Abstract
Unhappy couple relationships are associated with impaired individual health, an effect thought to be mediated through ongoing couple conflicts. Little is known, however, about the underlying mechanisms regulating psychobiological stress, and particularly autonomic nervous system (ANS) reactivity, during negative couple interaction. In this study, we tested the effects of the neuropeptide oxytocin on ANS reactivity during couple conflict in a standardized laboratory paradigm. In a double-blind, placebo-controlled design, 47 heterosexual couples (total n = 94) received oxytocin or placebo intranasally prior to instructed couple conflict. Participants’ behavior was videotaped and salivary alpha-amylase (sAA), a measure of sympathetic activity, and emotional arousal were repeatedly measured during the experiment. Oxytocin significantly reduced sAA during couple conflict in women, whereas men showed increases in sAA levels (sex × group interaction: B = −49.36, t = −2.68, P = 0.009). In men, these increases were related to augmented emotional arousal (r = 0.286, P = 0.028) and more positive behavior (r = 0.291, P = 0.026), whereas there was no such association in women. Our results imply sex-specific effects of oxytocin on sympathetic activity, to negative couple interaction, with the neuropeptide reducing sAA responses and emotional arousal in women while increasing them in men.
Keywords: intranasal oxytocin, autonomic nervous system, alpha-amylase, couple conflict, sex-specific
INTRODUCTION
Social integration and affectionate social interactions have a substantial influence on individual stress resilience (Robles and Kiecolt-Glaser, 2003), health (Uchino, 2006; Thoits, 2011) and longevity (House et al., 1988; Holt-Lunstad et al., 2010), making couple relationships, as the most prominent social relationship for nearly all adults, an important predictor of quality of life and health (Proulx et al., 2007). In line with this and in contrast to positive couple interaction (cf. Grewen et al., 2003; Coan et al., 2006; Ditzen et al., 2007; Holt-Lunstad et al., 2008), ongoing couple conflicts have been suggested to constantly increase stress and, thereby, to impair individual health (Kiecolt-Glaser and Newton, 2001).
Consequently, research interest has turned to the neurobiological mechanisms underlying the effects of couple interaction on stress and individual health-relevant outcomes (Uchino et al., 1996; Robles and Kiecolt-Glaser, 2003; Karelina and DeVries, 2011). Among other factors, this research points to the neuropeptide oxytocin (OT), which has been shown to improve social affiliation and attachment and to reduce anxiety and stress (Young et al., 2001; Donaldson and Young, 2008; Ross and Young, 2009; Insel, 2010). OT is synthesized in the hypothalamus, from where it exerts its effects on widespread areas in the central nervous system (CNS) (Gimpl and Fahrenholz, 2001; de Bono, 2003; Landgraf and Neumann, 2004). As no specific central nervous in-vivo markers are yet available, the interpretation of OT measures in humans is limited (Meyer-Lindenberg et al., 2011; Szeto et al., 2011). However, neuropeptides have been shown to gain access to the brain following intranasal administration (Born et al., 2002; Chang et al., 2012). Using this technique, an increasing number of human studies suggest effects of OT on the level of behavioral and neural systems, including social interaction, social memory and stress reactivity (Heinrichs et al., 2009; Bos et al., 2011; Meyer-Lindenberg et al., 2011). Notably, with regard to couple interaction, previous data from our laboratory indicate that intranasal OT augments the ratio of positive to negative communication behavior and leads to reduced endocrine stress activity, i.e. cortisol levels, during a couple conflict task (Ditzen et al., 2009). Within the context of social interaction research, couple conflict is a valuable research paradigm, because for all subjects it involves a highly relevant social interaction partner during instructed and observable real-time behavior. Indeed, in previous research, couple conflict was shown to increase autonomic nervous system (ANS) stress responses, which were identified with different measures. Kiecolt-Glaser et al. (1993) and, more recently, Nealey-Moore et al. (2007) observed increased heart rate (HR) and blood pressure (BP) in response to couple conflict in both men and women, as did Flor et al. (1995) in chronic pain patients and healthy controls. Ewart and colleagues found increased BP in response to couple conflict (1991) in high-BP patients. Interestingly, however, in women, these changes were related to affective content and quality of the interaction, whereas in men, these (overall smaller) changes were related to speech rate (Ewart et al., 1991). In line with BP and HR, levels of the catecholamines epinephrine (EPI) and norepinephrine (NE) in blood were found to raise during conflict tasks in the laboratory (Malarkey et al., 1994), although again with sex differences, as revealed by significantly higher responses in EPI levels in women than in men.
