Abstract
It is a unique human ability to regulate negative thoughts and feelings. Two well-investigated emotion-regulation strategies (ERSs), cognitive reappraisal and expressive suppression, are associated with overlapping prefrontal neural correlates, but differ temporally during the emotion-generation process. Although functional imaging studies have mainly investigated these ERS as a reaction to an emotion-inducing event, the intention to regulate upcoming negative emotions might already be associated with differences in neural activity. Hence, event-related functional magnetic resonance imaging was recorded in 42 participants while they completed an emotion-regulation paradigm. During this task, participants were instructed to proactively prepare to use a specific ERS knowing that a negative, high-arousing image would appear after the preparation period. As expected, the results demonstrated prefrontal and parietal activation while participants were suppressing or reappraising their emotions (family-wise error (FWE)-corrected). The intention to suppress emotions was associated with increased activation in the right inferior frontal gyrus, bilateral putamen, pre-supplementary motor area and right supramarginal gyrus (FWE-corrected). This enhanced proactive inhibitory control: (i) predicted decreased motoric activity during the actual suppression of emotional expressions and (2) trended toward a significant association with how successfully participants suppressed their emotions. However, neural correlates of preparatory control for cognitive reappraisal were not observed, possibly because contextual cues about the upcoming emotional stimulus are necessary to proactively start to cognitively reinterpret the situation.
Keywords: emotion regulation, cognitive reappraisal, expressive suppression, proactive and reactive control
It is a unique human ability to regulate emotions to influence which emotions we have, when we have them and how we experience and express them (Gross, 1998, pp. 224). Individuals might try to avoid behaviorally expressing their emotions or showing their sad feelings. This phenomenon is called expressive suppression (i.e. suppression) and refers to a so-called ‘poker face’. It is a behavioral strategy to regulate emotional responses after they have arisen. Another emotion-regulation strategy (ERS) is cognitive reappraisal (i.e. reappraisal), which involves a means of changing the way one thinks about an emotion-eliciting situation to reduce its emotional impact.
People commonly use these ERS to control negative feelings in response to life stress (Morris and Reilly, 1987). Habitual use of reappraisal, relative to suppression, to down-regulate negative feelings is associated with more positive feelings and psychological well-being (Gross and John, 2003). This is because reappraisal is an ‘antecedent-focused’ strategy that intervenes early in the process underlying the generation of (negative) emotions (Ochsner and Gross, 2005). As a result, this cognitive strategy makes it possible to down-regulate negative emotions by manipulating the input of the system and requires no sustained effort over time (Gross, 1998). Suppression, on the other hand, is a ‘response-focused’ strategy that simply changes the product (i.e. behavioral expression and verbal utterances) of the emotion-generation process, and habitual use is associated with increased depressive symptoms (Gross and John, 2003).
Besides the effects of habitual use of ERS on emotional well-being, adequate and efficient regulation of emotions is crucial for adaptive social interactions (Vingerhoets et al., 2008). Indeed, the acute usefulness of reappraisal and suppression largely depends on the immediate context and/or upcoming stimulus. For example, based on the social consequences, it might be important to behaviorally inhibit the expression of emotions (Gross, 1998). During the past decades, there has been a wealth of experimental research looking at the processes underlying, and effects of, the trial-by-trial use of reappraisal and suppression. In a standard experimental design, participants are shown aversive pictures from the International Affective Picture System (IAPS; Lang et al., 1997) that typically generate negative emotion. Functional imaging studies consistently found that reappraisal of emotions induced by these pictures activated the prefrontal cortex (PFC; e.g. increased dorsal PFC, ventrolateral PFC, posterior portion of the dorsomedial PFC/superior frontal gyrus and dorsal anterior cingulate cortex), associated with cognitive control, and deactivated the amygdala and/or insula (for a meta-analysis, see Kalisch, 2009). As compared with reappraisal, imaging studies on expressive suppression are scarce. A recent study investigated the regulation of facial expressiveness to acute pain (Kunz et al., 2011). These authors observed that suppression of pain expression was related to frontostriatal and motor neural activity. In another study, Lee et al. (2008) observed activities in the inferior frontal gyrus (IFG), insula and motor face areas when responding with the opposite facial emotional expression that was presented to the participant.
