Abstract
Repetition has long been known to facilitate memory performance, but its effects on event-related potentials (ERPs), measured as an index of recognition memory, are less well characterized. In Experiment 1, effects of both massed and distributed repetition on old–new ERPs were assessed during an immediate recognition test that followed incidental encoding of natural scenes that also varied in emotionality. Distributed repetition at encoding enhanced both memory performance and the amplitude of an old–new ERP difference over centro-parietal sensors. To assess whether these repetition effects reflect encoding or retrieval differences, the recognition task was replaced with passive viewing of old and new pictures in Experiment 2. In the absence of an explicit recognition task, ERPs were completely unaffected by repetition at encoding, and only emotional pictures prompted a modestly enhanced old–new difference. Taken together, the data suggest that repetition facilitates retrieval processes and that, in the absence of an explicit recognition task, differences in old–new ERPs are only apparent for affective cues.
Keywords: distributed repetition, recognition memory, emotion, ERPs
INTRODUCTION
Repetition effects on memory were first described by Ebbinghaus (1885) who noted that memory performance was facilitated when item repetitions were distributed over time (Melton, 1970). This robust performance enhancement has been consistently replicated using a wide range of materials and measures (Glenberg, 1979; Reder and Anderson, 1982; Dempster, 1987; Mäntylä and Cornoldi, 2002; Cepeda et al., 2006; Groh-Bordin et al., 2007; Xue et al., 2011). However, despite a large behavioral literature, effects of repetition on old–new differences measured using brain potentials have not been well characterized. Moreover, whether massed and distributed repetition, which impact memory performance differently, also differentially modulate brain potentials during recognition remains not only an important question but also could assist in addressing alternative theoretical interpretation of the performance enhancement following distributed repetition. In this study, we measured ERPs when people recognized old and new pictures that had been presented using massed or distributed repetition (or presented once) during encoding.
When effects of repetition on ERPs have been assessed at encoding for words (e.g. Besson et al., 1992; Besson and Kutas, 1993; Groh-Bordin et al., 2007), an enhanced late positive wave for repeated words is reported, which is sometimes interpreted as indicating successful retrieval of the item’s first presentation (Van Petten and Senkfor, 1996). On the other hand, in previous studies, we measured effects of repetition on ERPs during encoding of pictures of natural scenes (Codispoti et al., 2006a, 2007; Ferrari et al., 2010, 2011) and found that massed, compared with distributed, repetition greatly attenuated the amplitude of a centro-parietal late positive potential that is typically heightened when viewing emotional, compared with neutral pictures (Codispoti et al., 2006b; Ferrari et al., 2011). To determine whether these differences have implications for later memory performance, in this study we measured ERPs during an immediate recognition test following massed or distributed repetition of emotional and neutral pictures. Pictures were presented either once or four times during encoding, with the four repetitions occurring contiguously (i.e. massed) or distributed across the encoding phase. Incidental encoding, in which there is no expectation of a later memory test, was utilized so that differences mediated by differential input strategies, such as rehearsal, elaboration, etc., are attenuated.
Recognizing old, compared with new, items on a later recognition test is typically associated with an enhanced centro-parietal positivity for both words and pictures (400–700 ms; Wilding et al., 1995; Wilding and Rugg, 1996; Voss and Paller, 2008; Weymar et al., 2009; Curran and Doyle, 2011). In one of the only studies specifically investigating the effects of distributed repetition on later memory using ERPs, Finnigan et al. (2002) reported heightened late parietal positivity for repeated words, suggesting that distributed repetition might prompt an enhanced old–new difference in this study.
Although emotional pictures are better recalled than neutral pictures on an immediate free recall test (e.g. Bradley et al., 1992; Bradley, 1994; Dolcos et al. 2004), recognition of pictures is quite good and, in a previous study, we found no difference in memory performance or on the amplitude of old–new ERP differences for emotional, compared with neutral, pictures on an immediate recognition test (Versace et al., 2010). If distributed repetition generally facilitates memory, one hypothesis is that repetition at encoding will have similar beneficial effects on performance and ERPs for emotional and neutral pictures in Experiment 1.
Multiple mechanism(s) have been proposed to explain the facilitation in memory performance following distributed repetition (see Cepeda et al., 2006). For instance, multiple trace theory (Melton, 1970; Glenberg, 1979) hypothesizes that distributed, but not massed, repetition results in multiple encoding traces, which subsequently benefit retrieval performance. In attention theory (Hintzman, 1974), one variant of a deficient processing theory, suggests that when the time between repetitions is brief, as for massed repetition, processing of the second presentation is reduced. Recursive reminder theory (Hintzman, 2010) suggests that distributed repetition acts as instances of memory retrieval, strengthening the memory trace.
Testing between theories is often difficult because they make similar behavioral predictions. Event-related potentials provide a novel methodology for assessing alternative interpretations, as ERPs following distributed or massed repetition can be assessed not only during explicit memory tasks but also, and importantly, without requiring an explicit memory decision. If repetition primarily affects encoding or storage processes, for instance, the same pattern of ERP modulation is expected, regardless of whether explicit retrieval is required. Thus, in Experiment 1, we measured event-related potentials during immediate recognition of pictures of natural scenes that were presented once or repeated during encoding; in Experiment 2, we simply replaced the explicit recognition task with passive viewing of old and new pictures.
EXPERIMENT 1
Method
Participants
Participants were 28 right-handed students (16 women) from a General Psychology course at the University of Florida who participated for course credit. They had normal or corrected-to-normal visual acuity. Prior to participating, all subjects gave their informed consent in accordance with the UF Institutional Review Board guidelines. Of these, one subject (male) was excluded from behavioral analyses as he only responded to old trials, and two subjects (males) were excluded from the EEG analysis due to technical problems.
