Abstract
The ability to cognitively regulate emotional responses to aversive events is essential for mental and physical health. One prerequisite of successful emotion regulation is the awareness of emotional states, which in turn is associated with the awareness of bodily signals [interoceptive awareness (IA)]. This study investigated the neural dynamics of reappraisal of emotional responses in 28 participants who differed with respect to IA. Electroencephalography was used to characterize the time course of emotion regulation. We found that reappraisal was accompanied by reduced arousal and significant modulation of late neural responses. What is more, higher IA facilitated downregulation of affect and was associated with more pronounced modulation of underlying neural activity. Therefore, we conclude that IA not only advances the consolidation of somatic markers required for guiding individual behaviour but also creates processing advantages in tasks referring to these bodily markers.
Keywords: interoception, interoceptive awareness, embodied cognition, emotion regulation, reappraisal, evoked potentials
INTRODUCTION
Emotions are an essential part of our lives. However, due to social conventions or personal attitudes, we mostly attempt to regulate our emotions in some way: by denying, intensifying or even completely altering them. However, successful control of affect partly depends on the capacity to modulate emotional responses through the use of cognitive strategies (Gross and John, 2003; Goldin et al., 2008; Ochsner et al., 2004). There exist different strategies to regulate one’s emotions. Furthermore, individuals differ in their use of emotion regulation strategies with implications for their well-being and social functioning (Gross et al., 2003; Goldin et al., 2008). One important strategy is reappraisal, a re-interpretation of the meaning of a negative situation so that it no longer feels negative (Gross et al., 2003; Goldin et al., 2008). Gross and Thompson (2007) proposed a theory-driven process model that describes different strategies of emotion regulation which can be distinguished in terms of the time course of impact on the emotion-generative process. According to this model, cognitive reappraisal belongs to antecedent-focused strategies, which modulate emotional response tendencies early on, before they give rise to full-fledged responses (Gross et al., 2007). Reappraisal is a successful regulation strategy that pertains to positive emotion regulation in everyday life (Gross et al., 2003).
As emotion regulation is essentially associated with both attention to and awareness of one’s emotional state, it can be assumed that this process might also be linked to the awareness of one’s bodily state. Several theories of emotions (James, 1884; Damasio, 1994; Craig, 2004) propose such a close relationship between the perception of bodily changes (interoception) and emotional and cognitive processes. William James (James, 1884) was one of the first to present a psychological theory linking viscero-afferent feedback to emotional experience. He suggested that an emotional stimulus initiates specific visceral, vascular or somatic activities, such as changes of blood pressure and heart rate. Furthermore, he proposed that the perception of these bodily reactions may be the crucial component for mediating the emotional experience. Although the general association of physical changes and their perception as the basis of emotional experience is appealing, individuals differ substantially in their interoceptive awareness (IA). It can be hypothesized that a greater sensitivity for one’s bodily state will facilitate the regulation of emotional responses, as ongoing bodily changes can be detected more accurately, which might in turn create advantages in the discrimination and possible regulation of different emotional states. This assumption is in accordance to data from Feldman Barrett et al. (2001) who demonstrated that persons with highly differentiated emotion experience could better regulate their emotions.
A most common method to assess IA is the ability to perceive one’s heartbeats accurately (Critchley et al., 2004; Dunn et al., 2007). This ability can be quantified by using validated and reliable heartbeat perception tasks (Whitehead and Drescher, 1980; Schandry, 1981), in which participants are instructed to perceive their own heartbeats without feeling for their pulse. Today, there is evidence that primates represent homeostatic afferent activity that reflects the physiological condition of body tissues in ‘interoceptive centers’ in the brain, especially the insular and prefrontal and orbitofrontal cortices (Craig, 2003). Therefore, the awareness of emotional feelings is based on the neural representation of bodily cognitions, with ‘somatic markers’ evoking feeling states that influence cognition and behavior (Damasio, 1994, 1999). So these representations are one crucial prerequesite for emotional feelings. From this perspective, the foundation of an ‘awareness’ of and thus one prerequisite for emotional feelings lies in the neural representation of bodily conditions with somatic markers evoking feeling states that influence cognition and behavior (Damasio, 1994, 1999).
The idea that bodily states and their representations shape emotion, cognition and behavior is a core characteristic of many theories referring to the terms ‘embodiment’ or ‘embodied cognition’ (Barsalou, 2007; Niedenthal, 2007). Embodied cognition is based on two major assumptions: first, that higher cognitive processes operate on perceptual symbols. And second that concept use involves reactivations of the sensory-motor states that occur during experience with the world (Niedenthal et al., 2005; Barsalou, 2007). Barsalou (2007) argues that internal states and knowledge acquired from introspection are as important as external experience for affective and cognitive processes. Stressing the importance of embodiment for emotions, Niedenthal (2007) points out that embodied cognition also offers new ways to look at emotional processes. Following this, perceiving and thinking about emotion involve perceptual, somatovisceral and motoric re-experiencing of the relevant emotion in one’s self. This prediction corresponds with hypotheses derived from peripheral theories of emotions (James, 1884; Damasio, 1994; Craig, 2004) linking IA to differences in the processing of emotions.
