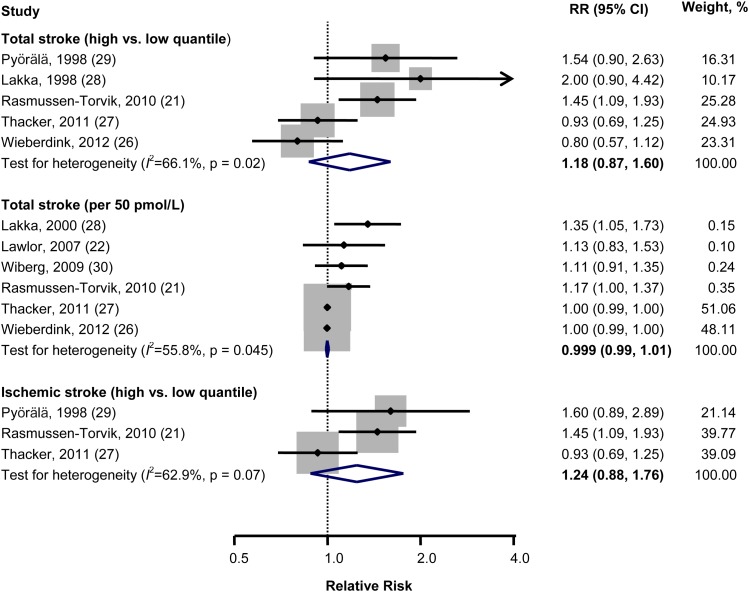
The pooled RR for the comparison of extreme quantiles of insulin concentrations was 1.18 (95% CI: 0.87, 1.60) for total stroke combining data from 6 studies. We saw high heterogeneity among studies (I2 = 66.1%, P = 0.02), and no evidence of publication bias (Egger's test, P = 0.44) (Figure 3).
FIGURE 3.
Multivariable-adjusted RRs and 95% CIs (horizontal lines) for incident stroke and its subtypes. The pooled estimates (diamond data markers) were obtained by using a random-effects model. The dots indicate the adjusted RRs by comparing high with low quantiles or per 50-pmol/L increase in fasting insulin concentrations. The size of the shaded square is proportional to the percentage weight of each study.
We found no linear trend, and the pooled RR from 6 studies was 0.999 (95% CI: 0.99, 1.01) for an increment of 50 pmol/L in insulin concentrations. There was high heterogeneity among the studies (I2 = 55.8%, P = 0.045). Because there was evidence of publication bias (Egger's test, P = 0.01), we used the Duval and Tweedie's nonparametric “trim and fill” method to account for the publication bias. An overall estimate of RR was 0.998 (95% CI: 0.99, 1.01).
In the stratified analysis, the pooled risk of those in the highest quantile was 1.24 (0.88, 1.76), with high heterogeneity among 3 studies (I2 = 62.9%, P = 0.07) for ischemic stroke. We observed no evidence of publication bias (Egger's P = 0.75). Only one study reported results on hemorrhagic stroke.
On the basis of 3 studies that reported results in men, we identified a positive (RR: 1.67; 95% CI: 1.07, 2.60; high compared with low quantiles) and linear (RR: 1.20; 95% CI: 1.03, 1.40; per 50-pmol/L increment) association between fasting insulin and risk of stroke. Only one study reported results on women separately. The duration of follow-up did not substantially modify the observed associations. Because all the studies were conducted in Western countries, we could not assess the heterogeneity between eastern and Western countries. Similar results were found in the studies conducted in US and non-US countries.