There is also evidence for an influence of OT mechanisms on ANS activation from animal and human research. In female prairie voles, peripheral OT administration reduced HR responses to social isolation (Grippo et al., 2009) and to social stress (resident intruder test) (Grippo et al., 2012). In humans, an association of OT receptor gene polymorphism rs53576 with cardiac pre-ejection period (PEP) was recently found in males, with A allele carriers displaying higher levels of sympathetic cardiac control, as revealed by lower resting PEP levels (Norman et al., 2012). Moreover, intranasal OT increased both sympathetic activity (assessed by PEP) and parasympathetic activity (assessed by high-frequency HR variability) (Norman et al., 2011). The authors did not find overall changes in HR following the administration of intranasal OT. Rilling et al. (2011) and Gamer and Buchel (2011) also failed to find such changes in HR. However, interestingly, in the latter study, phasic HR in OT-treated men increased specifically in response to pictures of fearful (and to a lesser extent neutral) faces. The authors interpret these data in terms of an intensified emotional valence of these pictures following the administration of OT. On a central level, OT might modulate ANS responses via the amygdala-cingulate circuit processing of emotional and cognitive responses to stress (Kirsch et al., 2005; Domes et al., 2007). However, substantial sex differences might be expected here, as women show different amygdala activation in response to OT than men (Domes et al., 2010). As parallel data on OT effects in both men and women are still rare and, in part, conflicting (for an overview, see Bartz et al., 2011), it is particularly important to investigate both sexes within the same research design. Interestingly, in a most recent study, Fischer-Shofty et al. (2012) detected a significant OT by sex interaction in social perception of kinship and competition in video sequences. The authors concluded that ‘gender differences in OT may be evident in different categories of social behaviors’ (Fischer-Shofty et al., 2012). Distinct effects of OT in men and women might also be evident in different physiological systems. We were thus interested to examine the effect of OT on ANS responses to couple conflict. The following might be hypothesized in this context: either decreased activation in women (as suggested by the studies in female prairie voles); increased activation in men due to increased emotional valence (as implied by the research in men); or no sex differences, based on the data from Norman et al. (2011).
With the dataset on OT nasal spray during an instructed couple conflict session (cf. Ditzen et al., 2009), we have a unique possibility to investigate the effects of this pharmacological modulation on real-time interpersonal behavior and physiology in men and women. We therefore went back to our data and investigated the effects of intranasally administered OT on ANS activation through repeated assessment of the salivary enzyme alpha amylase (sAA). SAA has been interpreted as an indirect marker of ANS activity and sympathetic nervous system (SNS) activity in particular (Ehlert et al., 2006; Nater et al., 2006; Nater and Rohleder, 2009). In order to capture possible modulators of ANS responses, emotional arousal was measured repeatedly and analyzed in relation to sAA and couple behavior.
METHODS AND MATERIALS
Forty-seven heterosexual couples (n = 94 subjects), aged 20–50 years, who were married or had been cohabiting for at least 1 year participated in the study. Exclusion criteria for participation were smoking, chronic mental or physical illness, medication intake and, for women, the intake of hormonal contraceptives, current pregnancy or breastfeeding. All women were investigated during the luteal phase of their menstrual cycle. Couples provided written informed consent and were offered 100 Swiss Francs for participation. The study was in line with the Declaration of Helsinki and approved by the ethics committee of the Canton of Zurich, Switzerland.
Equivalence among OT and placebo groups was assessed using the General Health Questionnaire (GHQ) (Goldberg and Williams, 1969), the Relationship Questionnaire (PFB) (Hahlweg, 1996) and the Short Chronic Stress Scale (SSCS) (Schulz et al., 2004). Experiments took place in the laboratories of the Department of Psychology at the University of Zurich between 5:00 pm and 7:30 pm to control for diurnal variation in sAA activity.
