Abstract
Osteoporosis is a major health problem in the aging population worldwide. Cross-sectional and retrospective evidence indicates that tea consumption may be a promising approach in mitigating bone loss and in reducing risk of osteoporotic fractures among older adults. Tea polyphenols enhance osteoblastogenesis and suppress osteoclastogenesis in vitro. Animal studies reveal that intake of tea polyphenols have pronounced positive effects on bone as shown by higher bone mass and trabecular bone volume, number, and thickness and lower trabecular separation via increasing bone formation and inhibition of bone resorption, resulting in greater bone strength. These osteoprotective effects appear to be mediated through antioxidant or antiinflammatory pathways along with their downstream signaling mechanisms. A short-term clinical trial of green tea polyphenols has translated the findings from ovariectomized animals to postmenopausal osteopenic women through evaluation of bioavailability, safety, bone turnover markers, muscle strength, and quality of life. For future studies, preclinical animal studies to optimize the dose of tea polyphenols for maximum osteoprotective efficacy and a follow-up short-term dose-response trial in postmenopausal osteopenic women are necessary to inform the design of randomized controlled studies in at-risk populations. Advanced imaging technology should also contribute to determining the effective dose of tea polyphenols in achieving better bone mass, microarchitecture integrity, and bone strength, which are critical steps for translating the putative benefit of tea consumption in osteoporosis management into clinical practice and dietary guidelines.
INTRODUCTION
Osteoporosis, a degenerative skeletal disorder, is characterized by low bone mass (osteopenia) and microarchitectural deterioration of bone tissue that results in compromised bone strength and increased risk of fractures (1). Among different types of fractures, hip fracture is the most severe consequence of osteoporosis, leading to reduced activities of daily living, lowered quality of life, and increased mortality (2). As the population ages worldwide, the increased life expectancy is accompanied with a higher prevalence of osteoporosis in the elderly population, such that osteoporosis has become a serious health concern in many countries (2, 3). For example, in the United States, it is estimated that by 2020, there will be >61 million women and men aged ≥50 y affected by osteoporosis or low bone mass (3); by 2025, the cost of osteoporosis-related expenses is projected to increase to $25.3 billion (4).
The etiology of osteoporosis includes both nonmodifiable (ie, genetic, sex, and age) and modifiable (ie, nutrition, excessive alcohol, tobacco smoking, and physical inactivity) factors. The risk of osteoporosis and related fractures can be reduced by modulating these modifiable factors. Nutrition influences the risk of osteoporosis and fractures through improving bone mineral density (BMD)6, microarchitecture, and strength (5). In the past decade, the awareness of complementary and alternative therapies for the prevention and/or treatment of osteopenia and osteoporosis, such as dietary supplements or functional foods, has been increasing among consumers (6).
The bone remodeling process involves both bone resorption (osteoclastogenesis) and bone formation (osteoblastogenesis) (7). Bone is resorbed by osteoclast cells and then new bone is formed by osteoblast cells. During bone remodeling, excessive osteoclastic activity and inadequate osteoblastic activity are the main reasons for osteoporosis development.
Reactive oxygen species (ROS) can lead to damage to DNA, protein, and lipids. Oxidative stress results from high amounts of ROS produced during normal cellular metabolism and from environmental stimuli perturbing the normal redox balance (8). With regard to osteoblastogenesis, excessive oxidative stress induces osteoblast and osteocyte apoptosis, decreases osteoblast number via extracellular signal-regulated kinases and extracellular signal-regulated kinase–dependent nuclear transcription factor κB (NF-κB) signaling pathways (9), and suppresses bone formation rate via Wnt/β-catenin signaling pathways (10). On the other hand, osteoblasts have been shown to upregulate antioxidant defenses such as glutathione peroxidase to stimulate transforming growth factor β, a growth factor involved in osteoclast differentiation and survival (11). Oxidative stress appears to enhance the differentiation and function of osteoclasts or directly contributes osteoclast-generated superoxide to bone degradation (12). A strong correlation exists between oxidative stress and bone loss during the development of osteoporosis (13, 14). Because oxidative stress can contribute to bone loss, lowering its status via antioxidant mechanisms could be an important strategy to mitigate or suppress osteoporosis-related bone loss.
Tea, the dried leaves of the plant Camellia sinensis species of the Theaceae family, is a popular beverage consumed all over the world with a production of 4.52 million tons in 2010, including black tea (78%) in Western countries, green tea (20%) in Asian countries, and oolong tea (2%) in southern China (15). Tea is an important source of dietary flavonoids, especially flavanols, and it has been associated with protecting against bone loss and reducing risk of fractures. In this article, we briefly summarize the state of knowledge regarding the effects of tea and its bioactive components on bone health and cover the findings from in vitro, animal, and human studies along with discussion of relevant future directions in translational research.