To date, only one functional magnetic resonance imaging (fMRI) study of Goldin et al. (2008) compared expressive suppression and cognitive reappraisal while female participants were watching negative, emotion-eliciting film fragments. They observed that both strategies elicited overlapping medial/inferior prefrontal brain activity as well as amygdala/insula activation. These authors also demonstrated that expressive suppression resulted in additional activation of the right ventrolateral PFC, a region related to inhibitory motor control (Garavan et al., 2006). Most importantly, results from Goldin et al. (2008) were in accordance with the theoretical framework that delineates antecedent-focused and response-focused ERS (Ochsner and Gross, 2005). These authors observed that reappraisal produced a rapid cognitive regulation-related medial/inferior prefrontal activation, and suppression resulted in a delayed component of medial/inferior prefrontal activation related to volitional motor inhibition as well as sustained amygdala activation.
An important observation is that most functional imaging studies to date have investigated the reactive aspects of emotion regulation (i.e. examining suppression and reappraisal subsequent to the presentation of the aversive picture or emotional face). An interesting question remains regarding the neural correlates when a negative event is anticipated and individuals are proactively preparing to use a specific ERS when confronted with this stimulus. This is because in everyday life, individuals often know that an upcoming event will elicit negative emotions, but they have the intention to control these expected distressing feelings. This proactive preparation is based on the intention to have control over predictable negative emotions and might influence the experience and expression of these emotions (Gross, 1998). For example, for the funeral of a close friend, what are the neural correlates supporting preparatory control associated with the intention to reappraise or suppress negative emotions? In other words, even though an individual does not know exactly what will happen during the upcoming funeral, one foresees that negative emotions will be elicited and intends to control these emotions in a specific way. Although this proactive aspect of behavior is largely underexplored (Aron, 2011), individuals might benefit from this anticipatory, control-supporting ERS to flexibly adapt to changing environmental situations and social demands. This anticipatory control could have an influence on the actual processing of negative material, for example, by interpreting the event as less threatening or inhibiting facial expressions more strongly.
Altogether, the primary goal of this study was to explore neural correlates of preparatory control associated with the purpose to suppress or reappraise upcoming negative emotions. Hence, a standard emotion-regulation paradigm was modified in a way that allowed participants time to anticipate a specific ERS. Imaging data were analyzed following the presentation of the preparatory cue and during the presentation of the emotional target. Based on prior research, the neural correlates during the emotion-regulation performance were hypothesized to be of prefrontal location (e.g. dorsal PFC, ventrolateral PFC, dorsomedial PFC and dorsal anterior cingulate cortex; Beauregard et al., 2001; Ochsner et al., 2004; Goldin et al., 2008) as well as the amygdala, which is responsible for emotion processing and has been implicated repeatedly in neuroimaging studies on emotion regulation (e.g. Urry et al., 2006). However, motor inhibitory areas could be expected as a possible target of expressive suppression in particular (Goldin et al., 2008; Lee et al., 2008; Kunz et al., 2011). Based on the temporal differences in medial/inferior prefrontal activation between reappraisal and suppression (Goldin et al., 2008), one might deduce that neural correlates of control might be most pronounced during the proactive anticipation period for reappraisal. This is because reappraisal has its impact early in the emotion-generation process (i.e. in the beginning of the generation of the emotion) by manipulating the input of the system (Gross, 1998). On the other hand, we expected neural correlates of control for suppression to only appear during the presentation of the aversive image because this latter ERS has its impact relatively late in the emotion-generation process (i.e. at the end of the generation of the emotion, right before the emotional response).
METHODS
Participants
Postings on the university website were used to recruit a group of 42 female participants with a mean age of 21.26 (s.d. = 2.29, [18–31]) years and without medical conditions that might influence the interpretation of brain functioning related to emotion and cognitive processing. Individuals with psychiatric disorders (and/or a history of) were excluded using the structured Mini-International Neuropsychiatric Interview (based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria: M.I.N.I.; Sheehan et al., 1997). All participants were right-handed, had normal or corrected-normal vision and were eligible for fMRI research.
Procedure
The study protocol was approved by the local Medical Ethics Committee of Ghent University Hospital. All participants were initially screened for inclusion/exclusion criteria (see participants’ characteristics) and gave written informed consent prior to the study. Subsequently, the emotion-regulation paradigm was administered in the fMRI scanner. Finally, after completing the paradigm, all participants rated the experimental IAPS pictures for valence and arousal. The entire experiment lasted approximately 2 h, for which participants received financial compensation.
Emotion-regulation paradigm
The stimuli set consisted of 63 negative pictures of the IAPS (Lang et al., 1997) with mean arousal values >6 and mean valence ratings <4, based on the normative ratings of female participants. Each image was shown only once for a given participant.