Materials and design
Overall, 216 color picture stimuli were selected from the International Affective Picture System (IAPS; Lang et al. 2008) and other sources, consisting of 108 emotional (half pleasant and half unpleasant of different contents) and 108 neutral pictures (faces, people in context and objects). Pictures were selected such that two sets of 108 stimuli included the same number of picture exemplars from each of 14 categories.1 Pleasant categories included erotic couples, romance, families–babies, puppies and sports; unpleasant categories included mutilations, human threat, animal threat, disgust and accident and neutral categories included people, urban and natural landscapes and objects. One set of pictures was presented in the encoding phase and then as the old pictures in the recognition phase, intermixed with the remaining 108 pictures that were not presented in the encoding phase (new pictures). The specific set of pictures serving as old or new was counterbalanced across participants.
In the encoding phase, 36 pictures were presented once during encoding (single) and the remaining 72 pictures were repeated four times each. Half of the repetitions were massed and half were distributed across the encoding phase. The 36 pictures selected for each condition (single, massed, distributed) were balanced for emotional content (18 emotional and 18 neutral). The presentation order of the massed repetitions was counterbalanced such that no more than three series of massed repetitions could occur consecutively. Distributed repetitions were intermixed with single pictures with a repetition of the same picture occurring approximately every 90 ± 40 trials.
Five buffer trials were added to the beginning and end of the encoding phase to avoid serial position effects and were not included in the recognition test. In addition, after each set of massed repetitions, a buffer picture (neutral content) was presented that was not included in the recognition phase or in final analyses, as a previous study indicated that these pictures attract heightened attention (see Ferrari, et al. 2010). The entire encoding phase included 370 trials.
Following a 15 min retention interval in which participants were engaged in a distractor task (visual oddball using geometric shapes; Sawaki and Katayama, 2007), the recognition test began. The 108 pictures presented during encoding, together with 108 new pictures, were presented for a total of 216 trials. Of the 108 ‘old’ trials, 36 (half emotional and half neutral) had been presented once during encoding, 36 had been presented four times in massed repetition and 36 had been presented four times using distributed repetition. The order of picture presentation was balanced such that each condition was presented in every block of 12 trials, with the constraint that no more than three old or new pictures and no more than four pictures of the same affective category were presented consecutively. Six stimulus sets were constructed that varied the specific picture presented in each of the condition (single, distributed, massed or new) across participants.
Each trial in the encoding and recognition phase consisted of a fixation cross presented at the center of the screen for 500 ms before picture onset, followed by a 2 s picture presentation. During encoding, picture offset was followed by a 2 s inter-trial interval (ITI). During the recognition test, picture offset was followed by the question ‘Have you seen this picture before?’ that appeared for 2 s and was followed by a 2 s ITI. When the question appeared, the participants pressed a button indicating ‘Yes’ or ‘No’.
All pictures were presented on a 19 inch CRT monitor situated approximately 100 cm from the participant. E-Prime software was used to present the pictorial stimuli and to record the behavioral response in the recognition phase.
EEG recording
EEG was measured from the scalp using a 128-channel system (Electrical Geodesics, Inc., Eugene, OR, USA) running NetStation software on a Macintosh computer. Scalp impedance for each sensor was kept below 50 kΩ. The EEG was recorded continuously with a sampling rate of 250 Hz, the vertex sensor as reference electrode and on-line bandpass filtered from 0.01 to 100 Hz. EEG data were analyzed offline using a MATLAB-based program (Junghöfer and Peyk, 2004). Continuous EEG data were low-pass filtered at 40 Hz using digital filtering, and artifact detection was performed by means of a dedicated algorithm that uses statistical parameters to determine trials with artifacts (Junghöfer et al., 2000). Processed data were then transformed to an average reference and baseline corrected (200 ms before picture onset) prior to subject averaging and analysis.
Data analysis
Behavioral responses during recognition were scored as correct if the appropriate response latency was not shorter or longer than the mean (within each subject and condition) ± 3 times the standard deviation of the mean (1.4% of trials were excluded). Because responses were delayed until the offset of the 2 s picture presentation to avoid motor potentials in the ERPs, reaction times were not informative. Memory performance (Table 1) was calculated on the basis of the number of hits for each participant and each condition. The discrimination index (Pr) as well as response bias (Br) were also determined using the number of hits and false alarms for emotional and neutral pictures.2
Table 1.
Recognition performance (% of accuracy and standard error) in the old–new recognition phase (Experiment 1) for emotional and neutral pictures as a function of repetition at encoding
| Emotional | Neutral | |
|---|---|---|
| Distributed repetition | 0.97 (0.09) | 0.96 (0.08) |
| Massed repetition | 0.94 (0.13) | 0.93 (0.13) |
| Single presentation | 0.90 (0.20) | 0.74 (0.28) |
| New | 0.93 (0.15) | 0.92 (0.10) |
ERPs were averaged separately for each channel and experimental condition. Trials with incorrect behavioral responses (misses or false alarms) were not used in the averaged data. Sensor clusters3 were selected based on first determining sensors that showed a difference >1µv between new and old pictures and then grouping these into centro-parietal and fronto-central regions. Statistical analyses were then conducted on the mean amplitude in a window 400–600 ms following picture onset in each region. An initial analysis also investigated laterality differences (left and right centro-parietal regions), but no significant differences were found and the final analyses were averaged across hemisphere.
Procedure
After arrival at the laboratory, participants signed an informed consent form. Participants were then seated in a recliner in a small, sound-attenuated, dimly lit room, and the EEG sensor net was attached. Participants were told that a series of pictures would be presented and that each picture should be viewed the entire time it was on the screen. No mention was made about the upcoming recognition test (incidental encoding). Between the encoding and the recognition test, participants performed a distractor task (visual oddball) that lasted about 12 min. In the recognition test, participants were instructed to press one button if the picture had been seen before and another if it had not. During both encoding and the recognition test, the participant was instructed to remain as still as possible and to maintain fixation on a cross at the center of the screen.