IN VITRO STUDIES
Theaflavins are the major polyphenols in black tea and are composed principally of theaflavin, theaflavin-3-gallate, theaflavin-3′-gallate, and theaflavin-3,3′-gallate (16). Oka et al (17) recently showed that theaflavin-3,3-gallate inhibits the formation and differentiation of osteoclasts via suppression of matrix metalloproteinases. Kim and Joo (18) reported that theaflavin inhibits LPS-induced IL-6, monocyte chemoattractant protein-1, and intracellular adhesion molecule-1 expression in bone marrow–derived macrophages through the blockade of NF-κB and mitogen-activated protein (MAP) kinase signaling pathways. These findings support an antiinflammatory role of black tea on bone health.
Compared with black tea, there are many more studies showing a positive impact of green tea on bone health. Green tea polyphenols (GTPs), including epigallocatechin-3-gallate (EGCG; the most abundant polyphenol in green tea), epigallocatechin, epicatechin-3-gallate (ECG), epicatechin, and catechins (19). In vitro studies have shown the antiresorptive properties of green tea's bioactive components through increasing apoptosis of osteoclasts, suppression of osteoclast formation, and inhibition of bone resorption. For instance, EGCG enhances osteoclastic cell death through the Fenton reaction (20) and caspase-3 activation (21). EGCG suppresses osteoclast formation by inhibiting the expression of matrix metalloproteinase-9 in osteoblasts (17, 22). EGCG inhibits bone resorption via inhibition of IL-6 production (23) or suppression of p44/p42 MAP kinase activation (24). EGCG suppresses osteoclastic differentiation via downregulation of the receptor activator of NF-κB ligand (RANKL)-induced expression of the nuclear transcription factor of activated T cells c1, thus blocking differentiation of monocytes into osteoclasts, followed by decreased bone resorption (25, 26). EGCG has also been shown to block RANKL signaling by suppressing RANKL-induced NF-κB transcription activity, a pathway essential to osteoclastogenesis (27).
Green tea's bioactive components have also been shown to benefit osteoblastogenesis by increasing osteoblastic survival, proliferation, differentiation, and mineralization. Observations from in vitro studies support such stimulatory effects of EGCG on osteoblastogenesis. For example, EGCG improves the survival of osteoblasts via suppression of TNF-α and IL-6 production (28). EGCG stimulates osteoblastogenesis by enhancing the mRNA expression of Runt-related transcription factor-2–mediated osteogenic genes and protein expression of alkaline phosphatase activity (a biomarker of osoteoblastic differentiation) and eventually stimulating mineralization (29, 30). EGCG also increases osteoblastic activity through Wnt signaling pathways, which modulate bone development and bone mass acquisition at all skeletal sites (31). EGCG upregulates prostaglandin F2α–stimulated vascular endothelial growth factor synthesis resulting from amplified activation of stress-activated protein kinase/c-Jun N-terminal kinase in osteoblasts (23, 32). Furthermore, EGCG-induced suppression of p44/p42 MAP kinase activation plays a protective role against bone resorption by downregulating the synthesis of IL-6, a potent bone resorptive agent in osteoblasts (24, 33).
Evidence from these in vitro studies suggests that tea polyphenols may benefit bone health by enhancing osteoblastogenesis and suppressing osteoclastogenesis through modulation of growth factors, cytokines, and chemokines and their receptors.
ANIMAL STUDIES
The effects of theaflavins and EGCG on in vitro osteogenesis have been confirmed in animal studies that used various bone-loss models. For example, Das et al (34–36) found that black tea extract has protective and restorative effects against ovariectomy-induced bone loss in rats by decreasing active osteoclastic number and bone turnover rate and increasing bone ash mineral content and strength. Karmakar et al (37) recently reported the potential role of black tea extract as a protective agent against nonalcoholic steatohepatitis-induced skeletal changes in bone metabolism in rats.
In a rodent model, Shen et al (38, 39) used a mixture of GTPs consisting of 480 mg EGCG, 160 mg ECG, 60 mg epicatechin, 103 mg epigallocatechin, and 30 mg catechin in 1000 mg GTPs confirmed by HPLC–electrochemical detection and HPLC-UV analyses. GTP supplementation (0.1% and 0.5%, weight/volume) resulted in increased urinary epigallocatechin and epicatechin concentrations in the sham and ovariectomized female rats in a dose-dependent manner. The treatment also mitigated bone loss (based on BMD measurement) and improved the integrity of bone microstructure and quality. The doses of 0.1% and 0.5% GTPs (equivalent to ∼1 and ∼4 cups green tea for human consumption/d, respectively) appear to be safe because no untoward changes were found in serum albumin, total protein, calcium, phosphate, glucose, blood urea nitrogen, creatinine, total bilirubin, alkaline phosphatase activity, creatinine phosphokinase, aspartate transferase, alanine transferase, globulin, amylase, and cholesterol.