Several studies demonstrated that higher IA was associated with more intense feelings and higher activation of underlying brain structures during emotional stimulation (Wiens, 2005; Pollatos et al., 2007; Dunn et al., 2010). What is more, IA was also associated with cognitive functions like decision making, selective attention or self-regulation during physical exercise (Pollatos et al., 2007; Dunn et al., 2010; Werner et al., 2009). Dunn et al. (2010) recently found that IA measured by heartbeat perception modulated the strength of the relationship between bodily reactions and cognitive-affective processing in an adaptive intuitive decision making task. These results highlight that interoception and awareness of interoceptive signals play a very important role beyond the field of emotions.
Taking these lines of evidence into account emotion regulation offers an optimal chance to assess both cognitive and emotional processes in relationship to IA by using an ecologically valid paradigm of high relevance for everyday functioning and mental health. As emotion regulation requires the awareness of one’s emotional and bodily state, it can be followed that a high awareness of interoceptive signals might facilitate emotion regulation by supporting the detection of early bodily reactions in response to emotional stimuli. Referring to the fact that different emotion regulation strategies exist, reappraisal is both a successful strategy and—importantly—it modulates emotional response tendencies early in the timeline, before they give rise to full-fledged responses (Gross et al., 2007). Therefore, IA probably shows the strongest association to this strategy of emotion regulation. To our knowledge, no former study investigated the interaction of IA on the effectiveness of reappraisal and underlying brain dynamics.
Recent imaging studies investigated the neural basis of the general reappraisal process (Goldin et al., 2008; McRae et al., 2010; Kanske et al., 2011). McRae et al. (2010) summarized that emotion regulation relies upon interactions between prefrontal regions, interpreted as implementing cognitive control, and ‘limbic regions’, such as the amygdala and the anterior cingulate cortex (ACC). The latter are assumed to mediate emotional responses. Although imaging approaches (e.g. fMRI, PET) allow identifying brain areas involved in emotional regulation with high spatial accuracy, they lack the adequate temporal resolution needed to investigate the time course of emotional regulation processes. The high temporal resolution or electroencephalography (EEG) allows determining the onset of (differences in) emotion regulation processes and as such allows for further insights into the time course of emotion processing and the regulation of emotions. This does not imply emotion regulation to start only at the time point of the onset of individual differences in the measured parameter (e.g. onset latency and amplitudes).
Such processes might start before the event-related brain potential (ERP) reveals significant differences. However, the use of ERPs provides the latest point in time where such individual differences are clearly observable in the electrocortical signature. Besides the topology of the measured signal, the topographic distribution provides tentative information about where such differences in the brain take place. Moser et al. (2009) assessed ERP under instructions to cognitively decrease and increase negative emotion using reappraisal techniques. The authors showed that reappraisal instructions modulated the amplitude in response to negative pictures starting at about 300 ms after picture onset.
On the basis of these results, it can be assumed that late ERP components like the P3 and the slow wave are modulated by emotion regulation. Both the P3 as well as the slow wave are associated with higher order aspects of cognitive information processing and appraisal (Hajcak and Olvet, 2008) and are strongly related to experienced intensity of feelings (Pollatos et al., 2007). Furthermore, recent studies using emotional pictures showed that IA modulated the P3 and slow wave amplitude in response to unpleasant content (Pollatos et al., 2007). Therefore, P3 and slow wave can be considered to be appropriate candidates for assessing the effects of IA on the time course of emotion regulation. Here, we investigated whether IA is associated with a greater ability to downregulate negative affect both on subjective as well as on levels of electrophysiological responses when emotion is regulated using a reappraisal strategy.
MATERIALS AND METHODS
Participants were screened for health status using an anamnestic questionnaire. They were only included if they did not have a history of any axis 1 disorders, in particular anxiety disorders or depression according to the Diagnostic and Statistical Manual of Mental Disorders. Drug use (except of contraceptives) was not allowed. All participants gave their written informed consent and received an amount of 30€ for their participation. In all, 28 participants (10 male; mean age 25.5 years, s.d. 3.2) were included in the main experiment. Experiments were conducted in accordance with the Declaration of Helsinki with the approval of the local ethics committee.