Participants were instructed to ‘suppress’, ‘reappraise’ or ‘appraise’ their emotions in response to aversive images. To standardize how participants applied the different instruction, they were intensely trained for ±20 min beforehand. The number of practice trials differed between participants and was dependent on when they achieved a minimum level of understanding or proficiency in suppression, reappraisal and appraisal (the same person trained all the participants). For the ‘suppression’ instruction, participants were trained to suppress displaying their feelings elicited by the picture. They were told that it was important that people in their environment would not be able to see what they were feeling (e.g. Gross and Levenson, 1997, pp. 97). For the ‘reappraisal’ instruction, participants were trained to reinterpret the situational event depicted by changing the emotions, actions and outcomes of individuals depicted in that situational context (e.g. Ochsner et al., 2002). Participants were trained to use a cognitive strategy to generate an alternative interpretation about each picture that would explain apparently negative events in a less negative way (e.g. women depicted crying outside of a church could be described as attending a wedding instead of a funeral; Ochsner et al., 2002, pp. 1225). The ultimate goal of this cognitive strategy was to down-regulate negative feelings elicited by the image and decrease emotional reactivity. For the ‘appraise’ instruction, participants were trained to simply look at the pictures, feel their natural feelings and not change anything.
Participants were trained in not only how to suppress, reappraise or appraise their feelings elicited by the negative and distressing image but also to enhance preparatory control supporting the strategy that would be employed. For reappraisal, participants were instructed to imagine that although the upcoming IAPS picture is unknown, they would be able to control their feelings elicited by this image. With this cognitive strategy, they prepared themselves to down-regulate their emotions, which gave them a feeling of control over an upcoming distressing image. For the suppression instruction, participants were asked to prepare not to show their feelings on an outward level, without holding their face already still beforehand. For the anticipation of reappraisal and suppression, we asked participants to think that they would be able to down-regulate or inhibit their negative feelings, respectively. This mindset of control was taught during the training block before the scanning procedure. We also instructed participants that anticipatory processes would help them with the actual ERS, making it worthwhile for them to anticipate both expressive suppression and cognitive reappraisal. With this preparatory control, we attempted to interfere in the emotion-generation process as early as possible. For the appraisal instruction, participants were instructed to do nothing specifically (no preparatory control), but wait until the picture was shown, knowing that they could react normally to the image.
For all the pictures, participants were told not to look away and to concentrate on the picture during the time it was projected. During this practice phase, participants first received a couple of examples to illustrate the reappraisal instruction (e.g. how to generate reinterpretations for several other sample pictures). Subsequently, participants were asked to verbally state what they were thinking during the preparation (cue) and picture (target) phase. This way, we were able to standardize the preparation and actual target phase over all participants. Moreover, participants were falsely instructed that facial movements would be recorded in the scanner to evaluate the feelings displayed. During the debriefing at the end of the study, all participants reported that they believed that a special camera had recorded their facial expressions/feelings and the social desirability response was low.
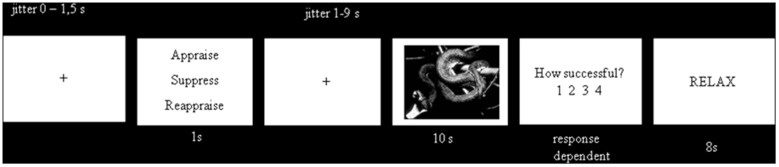
During the emotion-regulation paradigm (Figure 1), participants were cued to suppress, reappraise and appraise a series of 63 randomly intermixed trials in three blocks. The order of the instructions was semi-randomized (with three different instruction sequences), but with the same number of each instruction per block (21 trials in each block, a total of 7 trials per cue). Moreover, we controlled for the same number of repetitions between the different instructions across the whole task (no more than two repetitions/instructions). Each trial started with a fixation cross (0–1.5 s, jitter in steps of 500 ms) followed by a cue word (suppress, reappraise or appraisal). This cue word appeared centrally on the screen for 1 s, after which a blank screen was presented (1–9 s, jitter in steps of 500 ms and mean duration of 4.5 ms). This cue-offset time enabled participants to prepare for the instructed ERS. Subsequently, a negative, high-arousing image appeared centrally for 10 s. Although the image remained on the screen, participants performed the emotion regulation or appraisal specified by the prior instructional cue. Then, a rating scale appeared immediately after presentation of the photo. In a standard emotion-regulation study, participants provide a rating of their current negative affect immediately after the presentation of the image or movie (e.g. Ochsner et al., 2004), and emotion regulation success is indexed by self-reports of mood. In this study, however, we wanted to specifically evaluate how well participants applied a specific emotion-regulation/generation strategy to control or feel their negative affect, respectively. This way, we wanted to take individual differences in valence and arousal associated with each emotional picture into account. This need for online performance measures to control for adequate engagement with the task has already been highlighted by Carter (2009). Therefore, in this study, a Likert scale allowed participants to rate how successful they were in regulating or appraising their negative emotions (1 = not at all to 4 = very good). Successful cognitive reappraisal implies that the participant was able to down-regulate negative feelings, whereas successful expressive suppression implies that the participant was able to not show his/her feelings on an outward level. All together, this success rating resulted in an evaluation of ‘relative negative affect’ based on the application of an explicit emotion regulation/generation strategy.