In order to reflect ANS activity and, more precisely, sympathetic activity (Ehlert et al., 2006; Nater et al., 2006; Nater and Rohleder, 2009), sAA was repeatedly assessed from saliva samples. Salivette collection devices (Sarstedt, Sevelen, Switzerland) were taken at baseline (−50 min relative to the onset of the conflict discussion), at −20 min, immediately before conflict (−1 min) and after conflict (+10, +20, +30, +45 min). Saliva samples were stored at −20°C until required for analysis. AA activity was determined using the automatic analyzer Cobas Mira and assay kits obtained from Roche. The reagents in the kit contain the enzyme alpha amylase and alpha glucosidase, which convert the substrate ethyliden nitrophenyl to p-nitrophenol. The rate of formation of p-nitrophenol is directly proportional to the amylase activity. Activity is determined by measuring the absorbance at 405 nm. The assay is a kinetic colorimetric test. Inter- and intraassay variance was below 1%.
Following the baseline saliva assessment and a pregnancy test in women, all individuals rated their emotional arousal and specific emotions (anxiety, sadness, anger, joy) with a standard 14-item questionnaire to be evaluated on a seven-point Likert Scale (BSKE-EA, Janke et al., 1988). Sum-scores for emotional arousal (as described in Janke et al., 1988) were interpreted as dependent variables in the data analyses.
Couples were then asked to chose 2 out of 23 pre-determined areas of continuing disagreement in their own relationship (Hahlweg, 1996) for the later discussion (cf. Gottman, 1994; Weiss and Heyman, 1997; Kaiser et al., 1998). Following this procedure, in a double-blind design, couples self-administered either 40 IU (five puffs in each nostril) of OT (Syntocinon Spray, Novartis, Basel, Switzerland) or placebo intranasally under the supervision of the study coordinator. The dosage and timing of the nasal spray administration were chosen based on published research on intranasal OT, behavior and emotions in humans (Meyer-Lindenberg et al., 2011) and on Born and colleagues’ results on cerebrospinal fluid levels after intranasal vasopressin administration (Born et al., 2002). Assignment to groups was based on the couple level, meaning that both partners received either OT or placebo.
Forty-five minutes after drug administration, couples were asked to discuss the previously chosen conflict issue for the following 10 min. During this conflict discussion, couples were alone in the room and were videotaped. Videotapes were coded with a computer-aided system of behavior analysis (Computer Aided Observation System, CAOS) (Bourquard et al., 1992–2005). Positive conflict behavior was coded as the ratio of relative duration of positive/negative behavior according to Gottman’s balance theory (for details, see Gottman, 1994). Following the conflict discussion, participants were asked to evaluate the conflict on the dimensions ‘validity of the task, stressfulness of the task, positive own and partner’s behavior, negative own and partner’s behavior, and solution-oriented own and partner’s behavior’, using a standard evaluation questionnaire (cf. Hahlweg and Jacobson, 1984) and were again asked to rate their emotional arousal and specific emotions. During the following 60 min, saliva samples were taken repeatedly and couples watched a documentary (Komplett-Video, 2006) to prevent them from further talking or ruminating about the conflict. Finally, participants received the reimbursement and left the laboratory at 7:30 pm.
STATISTICAL ANALYSES
Baseline differences between groups were analyzed using ANOVAs. Time slopes of sAA were calculated as sAA = a + x1 (measure) + x2 (baseline sAA) + e. Associations of increases in sAA (difference scores were calculated as sAA after conflict, +10 min, −sAA prior to conflict instructions, −20 min) with group assignment (OT/placebo) and emotional arousal were calculated using hierarchical linear modeling (HLM), with sex, group, sex × group, BMI, age and baseline sAA included on level 1, and an empty couple level 2, in order to take into account the nested structure in the couple data (Kenny et al., 2006). Within sexes, associations of emotional arousal, sAA and behavior were calculated with correlations. Normal distribution of the data was assessed with the Kolmogorov–Smirnov test. As normal distribution of repeatedly assessed sAA was not violated, untransformed sAA levels were used in the analyses. Data on sAA during the course of the experiment were analyzed with repeated measures ANOVAs (groupOT/Pl × sexmen/women × time6 measures). In these analyses, couple data were also treated as repeated measures in order to account for dependency between partners. Mauchly’s test indicated that the assumption of sphericity had been violated, and therefore in repeated measures, multivariate tests using Pillai’s trace correction are reported (cf. Field, 2009). Data were analyzed using SPSS 19 (SPSS Inc., Chicago, IL, USA) and HLM 6 (Raudenbush et al., 2004).