Stimuli
A total of 120 pictures (40 neutral, 80 unpleasant) from the International Affective Picture System (IAPS) served as emotional stimuli. The IAPS is a standardized and well-characterized collection of visual images designed to evoke neutral, positive or negative emotional states (Lang et al., 1999). Unpleasant pictures depict, e.g., pictures of violent death, aimed guns, snakes, angry or starving people, while neutral pictures contain, e.g., common household objects. Pictures in the IAPS vary with respect to two primary dimensions, affective valence and arousal.
Procedure
Upon arrival, participants received extensive instruction on cognitive reappraisal from a trained psychologist. Emotion regulation instructions for the current study were adapted from former work by Ochsner and colleagues (Moser et al., 2006). This protocol was also successfully used in other studies (e.g. Ochsner et al., 2004; Moser et al., 2006) and was found to be effective in modulating neural responses to unpleasant pictures. The training session included interactive instruction with the psychologist to ensure that subjects could reappraisethe pictures quickly and effectively as well as practice trials with sample aversive pictures (not shown during EEG). Participants were instructed not to look away or distract themselves. After a break, psychophysiological recording including ECG and EEG was prepared by attaching the EEG cap and non-polarizable Ag-AgCl electrodes to the right mid-clavicle and lower left rib cage. First, IA was assessed using four heartbeat counting phases (varying in length) in accordance with the Mental Tracking Method suggested by Schandry (1981). Participants were asked to count their own heartbeats silently and to verbally report the number of counted heartbeats at the end of the counting phase. The beginning and the end of the counting intervals were signaled acoustically. IA was estimated as the mean heartbeat perception score according to the following transformation:
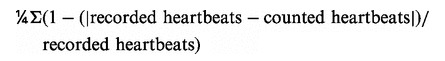 |
Subsequently, EEG recording started. Emotion regulation instructions for the current study were adapted from former work by Ochsner and colleagues, as they are effective in modulating neural responses to unpleasant pictures. The emotion regulation protocol employed three conditions, a negative maintain (NM), a negative reappraise (NR) and a neutral maintain (NeuM) condition. A block design with three picture presentation blocks consisting of 140 pictures of each condition randomized across subjects was used. A single trial always began with a fixation cross for 500 ms followed by a variable interstimulus interval of 250–500 ms before an IAPS slide was visible for 5 s. A varying time interval of 1.5–3 s was applied before the next trial started. At the end of each block, participants were asked to provide valence and arousal ratings on a nine-point scale using a non-verbal pictorial self-report, the Self Assessment Manikin (Bradley and Lang, 1994). Participants were asked to rate how pleasant vs unpleasant and how aroused vs calm they felt while watching the emotional pictures with scores ranging from 1 (very unpleasant or low arousing) to 9 (very pleasant or high arousing). In the negative reappraise condition they were also asked to rate how good they managed to downregulate negative affect during the ‘Reappraise’ trials on a nine-point rating scale.
EEG recording
EEG was recorded from 64 leads according to the 10% system with a DC amplifier (bandpass: 0.01–100 Hz; SYNAMPS, Neuroscan, Charlotte, NC, USA) and digitised at a sampling rate of 1000 Hz. Electrode positions were determined by an electrode cap (Falk Minow Services, Munich, Germany). The reference electrode was applied to scalp position Cz and the ground electrode placed on the left cheek. The EEG was re-referenced offline to linked mastoids. Horizontal and vertical eye movements were recorded with electrodes placed at the outer canthus of each eye (EOGH) and above and below the left eye (EOGV). Non-polarizable Ag-AgCl electrodes were used and electrode resistance was maintained below 8 KΩ.
EEG data reduction and data analyses
The EEG record was examined for EOG, muscle activity and other sources of electrophysiological artifacts. Blinks were corrected using the Gratton and Coles algorithm implemented in the analysis software (Vision Analyser, Brain Products, Munich, Germany). The EEG was filtered (bandpass 0.01–30 Hz) and averaged offline. EEG data were epoched with onset of the picture presentation for 1000 ms relative to a 200 ms pre-stimulus baseline. Mean amplitudes computed for the time window of the P3 (350–500 ms) and the slow wave (600–800 ms) at selected anterior, medial and posterior locations (FC1, FC2, FCZ, C3, C4, CZ, P1, P2, PZ, O1, O2, OZ) were examined using analyses of covariance (ANCOVAs) with three levels of ‘Condition’ and 12 levels of ‘Electrode’. IA was added as a covariate. In the ‘Results’ section, uncorrected F-values are reported together with the Greenhouse–Geiser epsilon values and corrected probability levels. In case of significant main or interaction effects of IA post hoc correlation analyses were performed between the IA score and subjective experience as well as mean P3/slow waves. Subjective valence and arousal were examined using ANCOVAs with three levels of Condition and IA as a covariate.