Similar findings of a protective effect by GTPs on bone health have been observed in various bone-loss models, such as testosterone-deficiency–induced bone loss and microstructural deterioration (40), chronic inflammation–induced bone loss and microstructural deterioration (41, 42), and obesity-induced deterioration of bone microstructure (43). In their animal models, Shen et al (38–43) evaluated the bioavailability of GTPs in biofluids, bone efficacy, and potential mechanistic profiles and concluded that GTP supplementation improves BMD and strength of the femur; increases trabecular volume, thickness, and number and bone formation of proximal tibia; and suppresses trabecular separation and bone erosion at both the proximal tibia and the tibia shaft, resulting in a larger net bone volume. The osteoprotective role of GTPs appears to be mediated in part via increases in antioxidant capacity (glutathione peroxidase activity), decreases in inflammation (TNF-α, cyclooxygenase-2, IL-6), and/or reduced oxidative stress DNA damage [8-hydroxy-2′deoxyguanosine (8-OHdG)].
Green tea extracts may act not only via antioxidant mechanisms but also serve as a prooxidant at high doses. Iwaniec et al (44) showed in growing male mice that green tea extracts at 1% and 2% in diets for 6 wk, which is equivalent to ∼7–14 servings/d for humans, is detrimental to bone growth during early bone development, as shown by a suppressed rate of bone accumulation resulting in shorter bone length, reduced total and cortical bone volume and thickness, and decreased bone mineral content of femur (44). It is worth contrasting these results to those by Shen et al (38–43), who used lower doses in adult animals, and worth considering the impact of interventions during different stages of bone metabolism, ie, modeling during growth compared with remodeling during aging.
Evidence from these animal studies suggests that moderate intake of tea polyphenols may mitigate bone loss and microstructural deterioration and improve bone strength and quality. This action may be partly mediated via stimulating anabolic local factors and systemic hormones, suppressing catabolic bone local factors and systemic hormones, decreasing bone resorption rates, and improving bone strength and mechanical quality. These results of animal studies suggest a potential prophylactic role of tea polyphenols in human bone health.
HUMAN STUDIES
In clinical application, dual-energy X-ray absorptiometry is generally used to measure BMD at the spine, hip, and femoral neck to predict fracture risk and to monitor the natural progression of diseases that affect BMD or to monitor the therapeutic response to osteoporosis-specific treatments 1–2 y after the inception of the therapies (45).
In addition to BMD, serum and urinary bone biochemical markers of bone formation [eg, bone-specific alkaline phosphatase (BAP), osteocalcin, and N-terminal propeptide of type I collagen] and bone resorption [eg, tartrate-resistant acid phosphatase (TRAP), type I collagen cross-linked N- and C-telopeptides, deoxypridinoline, and pyridinoline] can provide additional information and an indication of the effectiveness of certain therapies including antiresorptive agents (46, 47) and dietary supplements (48, 49) for mitigating BMD deterioration.
Biochemical markers of bone turnover have been shown to be promising in predicting fractures in the elderly up to 2 y before the event (50, 51). A higher bone resorption rate was strongly associated with faster BMD loss (50, 51). Decreased vertebral BMD and increased bone turnover have approximately equal power in predicting the risk of bone loss and the osteoporosis-related fracture rate in postmenopausal women (50, 51). Therefore, depending on the duration of treatment, both BMD and bone turnover biomarkers have been used clinically to monitor the effects of treatments on bone health in humans.
Whereas a body of evidence from animal models suggests a positive relation between tea intake and maintaining BMD, human studies of tea drinking and risk of osteoporosis and fracture have yielded inconsistent results. For instance, Hegarty et al (52) reported that the BMDs of postmenopausal women at the lumbar spine, trochanter, and Ward's triangle were higher in tea drinkers than in non–tea drinkers after adjustment for BMI and age. Similarly, a positive association between tea drinking and BMD was also reported among postmenopausal women in the United States (53), Canada (54), the United Kingdom (52), Australia (55), Denmark (56), and Japan (57) and among older Asian men and women (58). In a case-control study, Keramat et al (59) reported that regular consumption of ≥7 cups tea/d was found to be a significant protective factor for osteoporosis. On the other hand, Hamdi Kara et al (60) reported that habitual tea drinking was not found to be a significant factor for BMD in postmenopausal Turkish women (60). Furthermore, an inverse relation between tea consumption and BMD was found in a study in pre- and perimenopausal women (aged 50–60 y) in the United States (61).
Results from the MEDOS (Mediterranean Osteoporosis) Study showed that tea drinking was associated with a 30% reduction in the risk of hip fractures in both women (62) and men (63) >50 y of age. With the use of data from a longitudinal follow-up study, Chen et al (53) found that the current amount of tea consumption in the United States resulted in such a weak effect on BMD that it would be unlikely to have a significant impact on fracture risk among American postmenopausal women.
The discrepancy among these results may be due to different study designs, different populations, different tea categories (black, oolong, green, etc), incomplete adjustment of the confounding effects of lifestyle characteristics, and different skeletal sites for measuring BMD. Therefore, to evaluate tea polyphenols on bone health in humans, a translational approach in humans should consider the following: 1) a population at high risk of osteoporosis, 2) objective and quantitative assessments of characterizing tea consumption, 3) quantitative methods to assess tea bioavailability and bone treatment efficacy, 4) validated biomarkers of oxidative stress and inflammation to explore potential mechanisms of action, and 5) blood and/or urine analyses to monitor the safety of the intervention.