Results
Recognition accuracy
Table 1 lists recognition performance for old pictures that were presented during encoding either once, massed or distributed, and for new pictures. In the overall analysis, significant effects were obtained as a function of condition [4: single, massed, distributed, new; F(3,24) = 22, P < 0.0001, η2 = 0.73], content [2: emotional, neutral; F(1,26) = 34, η2 = 0.57] and their interaction [F(3,24) = 13, η2 = 0.62]. Simple main effects tests indicated a significant effect of condition for both emotional [F(3,24) = 8.7, P < 0.001, η2 = 0.52] and neutral pictures [F(3,24) = 20, P < 0.0001, η2 = 0.72]. In both cases, distributed repetition prompted a higher hit rate than single presentation [distributed: emotional F(1,26) = 17, P < 0.0001; neutral F(1,26) = 62, P < 0.0001]; as did massed repetition [massed: emotional F(1,26) = 9.5, P = 0.005; neutral F(1,26) = 52, P < 0.0001]. In addition, for emotional pictures, distributed repetition prompted better recognition than massed repetition [F(1,26) = 8.2, P < 0.01] with a trend in the same direction for neutral pictures [F(1,26) = 3.4, P = 0.08].
The significant interaction primarily reflects better recognition of emotional, compared with neutral, pictures only for items presented once during encoding [F(1,26) = 41, P < 0.0001, η2 = 0.61]. When pictures were repeated (either massed or distributed), emotional and neutral pictures were equally well recognized on the recognition test. Accuracy in recognizing new pictures did not differ as a function of emotional content.
Event-related potentials
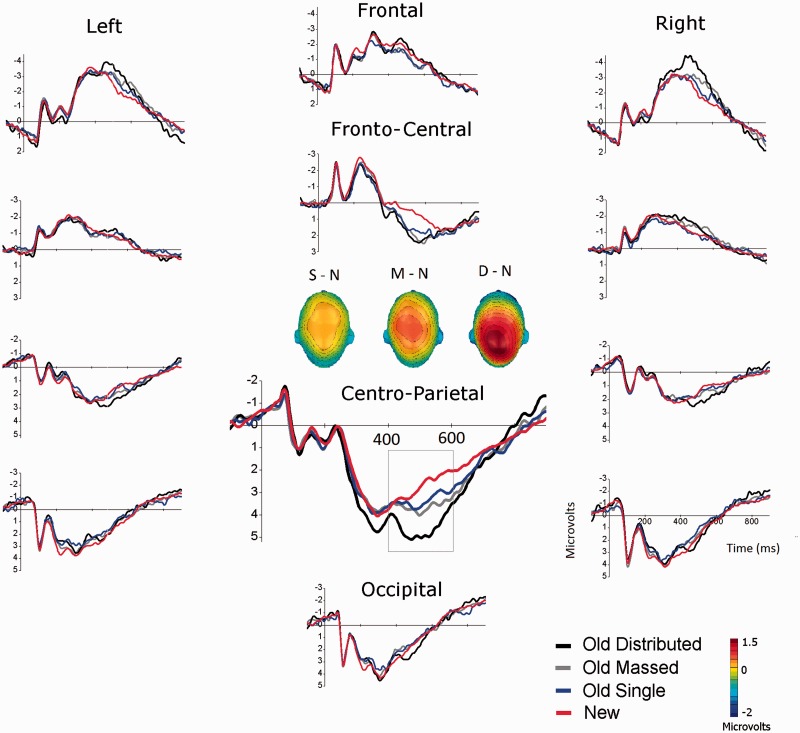
Figure 1 illustrates waveforms over representative subsets of sensors when viewing pictures that were presented once or repeated (massed, distributed) as well as new pictures during the recognition test, together with the resulting scalp topographies. Old–new ERP differences were apparent over two main scalp regions—a centro-parietal and a fronto-central region—that were examined in separate analyses. In each analysis, average ERPs in a 400–600 ms window following picture onset were analyzed using a MANOVA that included repetition condition (4: single old, massed, distributed and new) and picture content (2: emotional and neutral).
Fig. 1.
Experiment 1. Grand average ERP waveforms of representative scalp regions (sensor clusters) when viewing pictures that were presented once (single) or repeated (massed, distributed) during encoding and new pictures (new) during the old-new recognition test. (Inset) scalp topography (top view) of the difference in the 400–600 ms window between old pictures presented once (single: S – N); massed (M – N) or distributed (D – N) during encoding and new (N) pictures.
Centro-parietal region
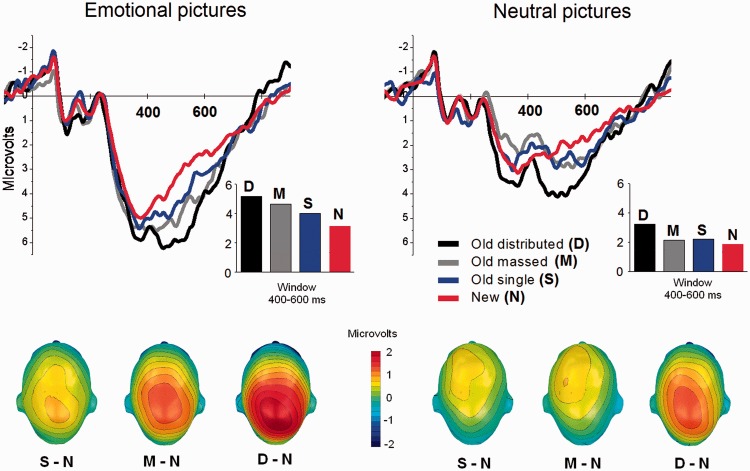
Main effects of condition [F(1,24) = 19, P < 0.0001, η2 = 0.72] and content [F(1,24) = 93, P < 0.0001, η2 = 0.80] were accompanied by a significant interaction [F(3,22) = 3.5, P = 0.03, η2 = 0.32]. Simple main effects tests indicated effects of condition on ERPs when recognizing both emotional [F(3,22) = 17.1, P < 0.0001, η2 = 0.70] and neutral [F(3,22) = 7.3, P = 0.001, η2 = 0.50] pictures. Figure 2 illustrates that distributed repetition prompted enhanced positivity, compared with new pictures, for both emotional [F(1,24) = 49.7, P < 0.0001, η2 = 0.67] and neutral pictures [F(1,24) = 18.4, P < 0.001, η2 = 0.44]. In addition, for emotional pictures, distributed repetition prompted more centro-parietal positivity than massed repetition [F(1,24) = 4.1, P = 0.05, η2 = 0.15] or single presentations, [F(1,24) = 18.8, P < 0.0001, η2 = 0.44] with a marginal difference in positivity between massed and single presentation [P < 0.05, one-tailed]. Although both massed repetition and single presentation prompted enhanced positivity compared with new stimuli for emotional pictures [massed, F(1,24) = 25, P < 0.0001, η2 = 0.51; single F(1,24) = 11.9, P < 0.005, η2 = 0.33], these old–new differences did not reach significance for neutral pictures (Fs < 1).