RESULTS
Experienced feelings during conditions
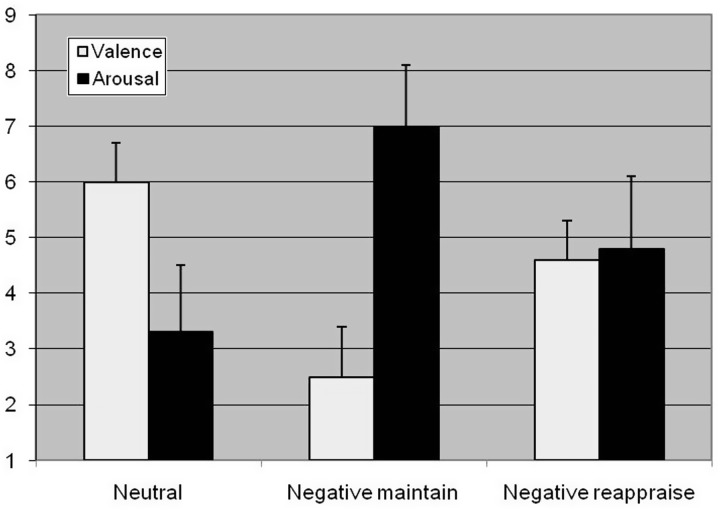
Valence and arousal ratings are depicted in Figure 1.
Fig. 1.
Mean pleasantness (1: highly unpleasant, 9: highly pleasant) and arousal (1: very calm, 9: highly aroused) scores contrasting neutral, negative maintain (NM) and negative reappraise (NR) conditions.
With respect to valence ratings (compare Figure 1), the statistical analysis revealed a highly significant effect for Condition (F(2,52) = 5.71; P < 0.01; η2 = 0.18; ε = 0.70). Valence during NM (mean 2.4) was significantly lower than during NR (mean 4.6) which was, in turn, significantly lower than NeuM (mean 6.0; Ps < 0.001).
Concerning arousal (compare Figure 1), a significant effect for Condition (F(2,84) = 4.56; P < 0.05; η2 = 0.15; ε = 1.00) occurred. Arousal during NM (mean 7.0) was significantly higher than during NR (mean 4.8), and arousal during NR (mean 4.8) appeared to be significantly higher than NeuM (mean 3.2).
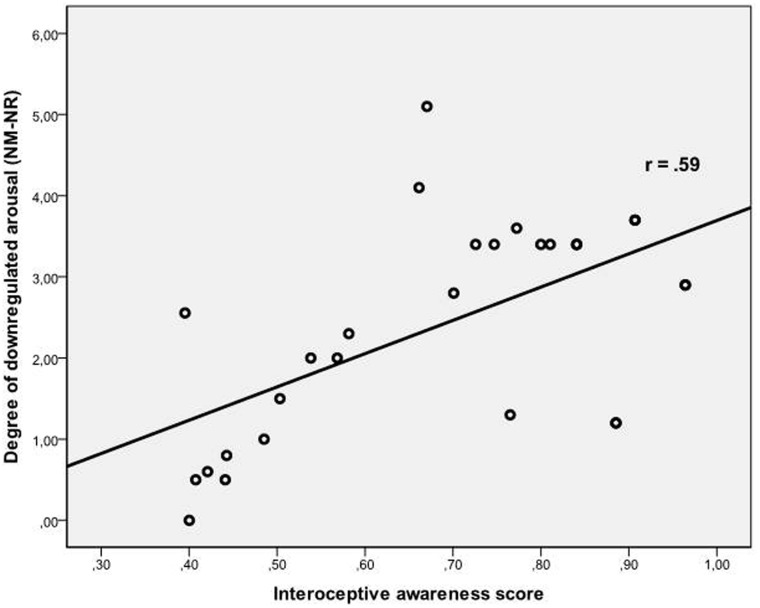
Additionally, a significant Condition × IA interaction (F(2,52) = 3.74; P < 0.05; η2 = 0.13; ε = 0.64) was observed (see Figure 2). Correlation analyses were performed between arousal scores (during NM, NR) and IA demonstrating that IA was positively correlated with arousal during NM (r = 0.38, P < 0.05) and negatively related to arousal during NR (r = −0.34, P < 0.05). We calculated a difference score (NM-NR) reflecting the perceived downregulation of arousal due to reappraisal which was highly significantly correlated to IA (r = 0.59, P < 0.01; compare Figure 2).
Fig. 2.
Scatter plot depicting degree of downregulated arousal and interoceptive awareness.
The perceived efficiency of downregulation by reappraisal on a scale ranging from 1 (no effect) to 9 (very good) was 6.8 (s.d. 1.4) and correlated positively with IA (r = 0.48, P < 0.01).