We translated the osteoprotective results of GTPs in ovariectomized rats to a short-term clinical trial to evaluate the effects of GTPs on bone turnover biomarkers in postmenopausal women with osteopenia (64). We assessed GTP bioavailability (65), safety (66), and molecular mechanisms (65) and participants’ quality of life (66) and muscle strength (64). In addition to GTP supplementation, the effects of tai chi (a mind-body exercise) as another treatment on the same outcome variables were also evaluated (64–67). In our study, 171 qualified postmenopausal osteopenic women [mean ± SD age: 57.5 ± 6.9 y; BMI (in kg/m2): 28.3 ± 5.4] were randomly assigned to 1 of 4 groups: 1) placebo (500 mg medicinal starch/d; n = 44); 2) GTP (500 mg GTP/d; n = 47); 3) placebo + tai chi training at 60 min/session, 3 sessions/wk (n = 42); and 4) GTP + tai chi training (n = 38) (64). On the basis of our laboratory analysis, the GTPs were 99.25% pure and contained 46.5% EGCG, 21.25% ECG, 10% epicatechin, 7.5% epigallocatechin, 9.5% gallocatechin-3-gallate, and 4.5% catechin. During the 6-mo intervention period, all participants were supplemented with 500 mg elemental calcium and 200 IU vitamin D (as cholecalciferol) daily. Bone treatment efficacy was assessed by measuring serum BAP and TRAP at baseline and at 1, 3, and 6 mo. GTP bioavailability was assessed by measuring serum and urine GTP components at baseline and at 1, 3, and 6 mo (65). Safety was monitored monthly by assessing serum liver enzymes (aspartate aminotransferase and alanine aminotransferase), kidney function (urea nitrogen and creatinine), alkaline phosphatase, total bilirubin, calcium, and inorganic phosphorus (66). Potential molecular mechanisms were assessed by measuring urinary 8-OHdG at baseline and at 1, 3, and 6 mo (65). Quality of life, assessed by using the Short Form-36 questionnaire (version 2) (66), and muscle strength, measured by using the wall-sit test (64), were evaluated at baseline and at 3 and 6 mo.
After 6 mo of intervention, 150 subjects completed the study with a 12% attrition rate. The compliance rates for placebo and GTP supplementation were 89% and 89%, respectively (64). The attendance rate for tai chi classes was 83%. Throughout the study period, neither GTP supplementation nor tai chi affected liver, kidney function, or any other blood variables (66). In addition, no adverse events as a result of the study treatment were reported by the participants (66). With regard to GTP bioavailability and compliance, we found the following: 1) no GTP components in serum or urine in any group at baseline, 2) no detectable GTPs in the placebo and placebo + tai chi groups at any collection time, and 3) higher serum EGCG and ECG as well as higher urinary epicatechin and ECG concentrations after 1, 3, and 6 mo in the GTP intervention groups (65).
With regard to bone treatment efficacy, we observed the following: 1) elevation in BAP due to GTP intake (at 1 mo) and tai chi exercise (at 3 mo) and 2) increases in the BAP:TRAP ratio due to GTP intake (at 3 mo) and tai chi (at 6 mo) (64). Compared with the placebo group, lower urinary 8-OHdG concentrations were found in the other 3 treatment groups at 3 and 6 mo in a time-dependent manner (65). The tai chi interventions, but not GTP intake alone, improved quality-of-life measures such as role-emotional and mental health (66). Relative to the placebo group, the 3 treatment groups showed improved muscle strength at 6 mo (64). These results suggest that GTP supplementation at 500 mg daily (equivalent to ∼4–6 cups/d) and tai chi exercise for 6 mo are safe for postmenopausal women with osteopenia. GTP supplementation and tai chi exercise benefit bone remodeling starting from an early stage of intervention in association with reduced oxidative stress and improved muscle strength.