Fig. 2.
Experiment 1. Grand average ERP waveforms over centro-parietal sensors when recognizing emotional (left panel) and neutral (right panel) old pictures presented once (single: S), massed (M) or distributed (D) during encoding and new (N) pictures. Insets show the mean amplitude of ERP in the window 400–600 ms from picture onset. (Bottom) scalp topography (top view) of the difference in the 400–600 ms window between old pictures presented once (single: S – N), massed (M – N) or distributed (D – N) during encoding and new (N) pictures.
Fronto-central region
Over fronto-central sensors, a main effect of condition [F(3,22) = 12.7, P < 0.0001, η2 = 0.63], indicated that old pictures generally prompted greater positivity than new pictures [Fs(1,24) = 17.6, 27.1 and 31.2, P < 0.0001, respectively], with no difference between single, massed and distributed repetition. These old–new differences were significant both for emotional [F(3,22) = 11.3, P < 0.0001, η2 = 0.61] and neutral [F(3,22) = 7.57, P < 0.005, η2 = 0.51] pictures. Emotional pictures also generally prompted more positivity than neutral pictures [F(1,24) = 79, P < 0.0001, η2 = 0.77]. There was no interaction between picture content and condition.
EXPERIMENT 2
Over centro-parietal sensors, effects of repetition were significant, with distributed repetition prompting the largest positivity, for both emotional and neutral pictures. Performance somewhat paralleled the ERP data: memory performance was enhanced following distributed repetition, compared with single presentation, for both emotional and neutral pictures. Beneficial effects of distributed repetition have been variously attributed to processes occurring at encoding, such as an increase in the amount of information stored in the memory trace (McClelland and Chappell, 1998; Murdock, 1982; Murdock et al., 2001) or to those occurring at retrieval, such as multiple trace theory, which proposes a higher probability of a cue contacting a memory trace at retrieval when multiple traces exist (Bower, 1967; Glenberg, 1979; Hintzman, 1988; Lansdale and Baguley, 2008).
Measuring ERPs following repetitive processing is a novel method for assessing these alternative hypotheses, as differences due to encoding should not be affected by whether or not an explicit memory decision is required. Furthermore, although effects of prior occurrence on ERPs during word processing have been assessed using implicit tasks that do not rely on memory, such as semantic judgments (Rugg et al. 1998), lexical decision (Curran, 1999) or categorization (Curran and Cleary, 2003), a more intriguing scenario for investigating how repetition at encoding affects old–new ERPs, given the relatively interesting, naturalistic visual scenes presented here, is to simply ask the participant to view these pictures. If effects of repetition on memory primarily reflect differential encoding (or storage), similar differences in the old–new ERP are expected. To the extent that repetition reflects explicit retrieval processes, a different pattern of modulation should be found.
Method
Participants
Participants were 24 right-handed students (13 women) from a General Psychology course at the University of Florida who participated for course credit. They had normal or corrected-to-normal visual acuity. Prior to participating, all subjects gave their informed consent in accordance with the UF Institutional Review Board guidelines.
Materials, design and procedure
The materials and design were identical to that described in Experiment 1. The only difference was that in the recognition phase, participants did not make an old/new decision, but were instructed to simply view each picture while it was on the screen and to maintain fixation at the center of the screen at all times.
Results
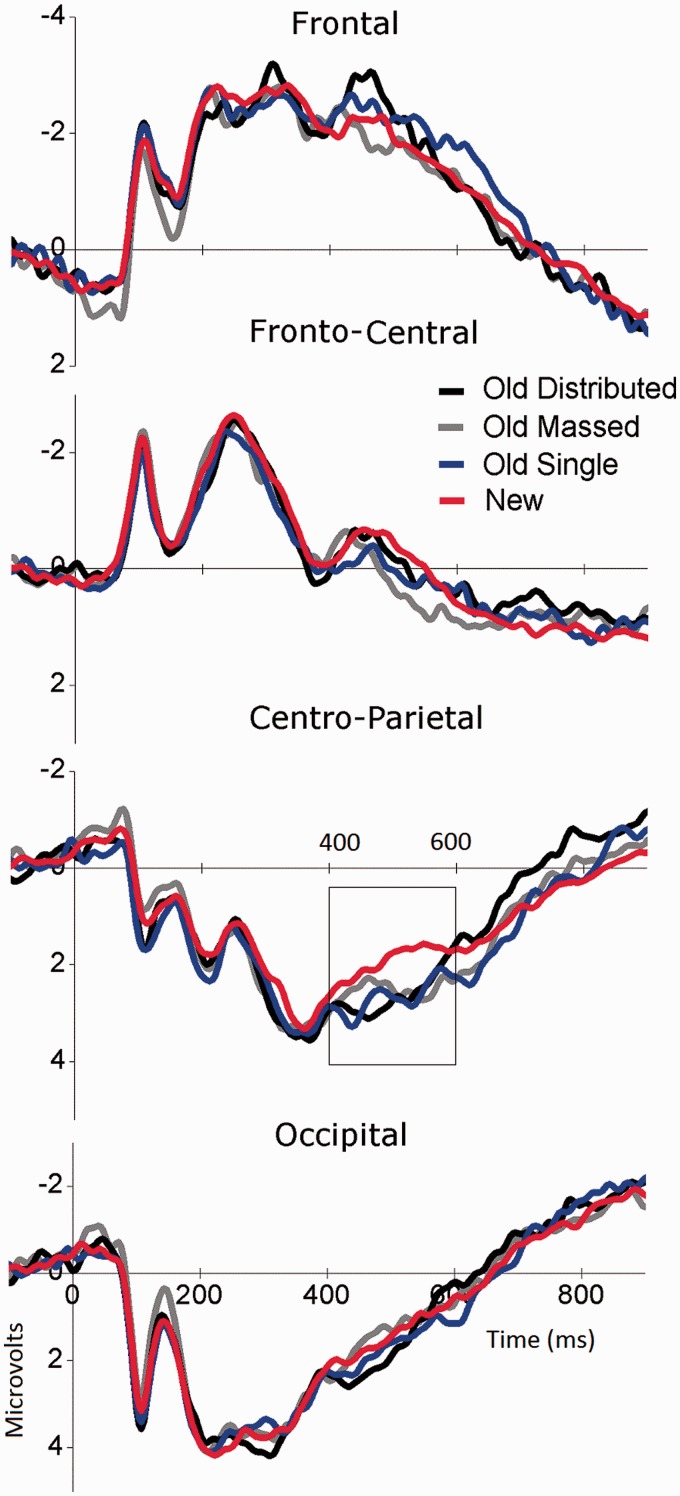
Figure 3 illustrates midline waveforms for pictures that were presented once (single) or repeated (massed, distributed) during encoding, and new pictures, averaged over emotion, when viewed without making an explicit recognition decision. Analyses were conducted using the same 400–600 ms time window over centro-parietal sensors and fronto-central sensors, separately, as in Experiment 1.