Fasting insulin concentrations and risk of CHD
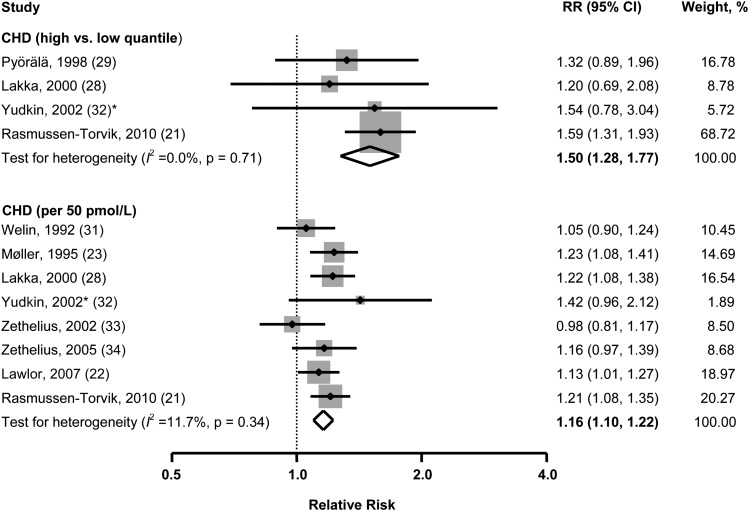
Ten studies were involved in the synthesis of the relation of fasting insulin concentrations with CHD (Figure 4). On the basis of available data from 4 studies, the pooled RR from a comparison of extreme quantiles of insulin concentrations for CHD was 1.50 (95% CI: 1.28, 1.77) with no significant heterogeneity among studies (I2 = 0.0%, P = 0.71). No evidence of publication bias was observed (Egger's test, P = 0.28).
FIGURE 4.
Multivariable adjusted RRs and 95% CIs (horizontal lines) for incident coronary heart disease. The pooled estimates (diamond data markers) were obtained by using a random-effects model. The dots indicate the adjusted RRs by comparing high with low quantiles or per 50-pmol/L increase in fasting insulin concentrations. The size of the shaded square is proportional to the percentage weight of each study. *De novo data for the meta-analysis. CHD, coronary heart disease.
According to available data from 8 studies, the pooled risk of CHD per 50-pmol/L increment in insulin concentration was 1.16 (95% CI: 1.10, 1.22), with modest heterogeneity among studies (I2 = 11.7%, P = 0.34) and no significant evidence of publication bias (Egger's test, P = 0.88).
Only one study reported results in women. On the basis of 6 studies that reported results in men, we identified a positive dose-response association (RR: 1.13; 95% CI: 1.02, 1.25; per 50-pmol/L increment). Similar results were found in the studies conducted in the United States and outside of the United States, and in studies with long-term (≥ the average time) and short-term (< the average time) follow-up periods.
Sensitivity analysis
The findings were consistent when a fixed-effects model was used (see Supplemental Table 1 under “Supplemental data” in the online issue). Omission of one study at a time and recalculation of the pooled RRs for the rest of the studies showed that none of the single studies substantially influenced the pooled RR for hypertension (see Supplemental Table 2 under “Supplemental data” in the online issue). For stroke, the pooled association changed from 1.18 (95% CI: 0.87, 1.60) to 1.29 (95% CI: 0.87, 1.91) and 1.31 (95% CI: 0.96, 1.80), respectively, when Thacker et al's study (27) and Wieberdink et al's study (26) were excluded. For CHD, the pooled estimate was attenuated from 1.50 (95% CI: 1.28, 1.77) to 1.32 (95% CI: 0.99, 1.77) with the omission of Rasmussen-Torvik et al's study (21). Omission of the other studies did not materially change the results.
DISCUSSION
In this quantitative meta-analysis of prospective cohort studies, we found significant, positive, and linear associations of fasting insulin concentrations with risk of hypertension and CHD, but not stroke. The observed associations were not materially modified by follow-up period. Our ability to access the potential modifications by sex or study region was limited by a lack of information from the primary studies.
Strengths and limitations
To date, this was the largest synthesis of prospective cohort studies to have assessed the associations of fasting insulin concentrations with incidence of hypertension, stroke, and CHD in one meta-analysis, which significantly increased the statistical power to detect potential associations. Also, our conclusions were strengthened by pooling data for estimating both dose-response relations and the RRs in a comparison of high with low concentrations of insulin. In addition, the positive association documented between fasting insulin concentration and risk of hypertension further supports the idea that elevated insulin concentrations may increase the risk of CHD through high blood pressure (BP).
Several limitations of this meta-analysis should be acknowledged. First, the inherent limitations of the primary studies may have affected our findings. For example, the possibility of residual confounding or bias due to systematic measurement errors or unmeasured factors cannot be ruled out. Second, the assay used to measure fasting insulin in a few studies (21, 28) was unspecific and had some cross-reactivity with pro-insulin, which may have confounded our findings. However, the results were not materially changed after exclusion of these studies in a sensitivity analysis. Third, the associations of fasting insulin concentrations with the outcomes of interest found in this meta-analysis were not completely independent of insulin resistance, because: 1) not all studies detected insulin resistance status in addition to fasting insulin concentrations, and 2) the effect of insulin resistance was impossible to be fully excluded from the models, whereas the exposure was fasting insulin concentrations. However, some included studies did adjust blood glucose concentrations in the model, which may have partially reduced this concern. In addition, we did not have enough information to assess the potential modifications by sex or study region (Eastern compared with Western countries).