RESULTS
The two groups (placebo vs OT) did not significantly differ in their BMI, duration of relationship, relationship quality, years of education, chronic stress levels, and number of general health symptoms or baseline sAA (−50 min relative to the onset of the conflict). However, male participants in the placebo group were slightly older than participants in the OT group. Emotional arousal prior to the conflict did not differ between groups or sexes (for statistical values and all baseline values, see Supplementary Table S1).
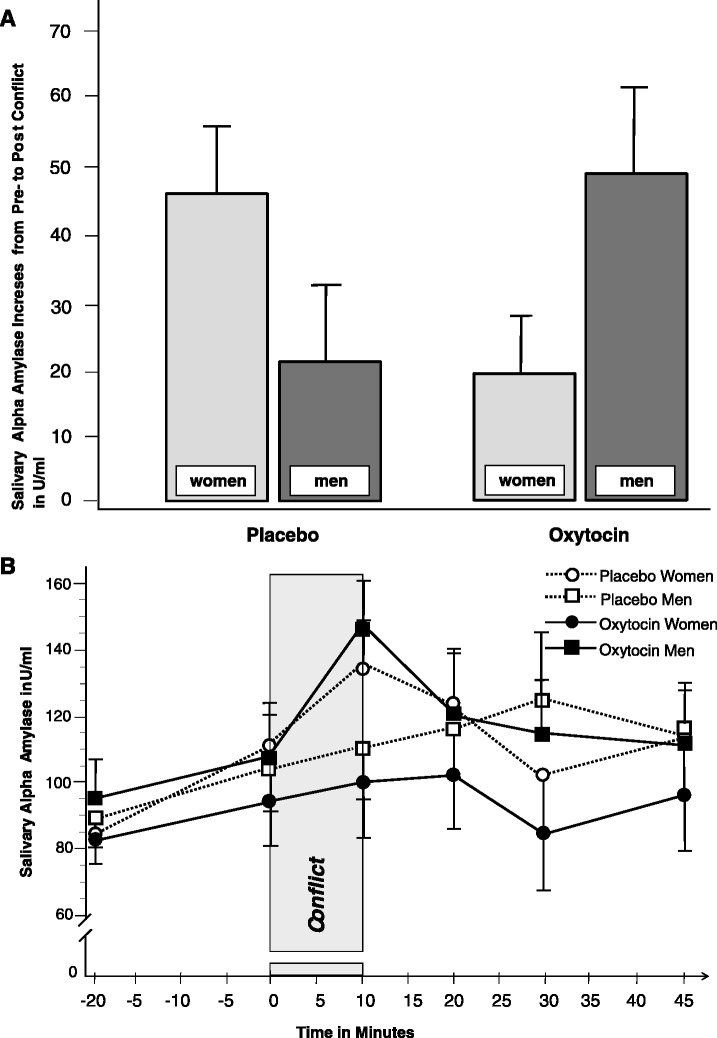
SAA significantly increased from pre- to post-couple conflict (time slope sAA −20 min, +10 min: B = 16.84, t = 6.10, P ≤ 0.001; mean sAA increaseplacebo condition = 33.70, s.d. = 51.47; mean sAA increaseOT condition = 33.98, s.d. = 52.12), with subsequent decreases (time slope sAA + 10 min, +20 min, +30 min, +45 min: B = −4.92, t = −2.52, P = 0.012; mean sAA decrease placebo condition = −10.22, s.d. = 51.79; mean sAA decrease OT condition = −17.13, s.d. = 48.42) during recovery in the total group. Interestingly, this effect was driven by men who had received OT. Results with regard to sAA increases from pre- to post-conflict show no significant group effect, no significant sex effect, but a significant sex × group interaction (Table 1). Whereas women with OT showed decreases in sAA as compared to placebo, men in the OT group showed increased sAA (Figure 1A).
Table 1.
HLM with increases in sAA as dependent variable
| Fixed effects | Coefficient; B | SE | T-ratio | d.f. | P-value |
|---|---|---|---|---|---|
| Intercept | 26.28 | 13.07 | 2.01 | 46 | 0.050* |
| Sex | 16.29 | 13.80 | 1.18 | 87 | 0.242 |
| Group | 23.17 | 17.19 | 1.35 | 87 | 0.181 |
| Sex × group | −49.36 | 18.38 | −2.68 | 87 | 0.009* |
| Age | −0.89 | .84 | −1.06 | 87 | 0.292 |
| BMI | −1.63 | 1.66 | −0.98 | 87 | 0.331 |
| Baseline sAA | −0.04 | 0.09 | −0.48 | 87 | 0.632 |
| Random effects | Variance component | s.d. | chi-square | d.f. | P-value |
| Intercept | 461.44 | 21.48 | 62.46 | 46 | 0.053 |
| Residual | 2135.87 | 46.21 | |||
Group: 0 = placebo, 1 = OT; sex: 0 = man, 1 = woman. Increases in sAA were calculated as delta sAA+10 min − sAA−20 min. The model explained 3.1% in sAA increases to couple conflict.