CONCLUSIONS AND FUTURE DIRECTIONS
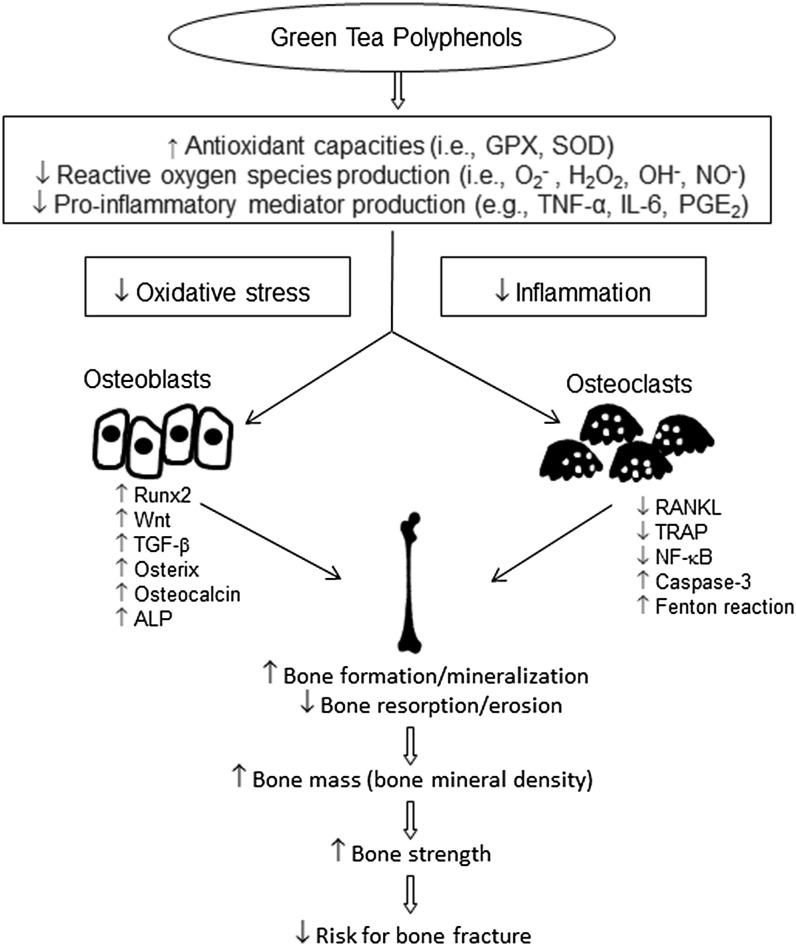
Osteoporosis results from a metabolic imbalance related to faster bone resorption than bone formation. The suppression of osteoclastogenesis and/or the enhancement of osteoblastogenesis may restore the balance and mitigate bone loss in the development of osteoporosis. The evidence from in vitro and animal studies of various bone-loss models strongly suggests that tea polyphenols, especially GTPs, are effective in protecting against osteoporosis. The possible mechanisms of action for GTPs in bone protection are shown in Figure 1. The most widely recognized characteristics of GTPs in bone protection are their antioxidant activities, stemming from their ability to scavenge ROS. In our previous studies, we showed that GTP supplementation suppressed the production of proinflammatory cytokines, inhibited oxidative stress damage, and enhanced antioxidant capacity in various bone-loss models (38–43). Tea and its polyphenol components act by increasing bone mass, trabecular bone volume, number, and thickness and reducing trabecular separation, as well as by suppressing bone resorption and enhancing bone formation, resulting in greater bone strength. However, there are some differences between animals and humans in terms of bone fracture. For example, the animal studies focused principally on long bones, instead of the spine and hip, the common sites of serious and frequent bone fracture in humans. In addition, animal studies are not able to evaluate antifracture capacity, an important indicator for osteoporosis management.
FIGURE 1.
Possible mechanisms of action for green tea polyphenols in bone protection. Green tea polyphenols increase antioxidant capacities and concomitantly suppress oxidative stress and inflammation. Consequently, Runx2, Wnt, TGF-β, osterix, osteocalcin, and ALP are upregulated in osteoblasts. RANKL, TRAP, and NF-κB activities are downregulated and caspase-3 and Fenton reaction activities are upregulated in osteoclasts. These actions may enhance bone formation and mineralization and reduce bone resorption and erosion, resulting in increased bone mass and bone mineral density, increased bone strength, and reduced risk of bone fracture. ALP, alkaline phosphatase; GPX, glutathione peroxidase; NF-κB, nuclear transcription factor κB; PGE2, prostaglandin E2; RANKL, receptor activator of nuclear transcription factor κB ligand; Runx2, Runt-related transcription factor 2; SOD, superoxide dismutase; TGF-β, transforming growth factor β TRAP, tartrate-resistant acid phosphatase; ↑, upregulated; ↓, downregulated.
The evidence supporting BMD improvement and the antifracture effect of tea from longitudinal studies is quite limited. Our 6-mo clinical trial showing that GTP favors bone formation at an early-intervention stage, improves muscle strength, and reduces oxidative stress in postmenopausal osteopenic women represents a first step toward translating the findings from animal studies to human applications (64–67). However, future research is necessary to better define the optimal dose of tea intake or GTP supplementation and to examine the potential interactions between tea polyphenols and other nutrients essential to bone health, including calcium and vitamin D; and it should be of sufficient duration to assess changes in BMD as well as biomarkers of bone turnover.
To better understand the effects of tea on bone health in people at high risk of osteoporosis and fracture, more basic research and human studies are warranted. For example, an animal study conducted to evaluate chronic toxicity, safety, and bone efficacy in ovariectomized rats supplemented with different dosages of tea polyphenols administered in drinking water will be essential to inform the design of new clinical trials. Similar short-term tea polyphenol dose-response studies could then be conducted in postmenopausal osteopenic women to determine the optimal dose on the basis of relevant bone-related outcomes (ie, bone turnover markers) along with a safety evaluation. Both of these approaches are necessary to prepare for a large-scale, long-term, placebo-controlled randomized trial with an optimal tea regimen. Such a study should be designed to 1) assess compliance and bioactivity with appropriate biomarkers, 2) monitor safety, and 3) quantify with sufficient power bone mass at different body sites and bone microarchitecture (ie, volumetric BMD, stress-strain index, and bone geometry) by using imaging technology. On the basis of the available preliminary data, it also appears worthwhile to determine other outcomes that are relevant to bone health, such as mobility, function, balance, and quality of life.