Comparison with other studies
No published meta-analysis has directly related fasting insulin to the incidence of hypertension. The current study was the first, and the findings are consistent with those of a meta-analysis (38) published in 1992, which reported that fasting insulin concentrations were associated with both increased systolic BP and diastolic BP.
Also, the current study was the first meta-analysis to link fasting insulin concentrations to risk of stroke. The finding for stroke was mainly influenced by 2 studies, ie, Thacker et al's study (27) and Wieberdink et al's study (26). When one of them was excluded, the combined association was somewhat strengthened, ie, the pooled RR changed from 1.18 (95% CI: 0.87, 1.60) to 1.29 (95% CI: 0.87, 1.91) and 1.31 (95% CI: 0.96, 1.80), respectively. One possible explanation is that, only in these 2 studies, SBP, antihypertensive medication use, and ≥3 lipids were simultaneously overadjusted in the final model to give a spurious relation because hypertension and dyslipidemia may be intermediate variables in addition to strong confounders. In addition, for subtypes of stroke, the nonsignificant association was likely attributable to a small number of cases.
Several meta-analyses investigated the associations of fasting insulin concentrations with the risk of CHD. One meta-analysis (4) published in 1998 reported a pooled RR of CVD (including myocardial infarction, death from CHD, and/or electrocardiography abnormalities) of 1.18 (95% CI: 1.08, 1.29) with every 50-pmol/L increment in fasting insulin by combining 10 cohort studies and nested case-control studies. Another meta-analysis (39) found a positive association between fasting insulin concentrations and CVD mortality based on 7 prospective studies conducted in European countries. In addition, a meta-analysis published in 2007 included 14 prospective cohort studies and nested case-control studies (40), which found no significant association between fasting insulin concentrations and risk of CHD (mixed incidence and mortality).
Notably, a recent meta-analysis published in 2012 focusing on insulin resistance and incidence of combined cardiovascular events found no association between fasting insulin concentrations and risk of CHD (41). The null association found in that study may be explained by 1) mixed prospective cohort studies and nested case-control studies, 2) combined multiple health endpoints, and 3) not having updated the findings from the Atherosclerosis Risk in Communities Study published in 2010 (21) with 960 CHD cases.
Potential mechanisms
Fasting insulin concentrations and risk of hypertension
Experimental studies suggest that insulin, a well-established inotropic agent (42), can increase cardiac output (43), blood volume through stimulating secretion of vasopressin (an antidiuretic) (44), and renal sodium retention (45). In addition, insulin can increase vascular tone by increasing basal concentrations of calcium in cytosol of vascular smooth muscle cells, stimulating the rennin-angiotensin system (46), and stimulating the secretion of endohelin-1 (47)—a vascular constrictor.
Although the likelihood of high BP causing elevated insulin concentrations cannot be excluded (48), the possibility should be small because changes in insulin concentrations usually precede the presence of obvious hypertension in metabolic diseases (49). In addition, a large body of evidence lends credence to an increase of BP by insulin (50, 51), but no existing evidence with respect to the underlying mechanism supports the idea that hypertension might cause hyperinsulinemia.
Fasting insulin concentrations and risk of CHD and stroke
In addition to traditional risk factors (eg, hypertension and dyslipidemia), insulin can increase risk of CHD and stroke through nontraditional CVD risk factors, including endothelial dysfunction, increased inflammation, and increased coagulation (52). First, insulin resistance is associated with endothelial dysfunction and the accumulation of reactive oxygen species in the vessel wall that help to initiate and maintain the atherosclerotic process. Second, insulin resistance can affect adipocyte function, giving rise to the generation of a number of inflammatory molecules (eg, C-reactive protein, complement C3), which play a crucial role in the atherosclerotic process. In addition, insulin resistance is associated with increased platelet activation and an increased concentration and activity of prothrombotic factors, which thereby ensures the presence of a prothrombotic milieu that could increase the risk of atherothrombotic events.