Fig. 3.
Experiment 2. Grand average ERP midline waveforms (sensor clusters) when passively viewing pictures that had been presented once (single) or repeated (massed or distributed) in encoding and new (new) pictures.
Centro-parietal region
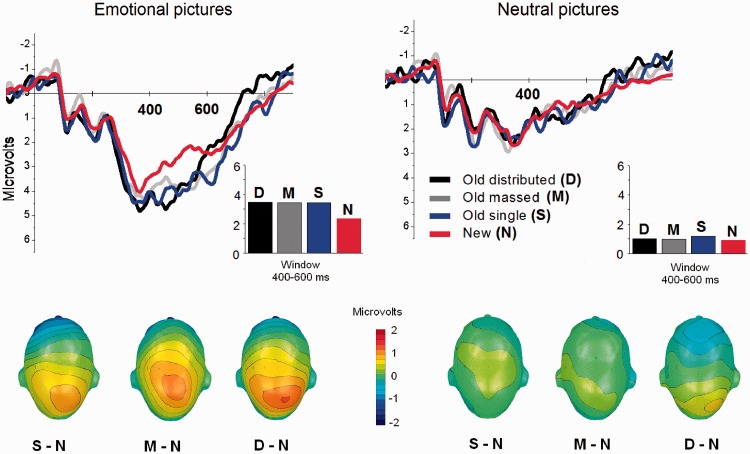
A significant interaction of condition and emotion [F(3,21) = 4.81, P < 0.05, η2 = 0.41] indicated that there were no differences in ERPs when viewing neutral pictures, whether these were new or had been presented in the encoding phase once, massed or distributed (see Figure 4). On the other hand, a main effect of condition for emotional pictures [F(3, 21) = 8.16, P < 0.005, η2 = 0.54] indicated that, compared with new pictures, enhanced positivity was found for pictures that were seen before, whether distributed [F(1,23) = 15.9, P < 0.005, η2 = 0.41], massed [F(1,23) = 8.2, P = 0.009, η2 = 0.26] or presented once [F(1,23) = 19.9, P < 0.0001, η2 = 0.46], which did not differ from each other.
Fig. 4.
Experiment 2. Grand average ERP waveforms over centro-parietal sensors when passively viewing emotional (left panel) and neutral (right panel) old pictures that had been presented once (single: S), massed (M) or distributed (D) during encoding and new (N) pictures. Insets show the mean amplitude of ERP in the window 400–600 ms from picture onset. (Bottom) scalp topography (top view) of the difference in the 400–600 ms window between old pictures presented once (single: S – N), massed (M – N) or distributed (D – N) during encoding and new (N) pictures.
Fronto-central region
Similar effects were found over fronto-central region: a significant interaction of condition and emotion [F(3,21) = 3.32, P < 0.05, η2 = 0.32] showed that there were no old–new differences in ERPs for neutral pictures, whereas old emotional pictures were associated with larger fronto-central positivity compared with new emotional pictures [F(3,69) = 4.9, P < 0.01, η2 = 0.17]. Again, this old–new difference was not modulated by the type of old pictures (distributed, massed or single).
DISCUSSION
During an immediate recognition task (Experiment 1), correct recognition of old, compared with new, scenes prompted enhanced positivity over centro-parietal sensors when pictures were presented using distributed repetition at encoding, compared with single presentations. When an explicit recognition task was replaced by passive viewing in Experiment 2, effects of repetition on ERPs were absent, and only emotional pictures showed small differences in positivity over centro-parietal sensors when viewing old, compared with new, pictures. Taken together, the data indicate that (1) in the context of an explicit recognition task, distributed repetition enhances positivity (in a 400–600 window) over centro-parietal sensors and (2) emotional, but not neutral, pictures show small effects of prior occurrence when an explicit recognition decision is not required.
Effects of repetition, particularly distributed, were prominent over a relatively posterior set of centro-parietal sensors. Posterior old–new differences have often been attributed to specific episodic recollection, in which recognition decisions reflect activation or retrieval of a specific episodic trace (e.g. Paller et al., 1995; Wilding and Rugg, 1996; Joyce et al., 1998; Allan et al., 2000; Paller et al., 2003; Rugg, and Curran, 2007; Voss and Paller, 2008; Curran and Doyle, 2011). That repetition, particularly distributed, may enhance the probability of specific episodic retrieval, consistent with multiple trace theories of repetition (e.g. Glenberg, 1979; Hintzman, 1988; Lansdale and Baguley, 2008), which propose that repeating items at encoding results in multiple, separable episodic traces, which facilitates the probability of contacting an episodic representation at retrieval.