Visual evoked potential to conditions
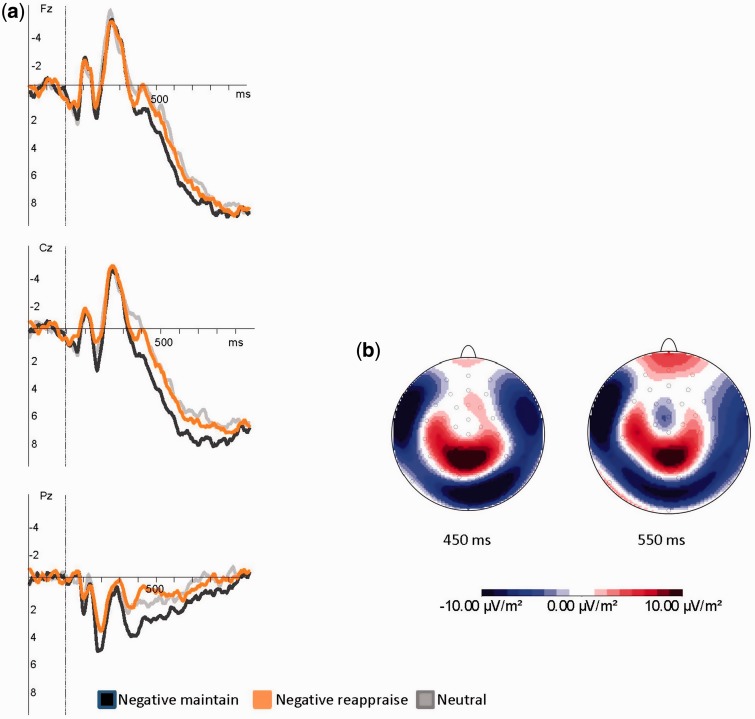
Figure 3 visualizes VEP midline electrodes, showing a clear modulation of the P3 and the slow wave during reappraisal when compared with negative maintain. Additionally, current source density maps of the difference potentials between conditions negative maintain minus negative reappraise were plotted. P3 and slow wave were examined in more detail.
Fig. 3.
(a) VEPs at midline electrodes contrasting neutral, negative maintain (NM) and negative reappraise (NR) conditions. (b) Current source density maps of the difference potentials (NM-NR).
P3
An ANOVA with P3 amplitudes as dependent measure revealed a significant main effect of Condition (F(2,52) = 4.82, P < 0.05; η2 = 0.14, ε = 0.76), indicating higher P3 amplitudes for the NM (mean 2.52 µV) when compared with both the NR condition (mean 0.79 µV; P < 0.01) and the NeuM condition (mean 0.75 µV; P < 0.01). The main effect Electrode (F(11,44) = 152.58, P < 0.001; η2 = 0.97, ε = 1.00) reflected most pronounced P3 amplitudes at parietal locations followed by central and frontal ones.
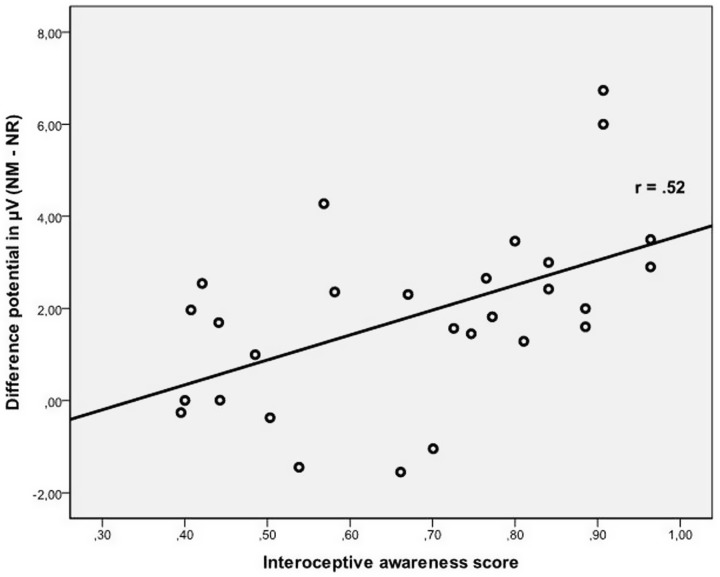
Besides, a significant main effect IA and a significant interaction IA × Condition (F(2,84) = 6.78, P < 0.01; η2 = 0.14, ε = 0.83) were observed. Post hoc correlation analyses between P3 amplitude (difference score between NM minus NR) and the IA score were performed. Due to multiple comparisons, all results were Bonferroni corrected. IA was significantly correlated to P3 amplitude during NM (r = 0.63, P <0.001) and, as a result of reappraisal, to the modulation of this component (difference potential, see Figure 4; r = 0.52, P < 0.01), while both correlations to NeuM (r = 0.34, P = n.s.) as well as NR (r = 21, P = n.s.) were not significant.
Fig. 4.
Scatter plot depicting modulation of P3 and interoceptive awareness.