Summary
In conclusion, the meta-analysis of prospective cohort studies found that an elevated fasting insulin concentration or hyperinsulinemia was significantly associated with an increased risk of hypertension and CHD. Our study suggests that early ascertainment of fasting insulin may help clinicians identify those who are potentially at high risk of CVD.
Supplementary Material
Acknowledgments
We thank John S Yudkin (University College London, United Kingdom) for providing de novo results for the Caerphilly Study and Margaret May (School of Social and Community Medicine, University of Bristol, United Kingdom) for analyzing the data for the de novo results from the Caerphilly Study.
The authors’ responsibilities were as follows―KH: funding support; PX: study concept and design, data analysis, and table and figure preparation; and PX and KH: literature search, study selection, data extraction, interpretation of the results, and drafting of the manuscript. All authors critically reviewed the manuscript for important intellectual content and approved the final version. None of the authors had any conflicts of interest to disclose.
Footnotes
Abbreviations used: BP, blood pressure; CHD, coronary heart disease; CVD, cardiovascular disease.
REFERENCES
- 1.Fernández-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev 2003;24:278–301. [DOI] [PubMed] [Google Scholar]
- 2.Hildreth KL, Van Pelt RE, Schwartz RS. Obesity, insulin resistance, and Alzheimer's disease. Obesity (Silver Spring) 2012;20:1549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue M, Tsugane S. Insulin resistance and cancer: epidemiological evidence. Endocr Relat Cancer 2012;19:F1–8. [DOI] [PubMed] [Google Scholar]
- 4.Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta-analysis. Circulation 1998;97:996–1001. [DOI] [PubMed] [Google Scholar]
- 5.Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy 2009;39:700–7. [DOI] [PubMed] [Google Scholar]
- 6.Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med 2001;249:225–35. [DOI] [PubMed] [Google Scholar]
- 7.Onat A, Ceyhan K, Sansoy V, Basar O, Erer B, Uysal O, Hergenc G. Fasting insulin levels independently associated with coronary heart disease in non-diabetic Turkish men and women. Int J Cardiol 2002;86:61–9. [DOI] [PubMed] [Google Scholar]
- 8.Bataille V, Perret B, Troughton J, Amouyel P, Arveiler D, Woodside J, Dallongeville J, Haas B, Bingham A, Ducimetiere P, et al. Fasting insulin concentrations and coronary heart disease incidence in France and Northern Ireland: the PRIME Study. Int J Cardiol 2006;108:189–96. [DOI] [PubMed] [Google Scholar]
- 9.Lempiäinen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation 1999;100:123–8. [DOI] [PubMed] [Google Scholar]
- 10.Pyörälä M, Miettinen H, Halonen P, Laakso M, Pyorala K. Insulin resistance syndrome predicts the risk of coronary heart disease and stroke in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Arterioscler Thromb Vasc Biol 2000;20:538–44. [DOI] [PubMed] [Google Scholar]
- 11.Rundek T, Gardener H, Xu Q, Goldberg RB, Wright CB, Boden-Albala B, Disla N, Paik MC, Elkind MS, Sacco RL. Insulin resistance and risk of ischemic stroke among nondiabetic individuals from the northern Manhattan study. Arch Neurol 2010;67:1195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronmal RA, Barzilay JI, Tracy RP, Savage PJ, Orchard TJ, Burke GL. The relationship of fasting serum radioimmune insulin levels to incident coronary heart disease in an insulin-treated diabetic cohort. J Clin Endocrinol Metab 2004;89:2852–8. [DOI] [PubMed] [Google Scholar]
- 13.Chul Sung K, Lim S, Rosenson RS. Hyperinsulinemia and homeostasis model assessment of insulin resistance as predictors of hypertension: a 5-year follow-up study of Korean sample. Am J Hypertens 2011;24:1041–5:. [DOI] [PubMed] [Google Scholar]