Multiple trace theory also predicts smaller facilitation for massed repetitions. Consistent with this, ERPs when recognizing pictures presented with massed repetition were most similar to those for scenes presented once, which is consistent with previous data indicating that massed repetition at encoding results in large attenuation of LPP amplitude compared with distributed repetition (Ferrari et al., 2011). On the other hand, memory performance was similarly enhanced for both massed and distributed repetition, compared with single presentations. Different patterns of modulation for behavioral and electrophysiological indices suggest that recognition may be mediated by different mechanisms. For instance, although repetition of either kind supported better recognition performance, the ERP data suggest that accurate performance following distributed repetition may be specifically mediated by recollective processes, whereas massed repetition may enhance stimulus familiarity, which is often associated with an early frontal old–new modulation (Rugg and Curran, 2007). Although not early, an old–new ERP difference found over fronto-central sensors in the same relatively late (400–600 ms) time window in the current study was not modulated by the type of repetition, and may reflect a different process (i.e. familiarity) involved in picture recognition.
Emotional pictures were only recognized more accurately than neutral pictures presented once. In fact, an emotional memory advantage is not always found on an immediate test for pictures, in which recognition is typically near the ceiling (M.M. Bradley, F. Versace and P.J. Lang, submitted for publication; Versace et al., 2010). One possibility is that the multiple repetitions of some pictures altered the recognition criterion (i.e. higher), resulting in particularly poor performance (74%) for neutral pictures presented only once during encoding, resulting in a significant advantage for emotional picture viewed only once. The relative absence of a significant parietal old–new difference for neutral pictures presented once is consistent with this hypothesis.
Conversely, effects of repetition on memory performance were somewhat more pronounced for neutral pictures. A similar facilitation in memory performance for neutral stimuli has been reported in several studies that varied the level-of-processing, finding that deeper processing more strongly impacts memory for neutral, compared with emotional, stimuli (Reber et al., 1994; Jay et al., 2008; Ritchey et al., 2011). Nonetheless, although emotional pictures presented once showed good performance, distributed repetition significantly facilitated recognition performance for these stimuli as well.
The null effects of repetition on old–new ERPs for either emotional or neutral pictures in the absence of an explicit recognition task (Experiment 2) provides further support that the centro-parietal repetition differences reflect processes occurring at retrieval. These data are not consistent with hypotheses that effects of repetition are due to differences at encoding (or storage), such as the amount or type of information encoded in a memory trace (Murdock, 1982; McClelland and Chappell, 1998; Murdock et al., 2001), as the identical encoding conditions in Experiments 1 and 2 predict similar effects on the old–new ERPs. The data also appear somewhat inconsistent with a ‘recursive reminder’ theory of repetition, which holds that repeated items automatically retrieve prior occurrence (Hintzman, 2010), as, again, no task-related differences are expected.
Although small old–new differences were found during passive viewing for emotional pictures in Experiment 2, in the absence of an explicit recognition task, all effects of prior presentation on ERPs were absent for neutral pictures. Because memory performance for neutral pictures was quite high (over type of repetition >80%) in Experiment 1, the retention interval brief (15 min), and memory for pictures generally good (Bradley et al., 1992; Dolcos et al., 2005; Talmi et al., 2008), it is unlikely that participants did not in some sense ‘recognize’ previously presented neutral pictures during passive viewing as ‘old’. Rather, it is only that this recognition was not reflected in the centro-parietal old–new ERP difference. Our interpretation is that the parietal old–new ERP difference does not reflect the mere existence or strength of a memory trace. Instead, the old–new ERP modulation reflects an active retrieval process, in which incoming information is matched with a stored representation. Based on this reasoning, the data suggest that (perhaps only a few) emotional pictures were spontaneously retrieved, whereas this was not true for neutral pictures when retrieval is not specifically required by the task. Single trial analysis would assist in testing this hypothesis (Li et al., 2009; Rousselet et al., 2009). Moreover, previous studies have found that, at encoding, both cortical (Schupp et al., 2004; Weinberg and Hajcak, 2010; De Cesarei and Codispoti, 2011) and peripheral responses (Bradley et al., 2001; Codispoti and De Cesarei, 2007) vary with emotional arousal for pleasant and unpleasant pictures. Assessing effects of specific content on recognition is somewhat more complex. First, in general, including semantically-related foils (‘new’ items) and/or multiple exemplars of the same content renders old–new decisions more difficult, increasing false positives (Versace et al., 2010). Second, assessing effects of category on memory critically relies on insuring that the relationship of foils to old items is equivalent across contents, which is difficult for pictures of natural scenes. Nonetheless, ongoing studies in our laboratories are attempting to grapple with these issues.
Taken together, the data indicate that (1) effects of repetition on old–new ERPs may reflect retrieval processes that are indexed by enhanced centro-parietal positivity indicating episodic recollection and (2) in the absence of an explicit memory task, only emotional stimuli show evidence of prior occurrence that can be measured in the amplitude of centro-parietal positivity, suggesting spontaneous retrieval. These findings are consistent with prior theory and data indicating enhanced attention to, and memory for, affectively salient cues which reflects learning and memory processes that have evolved to protect and sustain life (Bradley, 2009; Lang and Bradley, 2010). More generally, the current data suggest that observing a centro-parietal old–new difference in the ERP during recognition relies on retrieval of a prior episode and does not represent a task-free measure of memory storage or strength.
Acknowledgments
This research was supported, in part, by a grant from the National Institute of Mental Health (P50 MH 72850) to the Center for the Study of Emotion and Attention (CSEA) at the University of Florida.