Slow wave
We observed a significant main effect of Condition (F(2,50) = 4.29, P < 0.05; η2 = 0.15, ε = 0.71), with more positive going slow wave amplitudes for the NM (mean 3.29 µV) when compared with NR (mean 2.12 µV) and NeuM (mean 1.90 µV; Ps < 0.01) condition. NeuM and NR did not differ significantly. Additionally, a significant interaction IA × Condition (F(2,50) = 8.51, P < 0.01; η2 = 0.25, ε = 0.90) occurred.
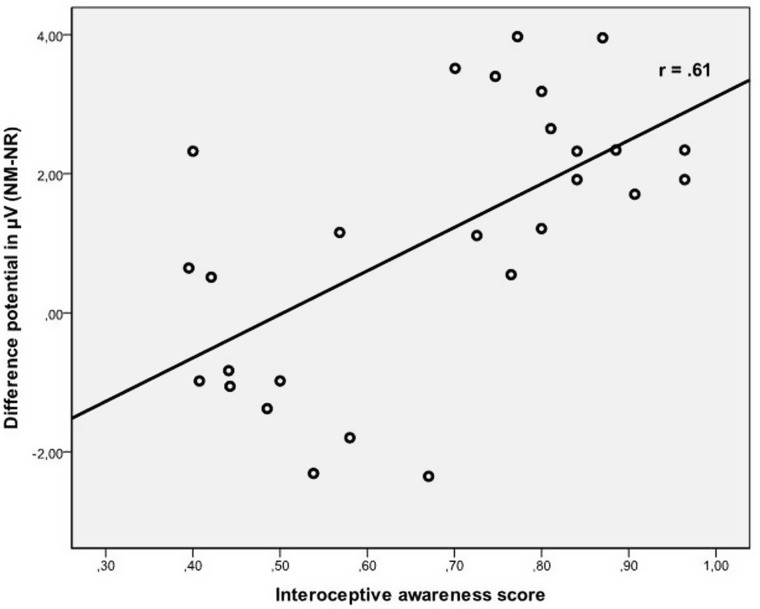
Next, post hoc correlation analyses were performed in the same manner as for the P3. IA was significantly correlated to mean slow wave amplitudes during NM (r = 0.33, P < 0.05) and to the modulation of this component due to reappraisal (difference potential, see Figure 5; r = 0.61, P < 0.01). Correlation coefficients to NeuM (r = 0.01, P = n.s.) as well as NR (r = −0.14, P = n.s.) were not significant.
Fig. 5.
Scatter plot depicting modulation of slow wave and interoceptive awareness.
DISCUSSION
Our data provide new electrophysiological evidence for the relevance of the perception of bodily signals (IA) for emotion regulation. When applying reappraisal as emotion regulation strategy, IA facilitated the downregulation of affect-related arousal. Reappraisal was accompanied by a significant reduction of P3 and slow wave amplitudes that correlated significantly with IA scores. These findings suggest that the more aware a person is of ongoing bodily processes, the more successful this person’s emotion regulation in response to negative affect will be.
It is important to stress that we selected reappraisal because this strategy is known to be successful and associated to psychological well-being and positive emotions in everyday life (Gross et al., 2003, 2007). Therefore, it can be followed that IA constitutes a protective factor for psychological well-being in everyday life as the ability to use a beneficial strategy like reappraisal is positively associated with interoceptive sensitivity. It is an open question whether the same effect is present when using another emotion regulation strategy like expressive suppression, directed toward inhibiting behaviors associated with emotional responding (e.g. facial expressions, verbal utterances, gestures (Gross et al., 2007; Goldin et al., 2008). Suppression is, by definition, implemented following emotion generation and can be described as response-focused strategy (Gross et al., 2007). It is associated with decreased expressive behavior, reduced emotion experience and increased sympathetic activation of the cardiovascular system (Gross, 2002; Gross and Levenson, 1993).
Referring back to the results of Feldman Barrett et al. (2001), the more emotionally differentiated participants were the more they reported a wide range of emotion regulation strategies used in daily life. It can be hypothesized that IA facilitates the efficiency of different emotion regulation strategies by providing a fine-tuned feedback of the actual emotional state across a variety of bodily variables. We therefore suggest that interoceptive sensitivity supports the effective downregulation of bodily arousal which might lead to lower ‘cost’ as measured by the degree of physical changes occurring during expressive suppression (see e.g. Gross et al., 1993). Although this hypothesis is intuitive, it has yet to be proven in further experiments comparing the use of different emotion regulation strategies. It can be followed that IA supports effective emotion regulation in daily social contacts which might be associated with a greater degree of positive emotions.
