Abstract
There is a need to evaluate the evidence about the health effects of tea flavonoids and to provide valid, specific, and actionable tea consumption information to consumers. Emerging evidence suggests that the flavonoids in tea may be associated with beneficial health outcomes, whereas the benefits and risks of tea extracts and supplements are less well known. The next steps in developing tea science should include a focus on the most promising leads, such as reducing the risk of cardiovascular disease and stroke, rather than pursuing smaller, more diffuse studies of many different health outcomes. Future tea research should also include the use of common reference standards, better characterization of intervention products, and application of batteries of biomarkers of intakes and outcomes across studies, which will allow a common body of evidence to be developed. Mechanistic studies should determine which tea bioactive constituents have effects, whether they act alone or in combination, and how they influence health. Clinical studies should use well-characterized test products, better descriptions of baseline diets, and validated biomarkers of intake and disease risk reduction. There should be more attention to careful safety monitoring and adverse event reporting. Epidemiologic investigations should be of sufficient size and duration to detect small effects, involve populations most likely to benefit, use more complete tea exposure assessment, and include both intermediary markers of risk as well as morbidity and mortality outcomes. The construction of a strong foundation of scientific evidence on tea and health outcomes is essential for developing more specific and actionable messages on tea for consumers.
INTRODUCTION
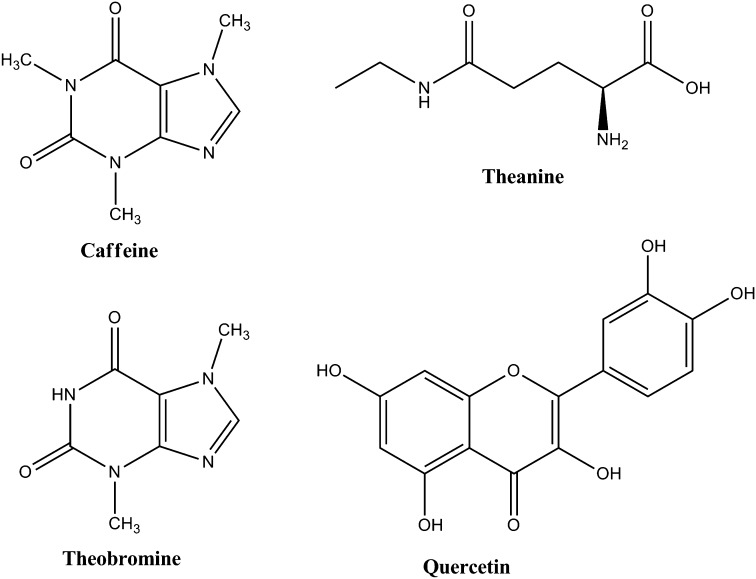
Tea is a fascinating, complex beverage and contains many bioactive ingredients including polyphenols as well as caffeine, theobromine, the amino acid theanine, inorganic salts, and elements. There is good evidence that certain classes of flavonoids may have beneficial health effects, so there is a great deal of research interest in tea flavonoids. This article presents the current science on tea flavonoids, suggests new research needed to provide evidence for a convincing case regarding health benefits and safety of the flavonoids and other bioactive components in tea, and discusses fruitful paths for communicating health messages about tea to consumers.
State of flavonoid science
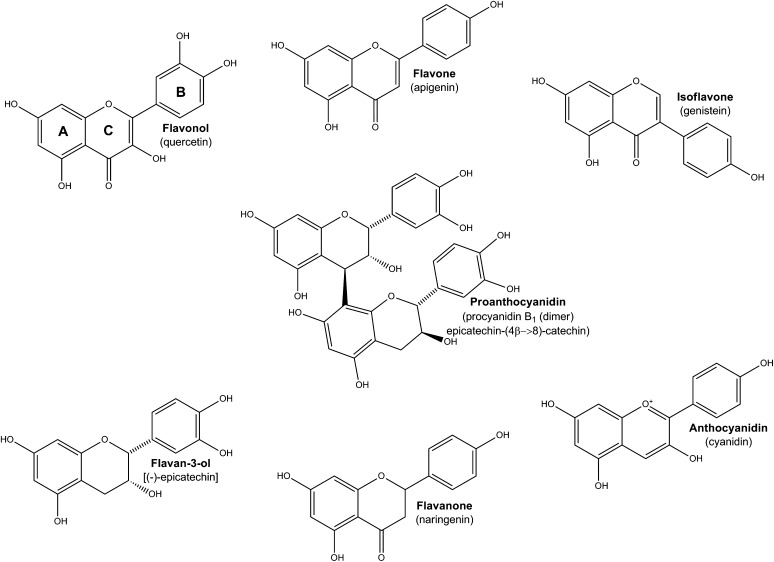
Flavonoids are phenolic compounds found in most plant foods and in particularly high concentrations in tea. Flavonoids appear to be the most plentiful and diverse of all the nonnutrient bioactive constituents of foods. Six classes of monomeric compounds and one class of oligomeric to polymeric flavonoid compounds are found in food (Figure 1). Flavonoids all share a common 3-ring structure and differ from each other in the location of their oxygen and hydroxyl moieties and by the presence or absence of double bonds on their carbon rings. The monomers in plants often appear as glycosides but are also found to be gallated or methylated. The notable exception is the flavan-3-ols, which are mainly present in the diet as aglycones.