Footnotes
1IAPS numbers: (set 1) pleasant, 1605, 1630, 1659, 2151, 2152, 2332, 2342, 2345, 2347, 2351, 3262, 4597, 4599, 4610, 4611, 4614, 4617, 4641, 4647, 4658, 4659, 4660, 4687, 4693, 4694, 4800 and 8031; unpleasant, 1111, 1120, 1205, 1300, 1304, 1930, 2717, 3000, 3001, 3015, 3140, 3170, 3195, 3212, 3261, 3350, 3400, 6212, 6230, 6570, 6821, 6834, 9120, 9301, 9326, 9412 and 9909; neutral, 2050, 2102, 2190, 2273, 2372, 2374, 2393, 2394, 2396, 2397, 2493, 2506, 2512, 2515, 2560, 2593, 2745, 5455, 5471, 5500, 5531, 5635, 5875, 5900, 7001, 7026, 7036, 7041, 7057, 7495, 7550, 7595 and 7632; (set 2) pleasant, 1460, 1595, 1710, 2060, 2075, 2314, 2340, 2360, 2655, 4604, 4616, 4624, 4626, 4640, 4645, 4653, 4666, 4668, 4676, 4680, 4690, 4692, 4697, 4698, 8179, 8370 and 8499; unpleasant, 1052, 1302, 1303, 1525, 1931, 2703, 2730, 3053, 3064, 3068, 3069, 3080, 3100, 3150, 3213, 3500, 6242, 6350, 6838, 9183, 9322, 9410, 9414, 9425, 9594, 9905 and 9940; neutral, 2039, 2217, 2221, 2305, 2370, 2382, 2383, 2390, 2410, 2435, 2500, 2501, 2513,, 2575, 2579, 2594, 2595, 5130, 5390, 5410, 6000, 7011, 7030, 7032, 7033, 7050, 7058, 7061, 7100, 7165, 7493, 7513, 7560, 7620, and 7950.
2This analysis revealed that discrimination index (Pr) was significantly higher for emotional compared to neutral pictures F(1,27) = 31, P < 0.0001, η2 = 0.53, as already suggested by the hit rate findings. Response bias was instead similar for emotional and neutral pictures.
3Geodesic sensor number for centro-frontal region: 6, 13, 113, 31, 7, 107, 106, 32, 129 and 81; for centro-parietal region: 54, 55, 80, 62, 61, 68, 79, 67, 73 and 78.
REFERENCES
- Allan K, Robb WG, Rugg MD. The effect of encoding manipulations on neural correlates of episodic retrieval. Neuropsychologia. 2000;38:1188–205. doi: 10.1016/s0028-3932(00)00013-0. [DOI] [PubMed] [Google Scholar]
- Besson M, Kutas M. The many facets of repetition: a behavioral and electrophysiological analysis of repeating words in same versus different sentence contexts. Journal of Experimental Psychology: Learning, Memory and Cognition. 1993;19:1115–33. doi: 10.1037//0278-7393.19.5.1115. [DOI] [PubMed] [Google Scholar]
- Besson M, Kutas M, Van Petten C. An event-related potential (ERP) analysis of semantic congruity and repetition effects in sentences. Journal of Cognitive Neuroscience. 1992;4:132–49. doi: 10.1162/jocn.1992.4.2.132. [DOI] [PubMed] [Google Scholar]
- Bower GH. A multicomponent theory of the memory trace. In: Spence KW, Spence JT, editors. The Psychology of Learning and Motivation. Vol. 1. New York: Academic Press; 1967. pp. 229–325. [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–98. [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory & Cognition. 1992;18:379–90. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Emotional memory: a dimensional analysis. In: Van Groot S, Van de Poll NE, Sargeant J, editors. The Emotions: Essays on Emotion Theory. Hillsdale, NJ: Erlbaum; 1994. pp. 97–134. [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: a review and quantitative synthesis. Psychological Bulletin. 2006;132:354–80. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- Codispoti M, De Cesarei A. Arousal and attention: picture size and emotional reactions. Psychophysiology. 2007;44:680–6. doi: 10.1111/j.1469-8986.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Research. 2006a;1068:213–20. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19:577–86. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, De Cesarei A, Cardinale R. Implicit and explicit categorization of natural scenes. Progress in Brain Research. 2006b;156:53–65. doi: 10.1016/S0079-6123(06)56003-0. [DOI] [PubMed] [Google Scholar]
- Curran T. The electrophysiology of incidental and intentional retrieval: ERP old/new effects in lexical decision and recognition memory. Neuropsychologia. 1999;37:771–785. doi: 10.1016/s0028-3932(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Curran T, Cleary AM. Using ERPs to dissociate recollection from familiarity in picture recognition. Cognitive Brain Research. 2003;15:191–205. doi: 10.1016/s0926-6410(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Curran T, Doyle J. Picture superiority doubly dissociates the ERP correlates of recollection and familiarity. Journal of Cognitive Neuroscience. 2011;23:1247–62. doi: 10.1162/jocn.2010.21464. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. Affective modulation of the LPP and alpha-ERD during picture viewing. Psychophysiology. 2011;48:1397–404. doi: 10.1111/j.1469-8986.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- Dempster FN. Effects of variable encoding and spaced presentations on vocabulary learning. Journal of Educational Psychology. 1987;79:162–70. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related potential study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos, F., LaBar, K.S., Cabeza, R. (2005). Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences USA, 102, 2626–31. [DOI] [PMC free article] [PubMed]
- Ebbinghaus H. Memory: A Contribution to Experimental Psychology. 1964. (H. R. C. Bussenius, Trans.). New York: Dover Publications. (Original work published 1885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ. Repetitive exposure: brain and reflex measures of emotion and attention. Psychophysiology. 2011;48:515–22. doi: 10.1111/j.1469-8986.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ. Detecting novelty and significance. Journal of Cognitive Neuroscience. 2010;22:404–11. doi: 10.1162/jocn.2009.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnigan S, Humphreys MS, Dennis S, Geffen G. ERP ‘old/new’ effects: memory strength and decisional factor(s) Neuropsychologia. 2002;40:2288–304. doi: 10.1016/s0028-3932(02)00113-6. [DOI] [PubMed] [Google Scholar]
- Glenberg AM. Component-levels theory of the effects of spacing of repetitions on recall and recognition. Memory & Cognition. 1979;7:95–112. doi: 10.3758/bf03197590. [DOI] [PubMed] [Google Scholar]