Fig. 1.
Timeline of the event-related emotion regulation task.
Finally, the word ‘RELAX’ appeared on the screen for 8 s so that participants knew they could relax until the presentation of the next trial.
At the end of the study (after the emotion-regulation paradigm), each participant rated all the pictures presented. They were asked to indicate how they felt while looking at the pictures using 9-point Likert scales for mood (1 = unhappy, 5 = neutral and 9 = happy) and arousal (1 = calm, 5 = intermediate and 9 = excited).
Scanning procedure
Images were collected with a 3T Magnetom Trio MRI scanner system (Siemens Medical Systems, Erlangen, Germany) using an eight-channel radiofrequency head coil. First, high-resolution anatomical images were acquired using a T1-weighted 3D Magnetization Prepared RApid Gradient Echo (3D MPRAGE) sequence (repetition time (TR) = 2530 ms, echo time (TE) = 2.58 ms, inversion time (TI) = 1100 ms, acquisition matrix = 256 × 256 × 176, sagittal field of view (FOV) = 220 mm, flip angle = 7°, voxel size = 0.86 × 0.86 × 0.9 mm3). Whole-brain functional images were collected using a T2*-weighted echo planar imaging (EPI) sequence sensitive to BOLD contrast (TR = 2000 ms, TE = 35 ms, image matrix = 64 × 64, FOV = 224 mm, flip angle = 80°, slice thickness = 3.0 mm, distance factor = 17%, voxel size 3.5 × 3.5 × 3 mm3 and 30 axial slices).
fMRI data pre-processing and general linear model analysis
The fMRI data were analyzed with statistical parametric mapping using SPM5 software (Wellcome Department of Cognitive Neurology, London, UK). The first four volumes of all EPI series were excluded from the analysis to allow the magnetization to approach a dynamic equilibrium. Data processing started with slice-time correction and realignment of the EPI datasets. A mean image for all EPI volumes was created, to which individual volumes were spatially realigned by rigid body transformations. The high-resolution structural image was co-registered with the mean image of the EPI series. Then the structural image was normalized to the Montreal Neurological Institute (MNI) template, and the normalization parameters were applied to the EPI images to ensure an anatomically informed normalization. During normalization, the anatomy image volumes were re-sampled to 1 × 1 × 1 mm3. A filter of 8 mm full-width at half maximum was used. Low-frequency drifts in the time domain were removed by modeling the time series for each voxel by a set of discrete cosine functions to which a cut-off of 128 s was applied. The subject-level statistical analyses were performed using the general linear model. The model contained separate regressors for the Cue Suppression, Reappraisal and Appraise cue (1–9 s) and the Suppression, Reappraisal and Appraise Target phase (e.g. implementation of the cue, duration of 10 s) and the response to the rating screen. The cue was modeled as an event with duration 0. The jitter was not separately modeled. Movement parameters were included to account for variance associated with head motion. All resulting vectors were convolved with the canonical hemodynamic response function and its temporal derivative to form the main regressors in the design matrix (the regression model).