FIGURE 1.
Flavonoid monomeric and oligomeric classes in foods.
Some flavonoids are associated with putative beneficial health effects. These are likely to be specific to certain flavonoid compounds and classes rather than to overall flavonoid intake. In vitro bioactivity includes antioxidant, antimutagenic, antimicrobial, and antiinflammatory properties as well as the capacity to inhibit platelet aggregation. Almost all of the in vitro studies have explored effects that are related to possible mechanisms of action of flavonoid compounds as they occur in food. The results of these studies may have little relevance to the true bioactivities in vivo because some flavonoids are metabolized before and after absorption to structurally distinct compounds. Few studies have explored the in vitro bioactivity of these compounds. In animal models, protective effects of flavonoids have been noted to decrease the initiation and/or progression of the pathogenesis of some common chronic degenerative diseases. Clinical evidence for a benefit of flavonoids is strongest for surrogate endpoints of cardiovascular disease such as flow-mediated dilation, biomarkers of inflammation, nitrous oxide production, and platelet aggregation. The observational evidence for flavonoid benefits on health is mixed, but relatively consistent, positive associations have been found for a decreased risk of coronary artery disease and stroke morbidity and mortality.
Food flavonoids and health outcomes
Early studies of the health effects of tea in the 1980s and 1990s took place at a time when databases of the flavonoid content of foods were incomplete. Nonetheless, case-control studies reported associations between diets high in flavonoid-rich tea, soy products, and cocoa. Differences in health outcomes between Asian and Western populations were thought to be due in part to these compounds. Cohort studies in Western populations showed associations between diets high in flavonoids, intakes of certain classes of flavonoids, and decreased risk of cardiovascular disease. Shortly after 2000, the USDA issued a series of more complete databases on isoflavonoids, other monomers, and proanthocyanidins; and a decade later, 2 other databases were released in Europe, the Eurofir project of the European Commission and the Phenol Explorer (1). The availability of these databases helped to accelerate progress in both observational and clinical research. Studies continued to report generally positive but inconsistent associations between foods such as tea, cocoa, wine, citrus, soy, and certain flavonoids with decreased risk of cardiovascular and some other chronic degenerative diseases. Adverse effects were not found from usual diets consisting of such foods and beverages. Progress continues to be made on increasing the accuracy and thoroughness of the databases for dietary polyphenols and other bioactive components as well as on developing valid biomarkers of flavonoid intake and metabolism.
Current evidence from cohort studies for health benefits of the flavonoids and flavonoid-rich foods such as tea appears to be strongest for coronary artery disease and stroke (2, 3). There is also a moderate amount of evidence that the flavonols contribute to cardiovascular health or are markers for other compounds that do have such effects. Investigations of the American Cancer Society's Cancer Prevention Study II (4) and the Iowa Women's Health Study (5) cohorts showed that intakes of anthocyanidins, anthocyanins, and flavanones were associated with decreased cardiovascular mortality risk. Inverse associations were also observed for the intake of flavan-3-ols and total flavonoids with mortality, but they were nonlinear, with most of the protective effects evident when consumption increased from virtually none to that in the second or third quintile of intake.
Incident hypertension was most closely associated with anthocyanin intake in another large cohort study, with some effect noted for the flavone apigenin in people <60 y of age and also for the flavan-3-ol catechin, although the effects were not strong (6). In a follow-up study with data from a twin registry, anthocyanin intake was associated with lower central systolic blood pressure and pulse-wave velocity, measures of cardiovascular risk, but the flavan-3-ols, which are high in tea, were not (7). There are a number of positive studies of cocoa flavan-3-ols with surrogate markers of decreased surrogate cardiovascular disease risk biomarkers such as flow-mediated dilation (8–11). Thus, the beneficial effects of the flavonoids on cardiovascular disease do not appear to be limited to a single food, a single class of flavonoids, or a single flavonoid.