*Significant on a 5% level.
Fig. 1.
(A) Increases in sAA in women (n = 23) and men (n = 23) with intranasal OT, or women (n = 24) and men (n = 24) with placebo during the 10-min conflict discussion. Increases in sAA were calculated as difference scores sAA+10 min − sAA−20 min. Error bars are s.e.m. (B) Courses of sAA before and following the 10-min conflict in women (n = 23) and men (n = 23) with OT, or women (n = 24) and men (n = 24) with placebo (intranasal administration). Error bars are s.e.m.
These results were confirmed in the repeated measures ANOVA. No main effect was found for OT on sAA over time (time × group interaction: F = 0.8575, 37, P = 0.519, NS) and no overall difference was detected between men and women in sAA over time (time × sex interaction: F = 0.6115, 37, P = 0.692, NS), but men and women significantly differed between OT and placebo group in their sAA levels over time (time × sex × group: F = 2.5815, 37, P = 0.042; Figure 1B).
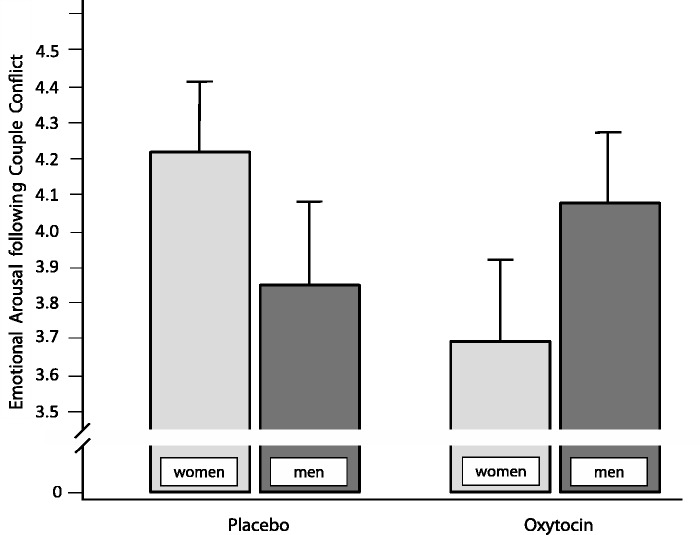
Subsequently, we tested whether OT increased emotional arousal during/following conflict, which might, in turn, be associated with higher sympathetic activity specifically in men. Results, as indicated by HLM analyses with emotional arousal as dependent variable, indeed suggest no main effects of either group or sex but, again, a significant sex × group interaction (Table 2). Men with OT reported higher emotional arousal to the couple conflict, whereas women with OT reported lower emotional arousal in comparison to placebo (Figure 2).
Table 2.
HLM with emotional arousal after conflict as dependent variable
| Fixed effects | Coefficient; B | SE | T-ratio | d.f. | P-value |
|---|---|---|---|---|---|
| Intercept | 3.87 | 0.22 | 17.69 | 46 | ≤0.001** |
| Sex | 0.34 | 0.26 | 1.30 | 85 | 0.197 |
| Group | 0.21 | 0.28 | 0.73 | 85 | 0.469 |
| Sex × group | −0.73 | 0.34 | −2.12 | 85 | 0.037* |
| Age | −0.02 | 0.02 | −1.16 | 85 | 0.251 |
| BMI | −0.02 | 0.04 | −0.59 | 85 | 0.553 |
| Random effects | Variance component | s.d. | chi-square | d.f. | P-value |
| Intercept | 0.09 | 0.30 | 51.15 | 46 | 0.278 |
| Residual | 0.85 | 0.92 | |||
Group: 0 = placebo, 1 = OT; Sex: 0 = man, 1 = woman. ‘Specific Emotions’ (anxiety, joy, sadness, anger), ‘Unspecific Emotions’ (mental wellbeing) and ‘Physical Wellbeing’, according to Janke et al., 1988, were included as control variables. The model explained 22.4% in emotional arousal.