- 14.Levin G, Kestenbaum B, Ida Chen YD, Jacobs DR, Jr, Psaty BM, Rotter JI, Siscovick DS, de Boer IH. Glucose, insulin, and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2010;172:1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lissner L, Bengtsson C, Lapidus L, Kristjansson K, Wedel H. Fasting insulin in relation to subsequent blood pressure changes and hypertension in women. Hypertension 1992;20:797–801. [DOI] [PubMed] [Google Scholar]
- 16.Tsuruta M, Hashimoto R, Adachi H, Imaizumi T, Nomura G. Hyperinsulinaemia as a predictor of hypertension: an 11-year follow-up study in Japan. J Hypertens 1996;14:483–8. [PubMed] [Google Scholar]
- 17.Cheung BM, Wat NM, Man YB, Tam S, Cheng CH, Leung GM, Woo J, Janus ED, Lau CP, Lam TH, et al. Relationship between the metabolic syndrome and the development of hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study-2 (CRISPS2). Am J Hypertens 2008;21:17–22. [DOI] [PubMed] [Google Scholar]
- 18.He J, Klag MJ, Caballero B, Appel LJ, Charleston J, Whelton PK. Plasma insulin levels and incidence of hypertension in African Americans and whites. Arch Intern Med 1999;159:498–503. [DOI] [PubMed] [Google Scholar]
- 19.Xun P, Liu K, Cao W, Sidney S, Williams OD, He K. Fasting insulin level is positively associated with incidence of hypertension among American young adults: a 20-year follow-up study. Diabetes Care 2012;35:1532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW, Lee WY. Impact of hyperinsulinemia on the development of hypertension in normotensive, nondiabetic adults: a 4-year follow-up study. Metabolism 2013;62:532–8. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen-Torvik LJ, Yatsuya H, Selvin E, Alonso A, Folsom AR. Demographic and cardiovascular risk factors modify association of fasting insulin with incident coronary heart disease and ischemic stroke (from the Atherosclerosis Risk In Communities Study). Am J Cardiol 2010;105:1420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawlor DA, Fraser A, Ebrahim S, Smith GD. Independent associations of fasting insulin, glucose, and glycated haemoglobin with stroke and coronary heart disease in older women. PLoS Med 2007;4:e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Møller LF, Jespersen J. Fasting serum insulin levels and coronary heart disease in a Danish cohort: 17-year follow-up. J Cardiovasc Risk 1995;2:235–40. [PubMed] [Google Scholar]
- 24.Arima H, Kiyohara Y, Kato I, Tanizaki Y, Kubo M, Iwamoto H, Tanaka K, Abe I, Fujishima M. Alcohol reduces insulin-hypertension relationship in a general population: the Hisayama study. J Clin Epidemiol 2002;55:863–9. [DOI] [PubMed] [Google Scholar]
- 25.Fagot-Campagna A, Balkau B, Simon D, Ducimetiere P, Eschwege E. Is insulin an independent risk factor for hypertension? The Paris Prospective Study. Int J Epidemiol 1997;26:542–50. [DOI] [PubMed] [Google Scholar]
- 26.Wieberdink RG, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM, Ikram MA. Insulin resistance and the risk of stroke and stroke subtypes in the nondiabetic elderly. Am J Epidemiol 2012;176:699–707. [DOI] [PubMed] [Google Scholar]
- 27.Thacker EL, Psaty BM, McKnight B, Heckbert SR, Longstreth WT, Jr, Mukamal KJ, Meigs JB, de Boer IH, Boyko EJ, Carnethon MR, et al. Fasting and post-glucose load measures of insulin resistance and risk of ischemic stroke in older adults. Stroke 2011;42:3347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakka HM, Lakka TA, Tuomilehto J, Sivenius J, Salonen JT. Hyperinsulinemia and the risk of cardiovascular death and acute coronary and cerebrovascular events in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Arch Intern Med 2000;160:1160–8. [DOI] [PubMed] [Google Scholar]
- 29.Pyörälä M, Miettinen H, Laakso M, Pyorala K. Hyperinsulinemia and the risk of stroke in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Stroke 1998;29:1860–6. [DOI] [PubMed] [Google Scholar]
- 30.Wiberg B, Sundstrom J, Zethelius B, Lind L. Insulin sensitivity measured by the euglycaemic insulin clamp and proinsulin levels as predictors of stroke in elderly men. Diabetologia 2009;52:90–6. [DOI] [PubMed] [Google Scholar]