The important role of bodily signals and their perception is emphasized in several theories proposing the embodiment of emotional and cognitive processes (Damasio, 1994; Barsalou, 2007; Niedenthal, 2007). Our data support this view and substantially extend former research underscoring the relevance of IA for the intensity of subjective emotions, emotion processing and decision making (Pollatos et al., 2007; Werner et al., 2009; Dunn et al., 2010). Moreover, we have demonstrated for the first time that emotion regulation is also shaped by interindividual differences in the ability to perceive internal signals. This result is in accordance to former data by Herbert et al. (2007) showing that IA is accompanied by a more finely tuned self-regulation of physical load in situations demanding the control of physical effort. This study demonstrated that within a context where persons are free to decide on their physical, cardiovascular load in an exercise situation, those who show a greater interoceptive sensitivity toward their cardiac signals, as measured by the same heartbeat tracking test, spend less physical effort according to their better perception of bodily feedbacks coming from their cardiac system. These findings suggest that more exact perception of signals of one bodily system such as the cardiac system enhances the ability to better regulate behavior associated with activation within the same physiological bodily system, which is, e.g., the case during physical exercise.
Additionally, recent results (Herbert et al., 2012) demonstrated that cardiac interoceptive sensitivity is positively related to the perception of other bodily signals, like felt hunger. This was proven in a situation using short-term food deprivation that was accompanied by physiological changes in different bodily systems producing somatic signals (cardiovascular and gastric feelings related to hunger). This suggests that mechanisms of interoception are associated across bodily systems in specific situations evoking reactivity in these different bodily systems. Hence, with regards to our data, it is to suggest that interoceptive sensitivity for bodily changes in one physiological system (such as the cardiac system) relevantly mediates emotion regulation in situations evoking physiological activity in these bodily systems which also can generalize to other interoceptive signals (such as, e.g., feelings of hunger). As a consequence, being aware of one’s bodily signals might therefore constitute a positive precondition for effective self-regulation of behavior.
It is interesting to note that IA correlated with P3 and slow wave in response to negative maintain condition which is in accordance to former studies using emotional material (Pollatos et al., 2005b). We did not observe a significant correlation to the negative reappraise condition, though, while the difference potentials between both negative conditions correlated with IA. One reason could be that IA is associated primarily with the modulation of activation during reappraisal and does not interact with the absolute activation during emotion regulation. It is possible that perceiving internal signals more precisely facilitates processes of comparisons of different internal states related to emotions and their regulation rather than the degree of arousal during reappraisal alone. Referring to this point, IA was related to higher subjective arousal during negative picture presentation as demonstrated in former studies (Herbert et al., 2007; Pollatos et al., 2007). The observed greater reduction of arousal during reappraisal is therefore in part due to the fact that participants with higher degrees of IA also started with more intense emotions.
Neuroanatomic evidence highlights the relevance of an ‘interoceptive neural network’ in the brain comprising the somatosensory and somatomotor cortices, the insular cortex, the ACC and prefrontal cortices (ventromedial prefrontal cortex, dorsolateral prefrontal cortex) (Critchley et al., 2004; Pollatos et al., 2005a; Gray et al., 2007; Pollatos et al., 2007; Craig, 2009). Within this network, the insular cortex and the ACC play crucial roles connecting interoceptive processes and emotions (Craig, 2002; Critchley et al., 2003). Their activation was found to be modulated by cognitive (Bush et al., 2000) and emotional factors in several studies (Bush et al., 2000; Critchley et al., 2000).
There is ample evidence that IA is positively correlated with the activation of the insula (Critchley et al., 2004; Pollatos et al., 2005a; Pollatos et al., 2007) and the ACC (Pollatos et al., 2007). What is more, both EEG components that were modulated by reappraisal—the P3 and the subsequent slow wave—have been shown to be associated with IA and underlying brain activity in insula and ACC (Pollatos et al., 2007). Furthermore, Critchley et al. (2004) reported a significant positive correlation between IA and volume of the insula, suggesting structural differences in the volume of this structure in relation to IA. As both the insula and the ACC are involved in reappraisal (e.g. Goldin et al., 2008; Kanske et al., 2011), it is very probable that these structures serve as interfaces between interoception and emotion regulation. It can be assumed that being aware of one’s bodily signals, which is associated with greater activity in insula and ACC (Critchley et al., 2001; Pollatos et al., 2007), facilitates the downregulation of bodily sensations provoked by negative emotional stimuli.
Support for this interpretation comes from Critchley et al. (2003). They demonstrated that functional activation of the ACC mediates context-driven modulation of bodily arousal states during effortful cognitive tests. According to the somatic marker theory of Damasio (Damasio, 1999; Damasio et al., 2000; Bechara and Naqvi, 2004), insula and somatosensory cortices can be classified as first-order representation areas involved in mapping ongoing bodily changes, while ACC and prefrontal cortices are second-order structures. They are assumed to re-map ongoing changes and connect them to activity in so-called emotional trigger sites like the amygdale, thereby creating a conscious state of feeling. A higher degree of IA might advance the consolidation of somatic markers required for guiding individual behaviour and might create a processing advantage in tasks referring to these bodily markers and their representation in first- and second-order structures (compare (Damasio, 1994; Damasio et al., 2000).