Acknowledgments
The authors’ responsibilities were as follows—C-LS: planned and drafted the article; and M-CC and J-SW: critically reviewed and revised the article. C-LS received an honorarium and travel support from the Tea Council of the USA for speaking at the Fifth International Scientific Symposium on Tea and Human Health and for preparing this article for publication. The authors declared no competing financial interests.
Footnotes
Abbreviations used: BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; ECG, epicatechin-3-gallate; EGCG, epigallocatechin-3-gallate; GTP, green tea polyphenol; MAP, mitogen-activated protein; NF-κB, nuclear transcription factor κB; RANKL, receptor activator of nuclear transcription factor κB ligand; ROS, reactive oxygen species; TRAP, tartrate-resistant acid phosphatase; 8-OHdG, 8-hydroxy-2′deoxyguanosine.
REFERENCES
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–95. [DOI] [PubMed] [Google Scholar]
- 2.Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab 2008;22:671–85. [DOI] [PubMed] [Google Scholar]
- 3.Boonen S, Dejaeger E, Vanderschueren D, Venken K, Bogaerts A, Verschueren S, Milisen K. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: a geriatric perspective. Best Pract Res Clin Endocrinol Metab 2008;22:765–85. [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465–75. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadieh H, Arabi A. Vitamins and bone health: beyond calcium and vitamin D. Nutr Rev 2011;69:584–98. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States. Adv Data 2004;343:1–19. [PubMed] [Google Scholar]
- 7.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys 2008;473:201–9. [DOI] [PubMed] [Google Scholar]
- 8.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med 2008;46:1550–5. [DOI] [PubMed] [Google Scholar]
- 9.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 2004;314:197–207. [DOI] [PubMed] [Google Scholar]
- 10.Manolagas SC. De-fense! De-fense! De-fense: scavenging H2O2 while making cholesterol. Endocrinology 2008;149:3264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ. A role for TGF-β in osteoclast differentiation and survival. J Cell Sci 2000;113:2445–53. [DOI] [PubMed] [Google Scholar]
- 12.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 1990;85:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syed FA, Ng AC. The pathophysiology of the aging skeleton. Curr Osteoporos Rep 2010;8:235–40. [DOI] [PubMed] [Google Scholar]
- 14.Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int 2010;87:226–35. [DOI] [PubMed] [Google Scholar]
- 15.Food and Agriculture Organization of the United NationsndashProduction FAOSTAT. Medium-term prospects for agricultural commodities. Available from: http://www.fao.org/docrep/006/y5143e/y5143e00.htm (cited 10 August 2010).
- 16.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr 1997;37:693–704. [DOI] [PubMed] [Google Scholar]
- 17.Oka Y, Iwai S, Amano H, Irie Y, Yatomi K, Ryu K, Yamada S, Inagaki K, Oguchi K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci 2012;118:55–64. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Joo YE. Theaflavin inhibits LPS-induced IL-6, MCP-1, and ICAM-1 expression in bone marrow-derived macrophages through the blockade of NF-κB and MAPK signaling pathways. Chonnam Med J 2011;47:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr 2000;130:2409–12. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, Lee IS, Nagai K. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun 2002;292:94–101. [DOI] [PubMed] [Google Scholar]
- 21.Hafeez BB, Ahmed S, Wang N, Gupta S, Zhang A, Haqqi TM. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol Appl Pharmacol 2006;216:11–9. [DOI] [PubMed] [Google Scholar]
- 22.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, Kim CK, Choi SH. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res 2004;39:300–7. [DOI] [PubMed] [Google Scholar]
- 23.Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Hosoi T, Harada A, Ohta T, Kozawa O. (-)-Epigallocatechin gallate suppresses endothelin-1-induced interleukin-6 synthesis in osteoblasts: inhibition of p44/p42 MAP kinase activation. FEBS Lett 2007;581:1311–6. [DOI] [PubMed] [Google Scholar]
- 24.Tokuda H, Takai S, Matsushima-Nishiwaki R, Akamatsu S, Hanai Y, Hosoi T, Harada A, Ohta T, Kozawa O. (–)-Epigallocatechin gallate enhances prostaglandin F2alpha-induced VEGF synthesis via upregulating SAPK/JNK activation in osteoblasts. J Cell Biochem 2007;100:1146–53. [DOI] [PubMed] [Google Scholar]
- 25.Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, Sakai Y, Kurosaka M, Kumagai S. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum 2008;58:2012–8. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Jin H, Shim HE, Kim HN, Ha H, Lee ZH. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol Pharmacol 2010;77:17–25. [DOI] [PubMed] [Google Scholar]