TEA FLAVONOIDS
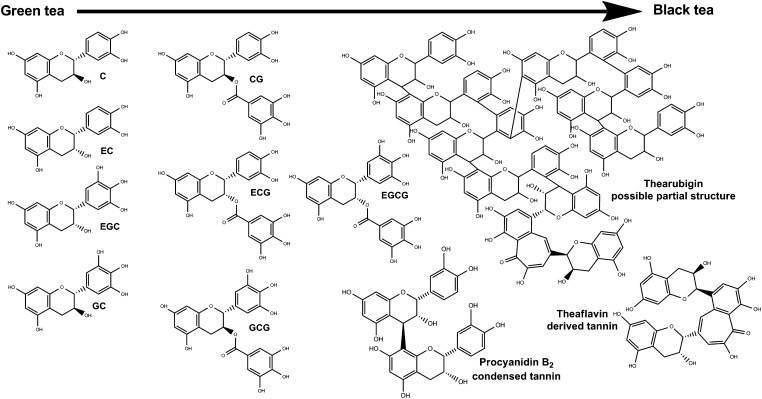
Tea is a member of the Camellia family. The process of fermentation in tea involves the oxidation of flavonoids present in the tea leaf caused by release of intracellular polyphenol oxidase. It transforms not only the flavonoids but also the color and taste of the product. Green tea is not fermented, oolong is partly fermented, and black tea is fermented. Pu'er tea, which is popular in some Asian countries, is wet fermented or composted. Tea is particularly rich in 3 flavonoid classes: flavan-3-ols (or catechins), oligimeric flavonoids (including thearubigins and theaflavins generated during fermentation), and flavonols (eg, quercetin) (see Figures 2 and 3) (12, 13). There are 6 unique “signature” monomer catechins in green tea that provide its characteristic chemical profile, including epigallocatechin-3-gallate and epicatechin-3-gallate. During the fermentation of green to black tea, the catechins form dimers called theaflavins (derived tannins), larger compounds called proanthocyandins (condensed tannins), and very large oligomers and polymers called thearubigens (condensed/derived tannins) (Figure 2). The chemical structures of these flavonoid oligomers in tea are very complex and have yet to be entirely characterized (14). Quercetin is the most common flavonol present in tea (15). Other characteristic bioactive components in tea (Figure 3) include theanine, an amino acid that has been hypothesized to reduce glutamate-related endothelial damage (16), caffeine, theobromine, and inorganic salts.
FIGURE 2.
Fermentation of green tea to black tea. C, catechin; CG, catechin-3-gallate; EC, epicatechin; ECG, epicatechin-3-gallate; EGC, epigallocatechin; EGCG, epigallocatechin-3-gallate; GC, gallocatechin; GCG, gallocatechin-3-gallate.
FIGURE 3.
Other characteristic bioactive components in tea.
Tea is a complicated beverage not only because of its many varietals, array of bioactive constituents, and several methods of fermentation and processing but also because of its varied techniques of preparation (13–15). Unblended teas vary by hybrid and by the geographic area in which they are produced (eg, Assam, China, Ceylon, Darjeeling, Kenya) and also differ in terms of their processing (eg, orthodox compared with “crush, tear, and curl”), type (eg, plain or flavored), and grade (size). The blend from which a brew is made has a major effect on flavonoid content. The blending and brewing of teas are intended to modify their aroma, color, and taste but directly affect their flavonoid profile as well. These factors and others, such as the addition of milk, lemon, or honey, complicate the assessment of the intake of tea flavonoids and other bioactive components in observational studies. Currently available flavonoid databases can help minimize measurement error and increase the contrast between consumers with different amounts of intake, but better efforts to characterize and define tea intake in epidemiologic studies and clinical trials are warranted.
The limitations associated with our current research approaches may account for some of the apparent discrepancy in study results. For example, whereas evidence from some cohort studies indicates a reduced risk of coronary artery disease with tea consumption of ≥3 cups daily (17), data on the effect of tea on the incidence and mortality from cardiovascular disease are limited, although the Cochrane Heart Group is now undertaking a meta-analysis of research studies on this topic (18). Observational studies suggesting that tea intake is inversely correlated with the risk of type 2 diabetes is compelling, but randomized clinical trials are necessary to establish causal inferences (19, 20). The evidence for a chemopreventive action of tea was not supported in a 2009 systematic review by Boehm et al (21). Although not an issue for tea drinking, new research is warranted because of concerns about the potential adverse effects of certain green tea extracts used in dietary supplements (30–32).
NEXT STEPS FOR TEA SCIENCE
Progress has been slow in achieving health claims for tea or other flavonoid-rich foods despite continued efforts both in the United States and in Europe (22). Approval for some statements about tea or other flavonoid-rich foods and their benefit to health seems likely at some point in the future. However, significant gaps in the research evidence remain today. Why is it that more definitive information on benefits is not available nearly a quarter century after the first studies linked tea and tea flavonoids with positive effects on cardiovascular disease risks?
It is possible that tea flavonoids have little or no efficacy and that the positive results from early studies simply reflected poor control of other influences or that the flavonoid-poor diets to which comparisons were made were also nutritionally poor in other ways. If so, the inverse association of flavonoid-rich diets with disease risk reduction may have been coincidental or due to the increased risk of poor outcomes in the comparison group. It is also possible that the observed beneficial effect was due to multicollinearity, ie, due to other bioactive compounds present in tea and/or other foods. A more likely possibility is that tea intake has a biologically significant but smaller effect than first reported, and that the number and quality of observational and clinical studies to date have not been sufficiently large or robust enough to show clear and consistent health benefits. As a natural plant-based beverage, the biological effects of tea are likely smaller than that of therapeutic drugs, and effects may be missed in studies that are powered for pharmacologic interventions. If only specific classes or combinations of flavonoids or other bioactive compounds are efficacious, and these exposures are poorly assessed in epidemiologic studies or vary too greatly in the available clinical trials, it is not surprising that results are inconsistent.