*Significant on a 5% level; **Significant on a 1% level.
Fig. 2.
Emotional arousal (BSKE-EA, Janke et al., 1988) in women (n = 23) and men (n = 23) with intranasal OT, or women (n = 24) and men (n = 24) with placebo, following the 10-min conflict discussion. Error bars are s.e.m.
In line with this, emotional arousal was positively correlated with increases in sAA (r = 0.286, P = 0.028) and with more positive observed behavior during couple conflict (r = 0.291, P = 0.026) in men. In women, there were no associations of sAA with emotional arousal or with observed conflict behavior.
DISCUSSION
In the current study, we found significant increases in sAA, as a proposed measure of SNS activation, in response to a standardized conflict discussion in couples. These responses were moderated by sex and central nervous availability of the neuropeptide OT: men with intranasal OT administration showed increased sAA responses in comparison to placebo, whereas women with OT showed decreases in sAA. Notably, in men, these increases were positively related to higher emotional arousal and to increased positive in relation to negative behavior.
Overall, these results confirm earlier results on ANS activation in response to couple conflict, such as increased HR, BP and catecholamines (Ewart et al., 1991; Flor et al., 1995; Nealey-Moore et al., 2007). However, with regard to underlying neuroendocrine mechanisms, in our study, OT did not show overall attenuating or stimulating effects but rather sex-specific influences on sAA. It is particularly this sex-specific nature of the OT effects which is salient in the present findings. In women, our findings are concurrent with animal data on OT application and reduced ANS activation in female prairie voles (Grippo et al., 2009, 2012). They also correspond to ‘befriending’ mechanisms in women, as opposed to the fight-or-flight physiology in men, as suggested by Taylor et al. (2000), and to the anxiolytic effects which were found in previous research in humans (Meyer-Lindenberg et al., 2011). However, the opposite is the case in men, in whom results strikingly resemble the data recently reported by Gamer and Buchel (2011). The authors assumed that in men, OT increased differential HR responses as a function of higher motivational value and followed that this might result in increased approach behavior. Indeed, it has been argued that besides anxiety reduction and affiliation, OT might modulate social salience (Bartz et al., 2011). According to the authors (Bartz et al., 2011), by increasing attention to social cues, OT might have socially more or less desirable consequences, depending on personal factors and the social context. In a most recent review paper, McCall and Singer (2012) presented the hypothesis that OT might modulate the motivational states approach and quiescence, and that these states should be distinguishable by their autonomic responses. Whereas quiescence is assumed to be associated with parasympathetic activity, approach would be characterized by SNS activity. Thus, in line with Bartz et al. (2011) and McCall and Singer (2012), in our study, OT might have driven quiescence in women and social salience and approach behavior in men. This increased social salience and approach behavior in men might have resulted in higher sympathetic activation, a result which is in line with the positive association of sAA levels and emotional arousal in our male participants.
However, caution is warranted with regard to the interpretation of causal effects. Although all variables were assessed repeatedly, continuous data sampling during the actual conflict was not possible. Hence, attempts to account for the causal nature of sAA, emotions or behavior in response to the conflict remain speculative. Moreover, effects of a single neuropharmacological challenge might differ considerably from endogenous OT mechanisms, which are modulated by number and sensitivity of OT receptors in different areas of the CNS (Bale et al., 2001; Landgraf and Neumann, 2004). In this regard, the sex-specific effects observed here might also be interpreted in the light of possible vasopressin receptor activity, and in interaction with steroid hormones. Neither of these points has so far been investigated in humans.
Taken together, the sex-specific response pattern to OT observed here is of relevance not only for future theory building but also for health-promoting marital interventions. Research in couples shows that women tend to express demand behavior more frequently, while men tend to show withdraw behavior (Gottman and Krokoff, 1989; Christensen and Heavey, 1990; Bodenmann et al., 1998), the latter being a behavioral pattern that is consistently predictive of decreases in relationship satisfaction (Heavey et al., 1995). This point leads to the interpretation of the positive associations of sAA with emotional arousal and positive behavior in men which were detected here. In a recent study, ANS responses were triggered by positive couple interactions (as compared to neutral interactions; Nealey-Moore et al., 2007), an effect which was attributed to the emotional nature of the task. With regard to the results presented here, this suggests a more refined interpretation of sympathetic activation during social interaction (cf. Porges, 2009). In fact, sAA responses during conflict might represent not only negative aspects of arousal, but also task engagement and emotional involvement in a positive sense.