- 27.Lin RW, Chen CH, Wang YH, Ho ML, Hung SH, Chen IS, Wang GJ. (-)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem Biophys Res Commun 2009;379:1033–7. [DOI] [PubMed] [Google Scholar]
- 28.Takai S, Matsushima-Nishiwaki R, Adachi S, Natsume H, Minamitani C, Mizutani J, Otsuka T, Tokuda H, Kozawa O. (-)-Epigallocatechin gallate reduces platelet-derived growth factor-BB-stimulated interleukin-6 synthesis in osteoblasts: suppression of SAPK/JNK. Mediators Inflamm 2008;2008:291808. [DOI] [PMC free article] [PubMed]
- 29.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int 2005;16:2039–45. [DOI] [PubMed] [Google Scholar]
- 30.Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem 2007;18:341–7. [DOI] [PubMed] [Google Scholar]
- 31.Rawadi G. Wnt signaling and potential applications in bone diseases. Curr Drug Targets 2008;9:581–90. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi K, Takai S, Matsushima-Nishiwaki R, Hanai Y, Kato K, Tokuda H, Kozawa O. (-)-Epigallocatechin gallate reduces transforming growth factor beta-stimulated HSP27 induction through the suppression of stress-activated protein kinase/c-Jun N-terminal kinase in osteoblasts. Life Sci 2008;82:1012–7. [DOI] [PubMed] [Google Scholar]
- 33.Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Yamauchi J, Harada A, Hosoi T, Ohta T, Kozawa O. (-)-Epigallocatechin gallate inhibits basic fibroblast growth factor-stimulated interleukin-6 synthesis in osteoblasts. Horm Metab Res 2008;40:674–8. [DOI] [PubMed] [Google Scholar]
- 34.Das AS, Mukherjee M, Mitra C. Evidence for a prospective anti-osteoporosis effect of black tea (Camellia Sinensis) extract in bilaterally ovariectomized rat model. Asia Pac J Clin Nutr 2004;13:210–6. [PubMed] [Google Scholar]
- 35.Das AS, Das D, Mukherjee M, Mukherjee S, Mitra C. Phytoestrogenic effects of black tea extract (Camellia sinensis) in an oophorectomized rat (Rattus norvegicus) model of osteoporosis. Life Sci 2005;77:3049–57. [DOI] [PubMed] [Google Scholar]
- 36.Das AS, Mukherjee M, Das D, Mitra C. Protective action of aqueous black tea (Camellia sinensis) extract (BTE) against ovariectomy-induced oxidative stress of mononuclear cells and its associated progression of bone loss. Phytother Res 2009;23:1287–94. [DOI] [PubMed] [Google Scholar]
- 37.Karmakar S, Majumdar S, Maiti A, Choudhury M, Ghosh A, Das AS, Mitra C. Protective role of black tea extract against nonalcoholic steatohepatitis-induced skeletal dysfunction. J Osteoporos 2011;2011:426863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen CL, Wang P, Guerrieri J, Yeh J, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int 2008;19:979–90. [DOI] [PubMed] [Google Scholar]
- 39.Shen CL, Yeh JK, Stoecker BJ, Chyu MC, Wang JS. Green tea polyphenols mitigate deterioration of bone microarchitecture in middle-aged female rats. Bone 2009;44:684–90. [DOI] [PubMed] [Google Scholar]
- 40.Shen CL, Cao JJ, Dagda RY, Tenner TE, Jr, Chyu MC, Yeh JK. Supplementation with green tea polyphenols improves bone microstructure and quality in aged, orchidectomized rats. Calcif Tissue Int 2011;88:455–63. [DOI] [PubMed] [Google Scholar]
- 41.Shen CL, Yeh JK, Samathanam C, Cao JJ, Stoecker BJ, Dagda RY, Chyu MC, Dunn DM, Wang JS. Green tea polyphenols attenuate deterioration of bone microarchitecture in female rats with systemic chronic inflammation. Osteoporos Int 2011;22:327–37. [DOI] [PubMed] [Google Scholar]
- 42.Shen CL, Yeh JK, Cao JJ, Tatum OL, Dagda RY, Wang JS. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J Nutr Biochem 2010;21:968–74. [DOI] [PubMed] [Google Scholar]
- 43.Shen CL, Cao JJ, Dagda RY, Chanjaplammootil S, Lu C, Chyu MC, Gao W, Wang JS, Yeh JK. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr Res 2012;32:448–57. [DOI] [PubMed] [Google Scholar]
- 44.Iwaniec UT, Turner RT, Koo SI, Kaur R, Ho E, Wong CP, Bruno RS. Consumption of green tea extract results in osteopenia in growing male mice. J Nutr 2009;139:1914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melton LJ, 3rd, Crowson CS, O'Fallon WM, Wahner HW, Riggis BL. Relative contributions of bone density, bone turnover, and clinical risk factors to long-tern fracture prediction. J Bone Miner Res 2003;18:312–8. [DOI] [PubMed] [Google Scholar]
- 46.Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 2002;87:1586–92. [DOI] [PubMed] [Google Scholar]
- 47.Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, Belleli R, Wright TM, John MR. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone 2009;45:1053–8. [DOI] [PubMed] [Google Scholar]
- 48.Grimnes G, Joakimsen R, Figenschau Y, Torjesen PA, Almås B, Jorde R. The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass—a randomized controlled 1-year trial. Osteoporos Int 2012;23:201–11. [DOI] [PubMed] [Google Scholar]