- Groh-Bordin C, Busch NA, Herrmann CS, Zimmer HD. Event-related potential repetition effects at encoding predict memory performance at test. Neuroreport. 2007;18:1905–9. doi: 10.1097/WNR.0b013e3282f2a61d. [DOI] [PubMed] [Google Scholar]
- Hintzman DL. Judgments of frequency and recognition memory in a multiple-trace memory model. Psychological Review. 1988;95:528–51. [Google Scholar]
- Hintzman DL. How does repetition affect memory? Evidence from judgments of recency. Memory & Cognition. 2010;38:102–15. doi: 10.3758/MC.38.1.102. [DOI] [PubMed] [Google Scholar]
- Jay T, Caldwell-Harris C, King K. Recalling taboo and nontaboo words. American Journal of Psychology. 2008;121:83–103. [PubMed] [Google Scholar]
- Joyce CA, Paller KA, McIsaac HK, Kutas M. Memory changes with normal aging: behavioral and electrophysiological measures. Psychophysiology. 1998;35:669–78. [PubMed] [Google Scholar]
- Junghöfer M, Peyk P. Analyzing electrical activity and magnetic fields in the brain. MATLAB News & Notes. 2004;2(4):14–5. [Google Scholar]
- Junghöfer M, Elbert T, Tucker D, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–32. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Rating of Measures and Instruction Manual. 2008. (Tech. Rep. No. A-6). Gainesville, FL: University of Florida. [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84:437–50. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdale M, Baguley T. Dilution as a model of long-term forgetting. Psychological Review. 2008;115:864–92. doi: 10.1037/a0013325. [DOI] [PubMed] [Google Scholar]
- Li R, Principe JC, Bradley M, Ferrari V. A spatiotemporal filtering methodology for single-trial ERP component estimation. IEEE Transactions on Biomedical Engineering. 2009;56:83–92. doi: 10.1109/TBME.2008.2002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntylä T, Cornoldi C. Remembering changes: repetition effects in face recollection. Acta Psychologica. 2002;109:95–105. doi: 10.1016/s0001-6918(01)00054-3. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Chappell M. Familiarity breeds differentiation: a subjective likelihood approach to the effects of experience in recognition memory. Psychological Review. 1998;105:724–60. doi: 10.1037/0033-295x.105.4.734-760. [DOI] [PubMed] [Google Scholar]
- Melton AW. The situation with respect to the spacing of repetitions and memory. Journal of Verbal Learning and Verbal Behavior. 1970;9:596–606. [Google Scholar]
- Murdock BB., Jr. A theory for the storage and retrieval of item and associative information. Psychological Review. 1982;89:609–26. doi: 10.1037/0033-295x.100.2.183. [DOI] [PubMed] [Google Scholar]
- Murdock BB, Jr., Smith D, Bai J. Judgments of frequency and recency in a distributed memory model. Journal of Mathematical Psychology. 2001;45:564–602. doi: 10.1006/jmps.2000.1339. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, McIsaac HK. Monitoring conscious recollection via the electrical activity of the brain. Psychological Science. 1995;6:107–11. [Google Scholar]
- Paller KA, Hutson CA, Miller BB, Boehm SG. Neural manifestations of memory with and without awareness. Neuron. 2003;38:507–16. doi: 10.1016/s0896-6273(03)00198-3. [DOI] [PubMed] [Google Scholar]
- Reber R, Perrig WJ, Flammer A, Walther D. Levels of processing and memory for emotional words. Swiss Journal of Psychology. 1994;53:78–85. [Google Scholar]
- Reder LM, Anderson JR. Effects of spacing and embellishment on memory for the main points of a text. Memory & Cognition. 1982;10:97–102. doi: 10.3758/bf03209210. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Labar KS, Cabeza R. Level of processing modulates the neural correlates of emotional memory formation. Journal of Cognitive Neuroscience. 2011;23:757–71. doi: 10.1162/jocn.2010.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet GA, Husk JS, Pernet C, Gaspar CM, Bennett PJ, Sekuler AB. Age-related delay in information accrual for faces: Evidence from a parametric, single-trial EEG approach. BMC Neuroscience. 2009;10:114. doi: 10.1186/1471-2202-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Science. 2007;11:251–7. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Allan K, Frith CD, Frackowiak RS, Dolan RJ. Neural correlates of memory retrieval during recognition memory and cued recall. NeuroImage. 1998;8:262–73. doi: 10.1006/nimg.1998.0363. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition and Emotion. 2004;18:593–611. [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learning and Memory. 2008;15:172–82. doi: 10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C, Senkfor AJ. Memory for words and novel visual patterns: repetition, recognition, and encoding effects in the event-related brain potential. Psychophysiology. 1996;33:491–506. doi: 10.1111/j.1469-8986.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Versace F, Bradley MM, Lang PJ. Memory and event-related potentials for rapidly presented emotional pictures. Experimental Brain Research. 2010;205:223–33. doi: 10.1007/s00221-010-2356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: the importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008;46:3021–9. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: Implications of examining electrocortical activity elicited by specific picture content. Emotion. 2010;10:767–82. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Melzig CA, Hamm AO. Enhanced long-term recollection for emotional pictures: evidence from high-density ERPs. Psychophysiology. 2009;46:1200–7. doi: 10.1111/j.1469-8986.2009.00869.x. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Doyle MC, Rugg MD. Recognition memory with and without retrieval of context: an event-related potential study. Neuropsychologia. 1995;33:743–67. doi: 10.1016/0028-3932(95)00017-w. [DOI] [PubMed] [Google Scholar]
- Xue G, Mei L, Chen C, Lu ZL, Poldrack R, Dong Q. Spaced learning enhances subsequent recognition memory by reducing neural repetition suppression. Journal of Cognitive Neuroscience. 2011;23:1624–33. doi: 10.1162/jocn.2010.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]