- 31.Welin L, Eriksson H, Larsson B, Ohlson LO, Svardsudd K, Tibblin G. Hyperinsulinaemia is not a major coronary risk factor in elderly men. The study of men born in 1913. Diabetologia 1992;35:766–70. [DOI] [PubMed] [Google Scholar]
- 32.Yudkin JS, May M, Elwood P, Yarnell JW, Greenwood R, Davey Smith G. Concentrations of proinsulin like molecules predict coronary heart disease risk independently of insulin: prospective data from the Caerphilly Study. Diabetologia 2002;45:327–36. [DOI] [PubMed] [Google Scholar]
- 33.Zethelius B, Byberg L, Hales CN, Lithell H, Berne C. Proinsulin is an independent predictor of coronary heart disease: Report from a 27-year follow-up study. Circulation 2002;105:2153–8. [DOI] [PubMed] [Google Scholar]
- 34.Zethelius B, Lithell H, Hales CN, Berne C. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia 2005;48:862–7. [DOI] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 37.Duval S, Tweedie RA. Nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- 38.Denker PS, Pollock VE. Fasting serum insulin levels in essential hypertension. A meta-analysis. Arch Intern Med 1992;152:1649–51. [PubMed] [Google Scholar]
- 39.Hu G, Qiao Q, Tuomilehto J, Eliasson M, Feskens EJ, Pyorala K. Plasma insulin and cardiovascular mortality in non-diabetic European men and women: a meta-analysis of data from eleven prospective studies. Diabetologia 2004;47:1245–56. [DOI] [PubMed] [Google Scholar]
- 40.Sarwar N, Sattar N, Gudnason V, Danesh J. Circulating concentrations of insulin markers and coronary heart disease: a quantitative review of 19 Western prospective studies. Eur Heart J 2007;28:2491–7. [DOI] [PubMed] [Google Scholar]
- 41.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS ONE 2012;7:e52036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieker RP, Lee JC, Downing SE. Positive inotropic action of insulin on piglet heart. Yale J Biol Med 1975;48:353–60. [PMC free article] [PubMed] [Google Scholar]
- 43.Liang C, Doherty JU, Faillace R, Maekawa K, Arnold S, Gavras H, Hood WB., Jr Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest 1982;69:1321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulmyer-Lacroix O, Anglade G, Grino M. Insulin-induced hypoglycaemia increases colocalization of corticotrophin-releasing factor and arginine vasopressin mRNAs in the rat hypothalamic paraventricular nucleus. J Mol Endocrinol 1994;13:313–20. [DOI] [PubMed] [Google Scholar]
- 45.Kageyama S, Yamamoto J, Isogai Y, Fujita T. Effect of insulin on sodium reabsorption in hypertensive patients. Am J Hypertens. 1994;7(5):409–15. [DOI] [PubMed]
- 46.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 1975;55:845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferri C, Pittoni V, Piccoli A, Laurenti O, Cassone MR, Bellini C, Properzi G, Valesini G, De Mattia G, Santucci A. Insulin stimulates endothelin-1 secretion from human endothelial cells and modulates its circulating levels in vivo. J Clin Endocrinol Metab 1995;80:829–35. [DOI] [PubMed] [Google Scholar]
- 48.Hu FB, Stampfer MJ. Insulin resistance and hypertension: the chicken-egg question revisited. Circulation 2005;112:1678–80. [DOI] [PubMed] [Google Scholar]
- 49.Barnard RJ, J, Roberts CK, Varon SM, Berger JJ . Diet-induced insulin resistance precedes other aspects of the metabolic syndrome. J Appl Physiol 1998;84:1311–5. [DOI] [PubMed] [Google Scholar]
- 50.Brands MW, Hildebrandt DA, Mizelle HL, Hall JE. Sustained hyperinsulinemia increases arterial pressure in conscious rats. Am J Physiol 1991;260:R764–8. [DOI] [PubMed] [Google Scholar]
- 51.Juan CC, Fang VS, Kwok CF, Perng JC, Chou YC, Ho LT. Exogenous hyperinsulinemia causes insulin resistance, hyperendothelinemia, and subsequent hypertension in rats. Metabolism 1999;48:465–71. [DOI] [PubMed] [Google Scholar]
- 52.Ajjan R, Kearney MT, Grant PJ. Insulin resistance and the pathogenesis of cardiovascular disease. In: Zeitler PS, Nadeau KJ, eds. Insulin resistance: childhood precursors and adult disease. Clifton, NJ: Humana Press, 2008:179–205.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.