- 49.Hooshmand S, Chai SC, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br J Nutr 2011;106:923–30. [DOI] [PubMed] [Google Scholar]
- 50.Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, Rizzoli R, Lipschitz S, Dimai HP, Witvrouw R, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 2012;367:1714–23. [DOI] [PubMed] [Google Scholar]
- 51.Löfman O, Magnusson P, Toss G, Larsson L. Common biochemical markers of bone turnover predict future bone loss: a 5-year follow-up study. Clin Chim Acta 2005;356:67–75. [DOI] [PubMed] [Google Scholar]
- 52.Hegarty VM, May HM, Khaw KT. Tea drinking and bone mineral density in older women. Am J Clin Nutr 2000;71:1003–7. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z, Pettinger MB, Ritenbaugh C, LaCroix AZ, Robbins J, Caan BJ, Barad DH, Hakim IA. Habitual tea consumption and risk of osteoporosis: a prospective study in the Women's Health Initiative observational cohort. Am J Epidemiol 2003;158:772–81. [DOI] [PubMed] [Google Scholar]
- 54.Hoover PA, Webber CE, Beaumont LF, Blake JM. Postmenopausal bone mineral density: relationship to calcium intake, calcium absorption, residual estrogen, body composition, and physical activity. Can J Physiol Pharmacol 1996;74:911–7. [PubMed] [Google Scholar]
- 55.Devine A, Hodgson JM, Dick IM, Prince RL. Tea drinking is associated with benefits on bone density in older women. Am J Clin Nutr 2007;86:1243–7. [DOI] [PubMed] [Google Scholar]
- 56.Vestergaard P, Hermann AP, Gram J, Jensen LB, Eiken P, Abrahamsen B, Brot C, Kolthoff N, Sørensen OH, Beck Nielsen H, et al. Evaluation of methods for prediction of bone mineral density by clinical and biochemical variables in perimenopausal women. Maturitas 2001;40:211–20. [DOI] [PubMed] [Google Scholar]
- 57.Muraki S, Yamamoto S, Ishibashi H, Oka H, Yoshimura N, Kawaguchi H, Nakamura K. Diet and lifestyle associated with increased bone mineral density: cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J Orthop Sci 2007;12:317–20. [DOI] [PubMed] [Google Scholar]
- 58.Wu CH, Yang YC, Yao WJ, Lu FH, Wu JS, Chang CJ. Epidemiological evidence of increased bone mineral density in habitual tea drinkers. Arch Intern Med 2002;162:1001–6. [DOI] [PubMed] [Google Scholar]
- 59.Keramat A, Patwardhan B, Larijani B, Chopra A, Mithal A, Chakravarty D, Adibi H, Khosravi A. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord 2008;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamdi Kara I, Aydin S, Gemalmaz A, Aktürk Z, Yaman H, Bozdemir N, Kurdak H, Sitmapinar K, Devran Sencar I, Başak O, et al. Habitual tea drinking and bone mineral density in postmenopausal Turkish women: Investigation of Prevalence of Postmenopausal Osteoporosis in Turkey (IPPOT Study). Int J Vitam Nutr Res 2007;77(6):389–97 [Published erratum appears in Int J Vitam Nutr Res 2008;78(3):166.] [DOI] [PubMed] [Google Scholar]
- 61.Hernández-Avila M, Stampfer MJ, Ravnikar VA, Willett WC, Schiff I, Francis M, Longcope C, McKinlay SM, Longcope C. Caffeine and other predictors of bone density among pre- and perimenopausal women. Epidemiology 1993;4:128–34. [DOI] [PubMed] [Google Scholar]
- 62.Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker J, Dilsen G, Gennari C, Lopes Vaz A, Lyritis G, et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res 1995;10:1802–15. [DOI] [PubMed] [Google Scholar]
- 63.Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, Dequeker J, Dilsen G, Gennari C, Vaz AL, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS Study. Mediterranean Osteoporosis Study. Osteoporos Int 1999;9:45–54. [DOI] [PubMed] [Google Scholar]
- 64.Shen CL, Chyu MC, Yeh JK, Zhang Y, Pence BC, Felton CK, Brismée JM, Arjmandi BH, Doctolero S, Wang JS. Effect of green tea and tai chi on bone health in postmenopausal osteopenic women: a 6-month randomized placebo-controlled trial. Osteoporos Int 2012;23:1541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian G, Xue K, Tang L, Wang F, Song X, Chyu MC, Pence BC, Shen CL, Wang JS. Mitigation of oxidative damage by green tea polyphenols and tai chi exercise in postmenopausal women with osteopenia. PLoS ONE 2012;7:e48090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen CL, Chyu MC, Pence BC, Yeh JK, Zhang Y, Felton CK, Doctolero S, Wang JS. Green tea polyphenols supplementation and tai chi exercise for postmenopausal osteopenic women: safety and quality of life report. BMC Complement Altern Med 2010;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen CL, Chyu MC, Yeh JK, Felton CK, Xu KT, Pence BC, Wang JS. Green tea polyphenols and tai chi for bone health: designing a placebo-controlled randomized trial. BMC Musculoskelet Disord 2009;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]