It might be hypothesized that decreased emotional arousal in women and increased emotional arousal along with more positive behavior in response to OT in men might help couples to overcome the above-described demand/withdraw patterns and possibly improve relationship satisfaction in the long term. However, in contrast to couples who seek treatment, a closer look at our study sample might lead this interpretation to be revised. It could be argued that OT increases emotional arousal and affiliative or genuine positive behavior toward the partner in men who associate their partner with positive memories and positive future expectations. In unhappy relationships, in contrast, emotional arousal might be coupled with considerable distress and hence may even be accompanied by contempt or aggressive behavior. These differential effects are beyond the scope of the present study, which included relatively happy couples who were not seeking treatment. However, they might help to refine our view of OT effects in couple interaction and thus be of utmost importance for couple therapy.
In sum, the results suggest sex-specific and more complex influences of OT on sympathetic and emotional reactivity in human pair-bonds than might be suggested by the neuropeptide’s advertisement and perception in the public domain.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
We thank Esther Goetz, MSc, Mirja Hemmi, MSc and Sabina Studhalter, MSc, for their excellent research assistance in conducting the study. We thank Sarah Mannion, MSc, for editing assistance. This work was supported by a Young Investigator Research Grant provided by the University of Zurich (No. 56233205) (to B.D.), by a Research Fellowship for Prospective Scientists provided by the Swiss National Science Foundation PBZH1-108392 (to B.D.), and by a grant from the Swiss National Science Foundation (PP001-114788) (to M.H.).
REFERENCES
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. Journal of Neuroscience. 2001;21:2546–52. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bodenmann G, Kaiser A, Hahlweg K, Fehm-Wolfsdorf G. Communication patterns during marital conflict: a cross-cultural replication. Personal Relationships. 1998;5:343–56. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5:514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe RM, Honk JV. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Frontiers in Neuroendocrinology. 2011;33:17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bourquard E, Bodenmann G, Perrez M. CAOS. Computer Aided Observation System. Fribourg: University of Fribourg; 1992–2005. [Google Scholar]
- Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proceedings of the National Academy of Sciences of the United States of America. 2012;109:959–64. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Heavey CL. Gender and social structure in the demand/withdraw pattern of marital conflict. Journal of Personality and Social Psychology. 1990;59:73–81. doi: 10.1037//0022-3514.59.1.73. [DOI] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychological Science. 2006;17:1032–9. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- de Bono M. Molecular approaches to aggregation behavior and social attachment. Journal of Neurobiology. 2003;54:78–92. doi: 10.1002/neu.10162. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, et al. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–74. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Bodenmann G, Gabriel B, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65:728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry. 2007;62:1187–90. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Erni K, Hebisch G, Nater U. Salivary alpha-amylase levels after yohimbine challenge in healthy men. Journal of Clinical Endocrinology and Metabolism. 2006;91:5130–3. doi: 10.1210/jc.2006-0461. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Taylor CB, Kraemer HC, Agras WS. High blood pressure and marital discord: not being nasty matters more than being nice. Health Psychology. 1991;10:155–63. doi: 10.1037//0278-6133.10.3.155. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering Statistics using SPSS. 3rd edn. London: Sage; 2009. [Google Scholar]
- Fischer-Shofty M, Levkovitz Y, Shamay-Tsoory SG. Oxytocin facilitates accurate perception of competition in men and kinship in women. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nsr100. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H, Breitenstein C, Birbaumer N, Fürst M. A psychophysiological analysis of spouse solicitousness towards pain behaviors, spouse interaction, and pain perception. Behavior Therapy. 1995;26:255–72. [Google Scholar]
- Gamer M, Buchel C. Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology. 2011;37:87–93. doi: 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Reviews. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Williams P. General Health Questionnaire (GHQ) Windsor: Nfer-Nelson; 1969. [Google Scholar]
- Gottman JM. What Predicts Divorce? The Relationship Between Marital Processes and Marital Outcomes. Hillsdale, NJ, England: Lawrence Erlbaum Associates Inc; 1994. [Google Scholar]