The statistical parameter estimates were computed separately for each voxel for all columns in the design matrix. Contrast images were constructed for each individual to compare the relevant parameter estimates for the regressors containing the canonical hemodynamic response function. Next, a group-level random effects analysis was performed. One-sample t-test was performed for each voxel of the contrasts Cue Suppression vs Cue Reappraisal, Cue Suppression vs Cue Appraisal and Cue Reappraisal vs Cue Appraisal. For the target, we contrasted Target Suppression vs Target Appraisal, Target Reappraisal vs Target Appraisal and finally, Target Reappraisal vs Target Suppression. The resulting statistical values were thresholded with a level of significance of P < 0.05, corrected for family-wise error (FWE, whole-brain analysis) with a cluster extent >5. Based on our a priori hypothesis on amygdala activation during ERS, we created masks by means of the Wake Forest University (WFU) PickAtlas (Maldjian et al., 2003). Finally, given the effects on motoric inhibition during the preparation phase for suppression (see Results, Cue Suppression > Cue Reappraisal), we investigated its relation to facial motor activity during the actual expressive suppression of negative emotion (as reflected in activation in the precentral gyrus during Target Suppression > Target Reappraisal). Therefore, the precentral gyrus region of interest (ROI) during Target Suppression was anatomically defined by means of the WFU PickAtlas and comprised the entire precentral gyrus.
The resulting maps were overlaid onto a normalized T1-weighted MNI template (colin27) and the coordinates reported correspond to the MNI coordinate system. To extract beta values for each participant for region and condition, we used Marsbar (http://marsbar.sourceforge.net/, Brett et al., 2002).
RESULTS
fMRI data
Target emotion regulation
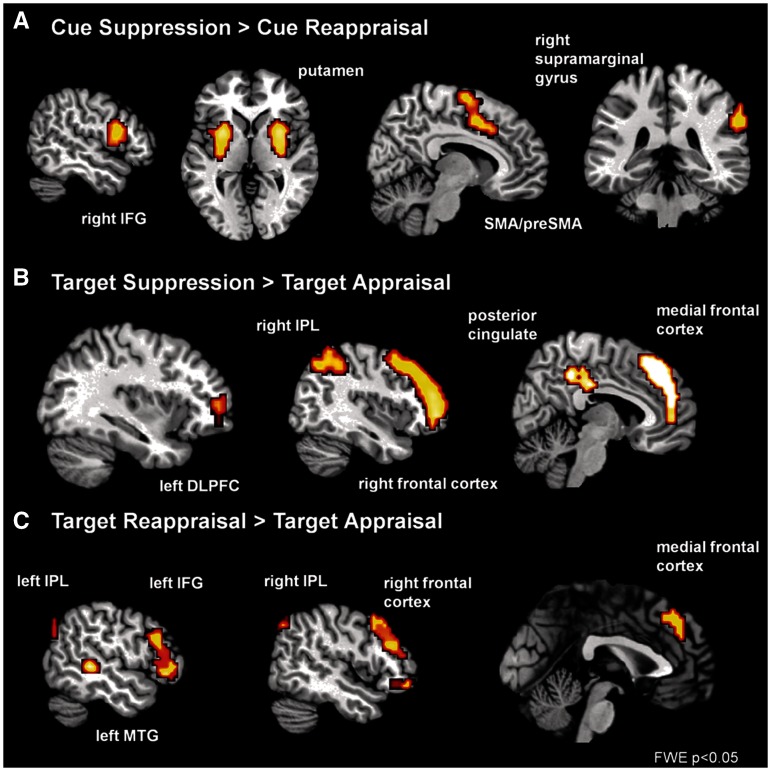
To explore brain activity during the expressive suppression itself, we compared activity for expressive suppression with the control condition appraisal (Target Suppression > Target Appraise). We found activity in the right frontal cortex, right inferior parietal lobe, lower precuneus and left dorsolateral PFC (Table 1; Figure 2). To explore brain activity during the reappraisal of emotions, we compared activity for cognitive reappraisal with appraisal (Target Reappraisal > Target Appraise). We found activity in the left and right middle temporal gyrus, left and right IFG, middle frontal gyrus and right inferior parietal lobe (Table 1; Figure 2). Finally, we compared brain activity during the expressive suppression with cognitive reappraisal (Target Suppression > Target Reappraisal). We found activity in the right IFG, right dorsolateral PFC, posterior cingulate, left superior temporal gyrus and left inferior parietal lobe (Table 3). For the contrast Target Reappraisal > Target Suppression, we observed motor area, visual cortex, middle temporal gyrus, calcarine and angular gyrus activation (Table 2).
Table 1.