The US Food and Drug Administration (FDA)6, the UK Food Standards Agency, the European Union's European Food Safety Administration (EFSA), and many other countries have developed procedures and regulations for permitting health claims on foods (23, 24). These administrative processes involve a review of generally acceptable scientific evidence that the claimed effect of the food is beneficial to health and that several other conditions are met. These include the following: 1) there is a cause-and-effect relation between consumption of the food and claimed effects in humans using generally accepted criteria, such as strength, consistency, temporal occurrence, specificity, and evidence of a dose-response relation, as well as biological plausibility of the relation; 2) a reasonable quantity of the food and the pattern of consumption is required to obtain the claimed effect, and this amount can be achieved as part of a balanced diet; 3) the specific groups on which evidence was obtained are described and they are representative of the larger target population for which the claim is made; and 4) the need to prevent consumer confusion as well as to protect the public's health must be met (25). For those planning to develop the evidence to craft health claims, it is worth examining the failed tea health claims to gain insight to guide future research in more productive directions. For example, some of the reasons for rejection of health claims for tea by regulatory agencies included the following: 1) insufficiently characterized active ingredients in relation to the claimed effects and 2) lack of an established cause-and-effect association between the tea beverage and/or its bioactive constituents and their claimed health effects if used as intended. However, these shortcomings are correctable and within the reach of existing scientific approaches (Table 1). For example, with regard to identifying key bioactive ingredients, Standard Reference Materials (SRMs) produced by the US National Institute of Standards and Technology now include for green tea the concentrations of 7 catechins and gallic acid, 3 xanthine alkaloids (including caffeine), theanine, and toxic elements (arsenic, cadmium, lead, and mercury) in SRM 3254 Camellia sinensis (Green Tea) Leaves, SRM 3255 Camellia sinensis (Green Tea) Extract, and SRM 3256 Green Tea–Containing Solid Oral Dosage Form.
TABLE 1.
Recommendations for improving epidemiologic and clinical studies of tea and tea flavonoids
| Issues | Recommendations |
| Observational studies | |
| Study common diseases where mechanistic and animal studies suggest strong effects | Emphasize study of cardiovascular disease, stroke, and type 2 diabetes with mortality as well as intermediary biomarkers of these diseases |
| Use appropriate populations with sufficient follow-up for morbidity and mortality outcomes | Avoid very ill or very old participants in whom other risk factors are likely to overwhelm dietary effects |
| Ensure power is adequate for reasonable effect size | Impact of tea is likely modest compared with druglike effect sizes |
| Use more detailed dietary assessment tools to assess exposures more precisely | Precise quantification of tea consumption (type, amount, frequency, brew strength, etc) is necessary; existing food-frequency questionnaires must be modified, or details on tea need to be asked separately |
| Improve exposure assessments | Both tea and total flavonoid intake should be measured |
| Improve bioactive databases so that they are more complete | Ensure details on tea and other flavonoid-rich foods are represented in databases and include dimers, trimers, and polymeric flavonoids |
| Clinical studies | |
| Baseline diets and test products | Should be well described and characterize all sources of flavan-3-ols, flavonols, proanthocyanidins, etc, so that total flavonoids can be calculated; assess content of the intervention tea product by using standard reference materials |
| Absorption and metabolism markers | Study variations in flavonoid absorption and use validated surrogate markers for metabolic and health effects |
| Intervention with test materials | Use well-characterized products and measure intervention precisely by using a battery of dietary tools and biomarkers |
| Intermediary and health outcomes | Track changes in valid, accepted surrogate outcomes or “hard” outcomes such as morbidity and mortality; batteries of agreed-on and validated intermediary outcomes used by several investigators facilitate comparisons between studies |
| Efficacy | Amass data on intake amounts that are efficacious in long-term studies to obtain relevant and validated health outcomes |
| Safety | Provide evidence of safe use and amass evidence for the absence of potential adverse effects |
Hypothesized causal pathways and mechanisms for the health effects of tea flavonoids and other tea bioactive components should also be guiding new investigations. In vitro studies exploring specific mechanisms should assess the effects of flavonoid compounds as they occur in vivo, rather than assessing effects only of the compounds found in foods and beverages. Of special importance is whether and how flavonoids bind at receptor sites and, if so, the nature of their bioactions. For example, by using a Bayesian approach, the dose of ingested epicatechin was found to explain most of the blood pressure–lowering effects of cocoa products; similar effects might be achieved by other foods rich in epicatechin such as tea (8). Synergy-directed fractionation techniques in appropriate animal models may also be worth pursuing to better explore the mechanisms of action of bioactive components of tea. The influence of genetics and epigenetics in determining responses to tea are also fertile research areas.
In clinical studies, more information is needed on the absorption, digestion, metabolism, and excretion of each of the bioactive components in tea that have been linked to health effects, including but not limited to the flavonoids. This approach can be helpful in refining hypotheses: eg, studies on the absorption of the bioactive components of tea found that procyanidins with a degree of polymerization of ≥2 were poorly absorbed (26). As noted above, the bioavailability of tea flavonoids can be influenced by preparation and brewing techniques and accompaniments such as milk; these factors must be taken into account because they affect the amount of catechins and other flavonoids that can reach their cellular target. As has already been investigated for wine, the effects of tea on the gut microflora also deserve further study (27).