We conclude that the perception of bodily states and their meta-representation as somatic markers are an essential prerequisite for adequate emotion regulation. Functional or structural deviations within the interoceptive neural networks might contribute to the observed effects and so connect the ‘body in the mind’ with basic mechanisms of the embodiment of affective and cognitive functions. Our study provides new evidence supporting the embodied mind approach to which mind, cognition and affect emerge from the reciprocal interactions of brain, body and world. Yet we extend the idea that perceiving and thinking about emotion involves somatovisceral re-experiencing of the relevant emotion in one’s self (Niedenthal, 2007). Perceiving one’s body and bodily signals also enables us more effectively to alter our emotions. We stress the notion that interoceptive processes and interindividual differences in IA provide powerful tools to characterize the access that we have to our body. Focusing on interoceptive processes emphasizes the affective dimension of the embodied mind and allows exploring the relationship between embodiment, affectivity and emotion regulation in more detail.
FUNDING
No national or international funds were used for this study.
ACKNOWLEDGEMENTS
We want to thank Julia Schneider for her help in manuscript editing. We also want to thank anonymous reviewers and Prof. Dr. Kevin Ochsner for their helpful comments which significantly improved the manuscript.
REFERENCES
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2007;59:617–45. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Bechara A, Naqvi N. Listening to your heart: interoceptive awareness as a gateway to feeling. Nature Neuroscience. 2004;7:102–103. doi: 10.1038/nn0204-102. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotions: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:45–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8:239–41. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel [mdash] now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nature Neuroscience. 2001;4:207–12. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason and the Human Brain. New York: Grosset/Putman; 1994. [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. Heartbeat perception in depression. Behaviour Research and Therapy. 2007;45:1921–30. doi: 10.1016/j.brat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, et al. Listening to your heart: how interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010;21:1835–44. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Feldman Barrett L, Gross J, Christensen TC, Benvenuto M. Knowing what your're feeling and knowing what to do about it: mapping the relation between emotion differntiation and emotion regulation. Cognition and Emotion. 2001;15:713–24. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Taggart P, Sutton PM, et al. A cortical potential reflecting cardiac function. Proceedings of the National Academy of Sciences. 2007;104:6818–23. doi: 10.1073/pnas.0609509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–86. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guliford; 2007. pp. 3–24. [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion. 2008;8:250–5. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Herbert C, Pollatos O, et al. Effects of short-term food deprivation on interoceptive awareness, feelings and autonomic cardiac activity. Biological Psychology. 2012;89:71–9. doi: 10.1016/j.biopsycho.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O, Schandry R. Interoceptive sensitivity and emotion processing: an EEG study. International Journal of Psychophysiology. 2007a;65:214–27. doi: 10.1016/j.ijpsycho.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Ulbrich P, Schandry R. Interoceptive sensitivity and physical effort: Implications for the self-control of physical load in everyday life. Psychophysiology. 2007b;44:194–202. doi: 10.1111/j.1469-8986.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21:1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings. Technical Report A-4, Center for Research in Psychophysiology. Gainesville/Florida: University of Florida; 1999. [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The Neural Bases of Distraction and Reappraisal. Journal of Cognitive Neuroscience. 2010;22:248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–6. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Krompinger JW, Dietz J, Simons RF. Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology. 2009;46:17–27. doi: 10.1111/j.1469-8986.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Barsalou LW, Winkielman P, Krauth-Gruber S, Ric F. Embodiment in attitudes, social perception, and emotion. Personality and Social Psychology Review. 2005;9:184–211. doi: 10.1207/s15327957pspr0903_1. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007a;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Herbert BM, Kaufmann C, Auer DP, Schandry R. Interoceptive awareness, anxiety and cardiovascular reactivity to isometric exercise. International Journal of Psychophysiology. 2007b;65:167–73. doi: 10.1016/j.ijpsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Human Brain Mapping. 2005a;26:54–64. doi: 10.1002/hbm.20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. On the relationship between interoceptive awareness, emotional experience, and brain processes. Cognitive Brain Research. 2005b;25:948–62. doi: 10.1016/j.cogbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007c;1141:178–87. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–8. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Werner NS, Jung K, Duschek S, Schandry R. Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology. 2009;46:1123–9. doi: 10.1111/j.1469-8986.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM. Perception of gastric contractions and self-control of gastric motility. Psychophysiology. 1980;17:552–8. doi: 10.1111/j.1469-8986.1980.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinion in Neurology. 2005;18:442–7. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]