- Gottman JM, Krokoff LJ. Marital interaction and satisfaction: a longitudinal view. Journal of Consulting and Clinical Psychology. 1989;57:47–52. doi: 10.1037//0022-006x.57.1.47. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Anderson BJ, Girdler SS, Light KC. Warm partner contact is related to lower cardiovascular reactivity. Behavioral Medicine. 2003;29:123–30. doi: 10.1080/08964280309596065. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Pournajafi-Nazarloo H, Sanzenbacher L, et al. Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress. 2012;15:149–61. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–53. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlweg K. Fragebogen zur Partnerschaftsdiagnostik (FPD) [Marriage diagnostic questionnaire] Gottingen: Hogrefe: 1996. [Google Scholar]
- Hahlweg K, Jacobson NS. Marital Interaction: Analysis and Modification. New York: Guilford; 1984. [Google Scholar]
- Heavey CL, Christensen A, Malamuth NM. The longitudinal impact of demand and withdrawal during marital conflict. Journal of Consulting and Clinical Psychology. 1995;63:797–801. doi: 10.1037//0022-006x.63.5.797. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology. 2009;30:548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic Medicine. 2008;70:976–85. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Medicine. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke W, Hüppe M, Kallus W, Schmidt-Atzert L. Mood Assessment with Categories and Attributes [Befindlichkeitsskalierung anhand von Kategorien und Eigenschaftswörtern]. BSKE, BSKE-EA, BSKE-EAK. Wurzburg: Institut für Psychologie; 1988. [Google Scholar]
- Kaiser A, Hahlweg K, Fehm-Wolfsdorf G, Groth T. The efficacy of a compact psychoeducational group training program for married couples. Journal of Consulting and Clinical Psychology. 1998;66:753–60. doi: 10.1037//0022-006x.66.5.753. [DOI] [PubMed] [Google Scholar]
- Karelina K, DeVries AC. Modeling social influences on human health. Psychosomatic Medicine. 2011;73:67–74. doi: 10.1097/PSY.0b013e3182002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic Data Analysis. New York, NY: Guilford; 2006. [Google Scholar]
- Kiecolt-Glaser JK, Malarkey WB, Chee M, et al. Negative behavior during marital conflict is associated with immunological down-regulation. Psychosomatic Medicine. 1993;55:395–409. doi: 10.1097/00006842-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komplett-Video. (2006). Wissen auf Video, Teil 3: Der Gesang der Wale [Knowledge on Video, Sound of the Whales]. In: Planet Ozean [Planet Ocean] (Series). Grunwald, Germany: Verlag Komplett-Media.
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–76. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Malarkey WB, Kiecolt-Glaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosomatic Medicine. 1994;56:41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nature Neuroscience. 2012;15:681–8. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, et al. Stress-induced changes in human salivary alpha-amylase activity – associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–96. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nealey-Moore JB, Smith TW, Uchino BN, Hawkins MW, Olson-Cerny C. Cardiovascular reactivity during positive and negative marital interactions. Journal of Behavioral Medicine. 2007;30:505–19. doi: 10.1007/s10865-007-9124-5. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biological Psychology. 2011;86:174–80. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Hawkley L, Luhmann M, et al. Variation in the oxytocin receptor gene influences neurocardiac reactivity to social stress and HPA function: a population based study. Hormones and Behavior. 2012;61:134–9. doi: 10.1016/j.yhbeh.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine. 2009;76(Suppl. 2):S86–90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx CM, Helms HM, Buehler C. Marital quality and personal well-being. A meta-analysis. Journal of Marriage and Family. 2007;69:576–93. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon R, du Toit M. HLM 6: Hiarchical Linear and Nonlinear Modeling. Lincolnwood, IL: SSI Scientific Software International, Inc; 2004. [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2011;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: pathways to health. Physiology and Behavior. 2003;79:409–16. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz P, Schlotz W, Becker P. Das Trierer Inventar zur Erfassung von chronischem Stress - Version 2 (TICS 2) [Trier Inventory for the Assessment of Chronic Stress] Gottingen: Hogrefe: 2004. [Google Scholar]
- Szeto A, McCabe PM, Nation DA, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Thoits PA. Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior. 2011;52:145–61. doi: 10.1177/0022146510395592. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29:377–87. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Weiss RL, Heyman RE. Couple interaction. In: Halford WK, Markman HJ, editors. Clinical Handbook of Marriage and Couples Intervention. New York: Wiley; 1997. pp. 13–41. [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Hormones and Behavior. 2001;40:133–8. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]