Tea was introduced to the Western world ∼450 y ago but has been consumed in the Eastern world for >4500 y, providing anecdotal evidence that its consumption in moderation is enjoyable and without untoward effects. Fortification of beverages and foods or supplementation with tea extracts has recently become popular, although their equivalence in efficacy and safety are not well established (28, 29). A potential for adverse effects exists for these products because their doses may be high, the processing techniques to prepare them (eg, aqueous compared with alcoholic extraction) can result in a profile of phytochemicals quite different from that of a tea beverage, and consuming them may occur in a different dietary context than typical tea drinking. Furthermore, many products combine tea extracts with a variety of other phytochemicals, nutrients, and herbal ingredients. Safety concerns, particularly the potential for hepatotoxicity (30–32) with regard to the use of green tea extracts, were summarized in a systematic review by the US Pharmacopeia (30). However, it is difficult to evaluate either beneficial or adverse effects for this category of products because they are not standardized. In addition to new preclinical safety studies, improved adverse event reporting is warranted in which factors such as prior experience, alternative etiologies, temporal correlations, correlations with dosage amounts, and dechallenge-rechallenge situations are considered. However, it is worthwhile to note that some small studies have shown a reduction in risk of liver disease and cancer associated with the use of green tea extracts (21, 33).
Epidemiologic investigations may be able to identify subpopulations that may particularly experience health benefits from drinking tea. However, better tools to ascertain beverage and food consumption and more complete databases are necessary to improve exposure assessment of tea and tea phytochemicals. There is also a need to use biomarkers of tea intake. Indeed, it is particularly important to ascertain intakes precisely so that the amount of flavonoids from the overall diet and from tea specifically can be assessed. This approach might be most successful with serial 24-h recalls, which allow for extensive probing for details on variety, blend, and brewing techniques. Food-frequency questionnaires are more often used in large cohort studies, but unless they are specially tailored for tea studies they contain only general questions on tea and make true differences in outcome difficult to measure. For example, items on food-frequency questionnaires usually ask about tea consumption per day, with teas and other beverages of very different flavonoid content amalgamated altogether in a single item (eg, ready-to-drink, brewed from bags of blended teas, loose-leaf tea), and a single average value is applied to all consumers. For these reasons, food-frequency questionnaires must be either purpose-built or modified to assess the consumption of tea and tea flavonoids. This approach is feasible, as Zhang et al (34, 35) have shown by developing and testing a detailed food-frequency questionnaire to assess dietary flavonols and flavones. Alternatively, one could use an ancillary questionnaire that obtains complete information on type of tea and brewing and preparation of the tea.
Databases for dietary flavonoids need to be expanded and updated by using similar definitions for categorizing compounds into various classes and sufficiently precise to avoid errors in quantification. When including or excluding studies from meta-analyses, standards are needed for the completeness and quality of the database used in the study; otherwise, gaps and errors in the databases may mask true effects and lead to null results. Moreover, other improvements to the design and conduct of epidemiologic research need to be implemented. Whereas these studies need to include a sufficient sample size, it is also important that tea intake be described and assessed precisely, confounders be suitably controlled or adjusted, and chronic disease endpoints or valid surrogates be used for chronic degenerative disease endpoints (36) as well as intermediary markers of risk (17, 37). One useful biomarker of black tea intake does exist (4-O-methylgallic acid). This biomarker probably also provides a good indicator of the level of exposure to black tea–derived polyphenols. The main problems with using this biomarker is that very few population studies collect 24-h urine samples, which is appropriate, and that in any event the compound is a better indicator of intake over 1–2 d rather than usual intake. However, there is no good biomarker of longer term intake that has been identified. Thus, self-reported estimates of intake are still superior to biomarkers for ascertaining usual intake. It would also be helpful if objective biomarkers of flavonoid intake could be developed. Because such health outcome studies are costly and time-consuming, a long-term research horizon must be contemplated.
COMMUNICATING TEA RESEARCH TO THE PUBLIC
As the science of tea becomes more mature and specific, actionable messages about consuming tea (and other flavonoid-rich foods) that are based on solid evidence and not misleading will be needed. Consumer communications are complicated, and it is usually difficult to prepare messages that are consonant with the science and that actually help, rather than confuse, consumers. What valid and useful information can be transmitted to consumers about tea and/or its flavonoid content in dietary guidance, on label contents, or through structure-function or qualified health claims? Can a dietary reference intake be established for the flavonoids found in tea (or other foods)? How can information derived from tea research fit within existing dietary guidance such as the “My Plate” food guide? Is evidence today sufficient for a general dietary guidance statement, such as recommending that individuals eat 5–9 servings of a variety of colorful fruit and vegetables and other good sources of flavonoids, such as tea? These are questions that are easier to ask than to answer. More specific guidance may have to await the results of new evidence derived from different research strategies.
Are more specific messages about flavonoid content appropriate based on the available evidence? Should tea products be labeled with their content of flavonoids or other bioactive components? Should teas be standardized to one or more of their bioactive constituents to ensure a specific health benefit? This question, however, will require more new information on the direct relation between one or more of these compounds and the putative benefits of tea. For example, is the apparent benefit of tea derived from its total polyphenol content or from its total flavonoids, total catechins, and/or some combination of these and other bioactive components that are also present, such as l-theanine, caffeine, theobromine, or other constituents? A particular advantage of black tea compared with green tea is that most of the black tea consumed worldwide is blended. This means that the contents of flavonoids, caffeine, theanine, and other compounds are reasonably similar for many of the teas consumed around the world. There is therefore less of a need to evaluate the product and more focus can be placed on how the tea is prepared and consumed. It is worth noting that consumers generally prefer scientists to talk more about foods than about specific nutrients or phytochemicals. However, once a bioactive constituent or constituents is identified as having beneficial effects, it may be helpful for manufacturers to identify their content on the food label. A glance at the labels of some teas now on the market finds them sometimes declaring the content of caffeine or flavonoids or “healthy antioxidants.” It is clear that agreement is lacking within the tea industry on which ingredients to highlight and to standardize against for stating content claims or health benefits.
Health claims are another means of communicating benefits. Should structure-function claims for tea flavonoids be used? Would such statements help consumers, even if substantiated, truthful, and not misleading? Beyond the basic issues regarding the association of the evidence to the claim and the quality and totality of the evidence, there are also communications issues that must be wrestled with. What may be permitted under the law and what consumers understand may be quite different. For example, it is unclear if consumers understand the distinction between nutrient content, structure-function, and other health claims or between different levels of claims (23).
The FDA allows a number of health claims related to plant foods including the following: 1) soy protein and reduced risk of coronary heart disease, 2) fruit and vegetables and reduced risk of some cancers, and 3) fruit, vegetables, and grains containing fiber (especially soluble fiber) and reduced risk of coronary heart disease (24). The FDA denied proposed health claims for green tea and decreased risk of cardiovascular disease, gastric cancer, colorectal cancer, and esophageal, pancreatic, and other cancers. For green tea and cancer, the FDA concluded that it was highly unlikely that green tea decreased breast and prostate cancer risks. Similarly, EFSA concluded that the substantiation for the health claims related to tea did not suffice in several recent submissions the agency received. The claims denied included those that catechins from green tea contribute to the maintenance or achievement of a normal body weight and increased β oxidation of fatty acids leads to a reduction in body fat mass and helps maintain normal blood glucose concentrations (38). The EFSA also denied claims related to tea and the catechins and intermediary biomarkers of outcomes such as improvement of endothelium-dependent vasodilation, maintenance of normal blood pressure, maintenance of normal blood glucose concentrations, maintenance of normal blood LDL-cholesterol concentrations, protection of the skin from UV-induced damage (including photo-oxidation), protection of the DNA from oxidative damage, and protection of lipids from oxidative damage. Additional claims that did not fare well included ones that catechins contributed to normal cognitive function, normal cardiovascular system functions, bodily invigoration, decreasing potentially pathogenic gastrointestinal microorganisms, immune health, and oral health (39). The dossiers denied on substantiation of health claims related to tea also included proposed associations of catechins in green tea and tannins in black tea with protection of DNA, proteins, and lipids from oxidative damage; reduction in acid production in dental plaque; maintenance of bone health; decreases in potentially pathogenic intestinal microorganisms; maintenance of vision; maintenance of normal blood pressure; and maintenance of normal blood cholesterol concentrations (40). The EFSA also rejected claims for l-theanine in improving cognitive function, alleviating psychological stress, helping maintain normal sleep, and reducing menstrual discomfort (41).
Many other questions arise as we consider these issues, including whether health claims for tea/flavonoids are likely to contribute positively to the health of the population, and whether science can be communicated to the general public without the need for health claims. These difficult issues need to be addressed and answered in the future.
Is there sufficient evidence to establish a Dietary Reference Intake for flavonoids? Can an Estimated Average Requirement or Adequate Intake be established for flavonoids—or only for some classes or specific compounds? It is clear from a body of in vivo research that using simple criteria for the mechanism of action of flavonoids as antioxidants cannot readily be applied to dietary allowances for these compounds because they can possess both antioxidant and prooxidant properties (42–44) as well as an array of other bioactions. It is likely that dietary allowances would eventually need to focus more on the more selective mechanisms and physiologic functions associated with specific flavonoid classes and/or individual compounds. Tolerable Upper Levels of safe intake would also need to be considered. At a more practical level, much of the funding for the Dietary Reference Intake process comes from the federal government, which must choose among many pressing nutrition research priorities, and funding is always limited.
CONCLUSIONS AND FUTURE DIRECTIONS
The continuation of high-quality research on the health benefits of tea is critical to support valid, specific, and actionable information about consumption to consumers. Future efforts should include examining the best available evidence on tea and health to identify the most promising leads for more concentrated study. Clinical investigations will require better characterized baseline diets and test products, more complete phytochemical databases, better assessment of exposure, larger populations, longer study durations, useful biomarkers for tea intake/exposure, validated intermediary biomarkers of disease risk as well as ultimate health outcomes, and better safety monitoring. The use of common biomarkers and other measures across studies should be considered so that results can be better shared and aggregated. This scientific evidence must be firmly in place before it will be possible to develop specific and actionable messages for consumers on consumption of tea and other flavonoid-rich foods that are credible and widely accepted in both scientific and consumer circles.
Acknowledgments
The authors’ responsibilities were as follows—JTD: designed the review and had primary responsibility for preparing the final manuscript and for final content; and JTD and JP: collected and analyzed the data and wrote the manuscript. Both authors read and approved the final manuscript. JTD performed this work as a contractor at the Office of Dietary Supplements, NIH. JP received a grant for travel and accommodations to attend the symposium from the Tea Council of the USA. The authors declared no competing financial interests.
Footnotes
Abbreviations used: EFSA, European Food Safety Administration; FDA, US Food and Drug Administration; SRM, Standard Reference Material.
REFERENCES
- 1.Scalbert A, Andres-Lacueva C, Arita M, Kroon P, Manach C, Urpi-Sarda M, Wishart D. Databases on food phytochemicals and their health-promoting effects. J Agric Food Chem 2011;59:4331–48. [DOI] [PubMed] [Google Scholar]
- 2.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:38–50. [DOI] [PubMed] [Google Scholar]
- 3.Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev 2012;70:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullough ML, Peterson JJ, Patel R, Jacques PL, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr 2012;95:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007;85:895–909. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy A, O'Reilly ÉJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 2011;93:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelja M, Spector T, Macgregor A, et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr 2012;96:781–8. [DOI] [PubMed] [Google Scholar]
- 8.Ellinger S, Reusch A, Stehle P, Helfrich HP. Epicatechin ingested via cocoa products reduces blood pressure in humans: a nonlinear regression model with a Bayesian approach. Am J Clin Nutr 2012;95:1365–77. [DOI] [PubMed] [Google Scholar]
- 9.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart EE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short term studies. J Nutr 2011;141:1982–8. [DOI] [PubMed] [Google Scholar]
- 10.Tokede OA, Gaziano JM, Djousse L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr 2011;65:879–86. [DOI] [PubMed] [Google Scholar]
- 11.Zomer E, Owen A, Magliano DJ, Liew D, Reid CM. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ 2012;344:e3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 2003;133(suppl):3248S–54S. [DOI] [PubMed] [Google Scholar]
- 13.Peterson J, Dwyer J, Bhagwat S, Haytowitz D, Holden J, Eldridge AL, Beecher G, Aladesnmi J. Major flavonoids in dry tea. J Food Comp Anal 2005;18:487–501. [Google Scholar]
- 14.Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res 2009;53:S310–29. [DOI] [PubMed] [Google Scholar]
- 15.Peterson J, Dwyer J, Jacques P, Rand W, Prior R, Chui K. Tea variety and brewing techniques influence flavonoid content of black tea. J Food Comp Anal 2004;17:397–405. [Google Scholar]
- 16.Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke: a meta-analysis. Stroke 2009;40:1786–92. [DOI] [PubMed] [Google Scholar]
- 17.Ruxton CHS, Mason P. Is black tea consumption associated with a lower risk of cardiovascular disease and type 2 diabetes? Nutr Bull 2012;37:4–15. [Google Scholar]
- 18.Hartley L, Flowers N, Clarke A, Stranges S, Hooper L, Rees K. Green and black tea for primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2012 i(6) Art. No: CD009934. [DOI] [PMC free article] [PubMed]
- 19.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med 2009;169:2053–63. [DOI] [PubMed] [Google Scholar]
- 20.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine and risk of type 2 diabetes: a prospective cohort study in younger and middle aged U.S. women. Diabetes Care 2006;29:398–403. [DOI] [PubMed] [Google Scholar]
- 21.Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Miliazzo S, Horneber M. Green tea for the prevention of cancer. Cochrane Database Syst Rev 2009;3:CD005004. [DOI] [PMC free article] [PubMed]
- 22.Gallagher AM, Meijer GW, Richardson DP, Rondeu R, Skarp M, Stasse-Wolthuis M, Tweedie GC, Witkamp R. A standardised approach towards PROving the efficacy of foods and food constitutents for health CLAIMs (PROCLAIM): providing guidance. Br J Nutr 2011;106:S16–28. [DOI] [PubMed] [Google Scholar]
- 23.Verhagen H, Vos E, Francl S, Heinonen M, van Loveren H. Status of nutrition and health claims in Europe. Arch Biochem Biophys 2010;501:6–15. [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. Claims that can be made for conventional foods and dietary supplements. September 2003. Available from: http://www.fda.gov/food/labelingnutrition/labelclaims/ucm111447.htm (cited 23 January 2013).
- 25.Aggett PJ, Hathcock J, Jukes D, Richardson DP, Calder PC, Bischoff-Ferrari H, Nicklas T, Mühlebach S, Kwon O, Lewis J, et al. Nutrition issues in Codex: health claims, nutrient reference values and WTO agreements: a conference report. Eur J Nutr 2012;51:S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottaviani JI, Kwik-Uribe C, Keen CL, Schroeter H. Intake of dietary procyanidins do not contribute to the pool of circulating flavanols in humans. Am J Clin Nutr 2012;95:851–8. [DOI] [PubMed] [Google Scholar]
- 27.Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ. Influence of red wine polyphonols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 2012;95:1323–34. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Chen P, Lin LZ, Harnly JM. A non-targeted approach to chemical discrimination between green tea dietary supplements and green tea leaves by HPLC/MS. J AOAC Int 2011;94:487–97. [PMC free article] [PubMed] [Google Scholar]
- 29.Egert S, Rimbach G. Which sources of flavonoids: complex diets or dietary supplements? Adv Nutr 2011;2:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, Marles RJ, Pellicore LS, Giancaspro GI, Low Dog T. Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf 2008;31:469–84. [DOI] [PubMed] [Google Scholar]
- 31.Otera H, Tada K, Sakurai T, Hashimoto K, Ikeda A. Hypersensitivity pneumonitis associated with inhalation of catechin-rich green tea extracts. Respiration 2011;82:388–92. [DOI] [PubMed] [Google Scholar]
- 32.Verhelst X, Burvenich P, Van Sassenbroeck D, Gabrial C, Lootens M, Baert D. Acute hepatitis after treatment for hair loss with oral green tea extracts (Camellia Sinensis). Acta Gastroenterol Belg 2009;72:262–4. [PubMed] [Google Scholar]
- 33.Masterjohn C, Bruno RS. Theraperutic potential of green tea in nonalcoholic fatty liver disease. Nutr Rev 2012;70:41–56. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Cao J, Chen W, Yang J, Hao D, Zhang Y, Chang P, Zhao X. Reproducibility and relative validity of a food frequency questionnaire to assess intake of dietary flavonol and flavone in Chinese university campus population. Nutr Res 2010;30:520–6. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Li Y, Cao C, Cao J, Chen W, Zhang Y, Wang C, Wang J, Zhang X, Zhao X. Dietary flavonol and flavone intakes and their major food sources in Chinese adults. Nutr Cancer 2010;62:1120–7. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Jiménez J, Hubert J, Hooper L, Cassidy A, Manach C, Williamson G, Scalbert A. Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr 2010;92:801–9. [DOI] [PubMed] [Google Scholar]
- 37.Mack A, Balogh E, Micheel C. Perspectives on biomarker and surrogate endpoint evaluation: discussion forum summary. Washington, DC: National Academies Press, 2011. [PubMed] [Google Scholar]
- 38.European Food Safety Administration Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to Camellia sinensis (L.) Kuntze (tea), including catechins from green tea, and contribution to the maintenance or achievement of a normal body weight (ID 1107, 1112, 1544, 2716), increased beta-oxidation of fatty acids leading to a reduction in body fat mass (ID 1123, 1124, 3698), and maintenance of normal blood glucose concentrations (ID 1115, 1545) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. European Food Safety Agency Journal 2010;8:1791 DOI:10.2903/j.efsa.2010.1791. [Google Scholar]
- 39.European Food Safety Administration Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to Camellia sinensis (L.) Kuntze (tea), including catechins in green tea, and improvement of endothelium-dependent vasodilation (ID 1106, 1310), maintenance of normal blood pressure (ID 1310, 2657), maintenance of normal blood glucose concentrations (ID1108), maintenance of normal blood LDL-cholesterol concentrations (ID2640), protection of the skin from UV induced (including photo-oxidative) damage (ID 1110,1119), protection of DNA from oxidative damage (ID 1120,1121), protection of lipids form oxidative damage (ID 1275), contribution to normal cognitive function (ID 1117, 2812), “cardiovascular system” (ID 2814), “invigoration of the body” (ID 1274, 3280), decreasing potentially pathogenic gastrointestinal microorganisms (ID 1118), “immune health” (ID 1273) and “mouth” (ID 2813) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. European Food Safety Agency Journal 2011;9:2055 DOI:10.2903/j.efsa.2011.2055. [Google Scholar]
- 40.European Food Safety Administration Panel on Dietetic Products. Scientific opinion on the substantiation of health claims related to Camellia sinensis (L.) Kuntze (tea), including catechins in green tea and tannins in black tea, protection of DNA, proteins and lipids from oxidative damage (ID1103,1276,1311,1708,2664), reduction of acid production in dental plaque (ID1105, 1111), maintenance of bone (ID1109), decreasing potentially pathogenic intestinal microorganisms (ID1116), maintenance of vision (ID 1280), maintenance of normal blood pressure (ID1546), and maintenance of normal blood cholesterol concentrations (ID1113, 1114) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. European Food Safety Authority Journal 2010;8:1463 DOI:10.2903/j.efsa.2010.1463. [Google Scholar]
- 41.European Food Safety Administration Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to L-theanine from Camellia sinensis (L.) Kuntze (tea) and improvement of cognitive function (ID 1104, 1222, 1600, 1601, 1707, 1935, 2004, 2005), alleviation of psychological stress (ID 1598,1601), maintenance of normal sleep (ID 1222, 1737,2004), and reduction of menstrual discomfort (ID 1599) pursuant to Article 13(1) of Regulation (EC) No 1924-2006. European Food Safety Agency Journal 2011;9:2338 DOI:10.2903/j.efsa.2011.2238. [Google Scholar]
- 42.Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011;82:513–23. [DOI] [PubMed] [Google Scholar]
- 43.Finley JW, Kong AN, Hintze KJ, Jeffery EH, Ji LL, Lei XG. Antioxidants in foods: state of the science important to the food industry. J Agric Food Chem 2011;59:6837–46. [DOI] [PubMed] [Google Scholar]
- 44.Hollman PC, Cassidy A, Comte B, Heinonen M, Richelle M, Richling E, Serafini M, Scalbert A, Sies H, Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr 2011;141(suppl):989S–1009S. [DOI] [PubMed] [Google Scholar]