Areas showing significant activation during the target-phase within the contrasts Target Suppression > Target Appraise and Target Reappraisal > Target Appraise (FWE, P < 0.05)
| Area | BA | Peak coordinates (MNI) | Z-score | Voxel extent |
|---|---|---|---|---|
| Target Suppression > Target Appraise | ||||
| Right frontal cortex | 44, 45, 46, 8, 9, 6 | 42, 49, –4 | 7.11 | 1085 |
| Right inferior parietal lobe | 39, 40, 7 | 56, –49, 39 | 6.78 | 335 |
| Lower precuneus | 23 | 7, –42, 42 | 5.32 | 45 |
| Left dorsolateral prefrontal cortex | 46, 10 | –39, 49, –7 | 5.14 | 30 |
| Target Reappraisal > Target Appraise | ||||
| Left middle temporal gyrus | –53, –39, 0 | 6.28 | 54 | |
| Right middle frontal gyrus | 8, 9 | 25, 25, 60 | 6.17 | 91 |
| Left inferior frontal gyrus | 44, 45, 46 | –53, 32, –7 | 5.77 | 96 |
| Right inferior frontal gyrus | 44, 45, 46 | 53, 28, 28 | 5.71 | 129 |
| Middle frontal gyrus | 6, 32 | –7, 14, 60 | 5.59 | 128 |
| Right inferior frontal gyrus | 47 | 49, 45, –14 | 5.32 | 23 |
| Right inferior parietal lobe | 39 | 39, –70, 49 | 5.28 | 43 |
Fig. 2.
Brain activity of the contrast Cue Suppression > Cue Reappraisal (FWE, P < 0.05) mapped onto a T1-weighted MNI single-subject template (colin27). IFG = inferior frontal gyrus, SMA = supplementary motor area.
Table 3.
MNI coordinates of the contrasts Cue Suppression > Cue Appraisal, Cue Reappraisal > Cue Appraisal (FWE corrected P < 0.05, cluster > 5)
| Area | BA | Peak coordinates (MNI) | Z-score | Voxel extent |
|---|---|---|---|---|
| Cue Suppression > Cue Appraisal | ||||
| Right putamen | 32, –7, –7 | 4.95 | 14 | |
| Left putamen | –28, –4, –7 | 4.95 | 8 | |
| Right visual cortex | 19 | 18, –84, 18 | 4.94 | 7 |
| Left premotor cortex | 6 | –49, –14, 53 | 4.89 | 5 |
| Right inferior frontal gyrus/precentral gyrus | 44, 6 | 63, 0, 28 | 4.84 | 7 |
| Left putamen | 25, 4, 7 | 4.73 | 8 | |
| Cue Reappraisal > Cue Appraisal | ||||
| Right visual cortex | 14, –88, 0 | 6.82 | 209 | |
Table 2.
MNI coordinates of the contrasts Target Suppression > Target Reappraisal and Target Reappraisal > Target Suppression (FWE corrected P < 0.05, cluster > 5)
| Area | BA | Peak coordinates (MNI) | Z-score | Voxel extent |
|---|---|---|---|---|
| Target Suppression > Target Reappraisal | ||||
| Right inferior frontal gyrus | 44, 6 | 63, 7, 11 | 6.86 | 859 |
| Right dorsolateral prefrontal cortex | 9, 46 | 32, 39, 35 | 6.59 | 286 |
| Posterior cingulate | 23 | 7, –25, 49 | 6.53 | 687 |
| Left superior temporal gyrus | 48 | –39, –32, 18 | 6.39 | 320 |
| Left dorsolateral prefrontal cortex | 9, 46 | –32, 35, 35 | 5.59 | 57 |
| Left inferior parietal lobe | 40 | –32, –49, 42 | 4.78 | 8 |
| Target Reappraisal > Target Suppression | ||||
| Pre-supplementary motor area | 6 | –7, 14, 60 | 6.88 | 47 |
| Right visual cortex | 18 | 32, –95, 7 | 5.70 | 73 |
| Left middle temporal gyrus | 37 | –53, –35, –4 | 5.37 | 11 |
| Left visual Cortex | 18 | –25, –95, 4 | 5.24 | 28 |
| Calcarine | 17 | 4, –88, 0 | 5.03 | 33 |
| Left angular gyrus | 39 | –49, –67, 25 | 3.99 | 12 |
Cue Emotion Regulation
To explore brain activity during the preparation for expressive suppression, we compared activity for expressive suppression with appraisal (Cue Suppression > Cue Appraise). We found activity in the putamen, right visual cortex, left premotor cortex and the right IFG/precentral gyrus (Table 3). To explore brain activity during the preparation for reappraisal, we compared activity for cognitive reappraisal with appraisal (Cue Reappraisal > Cue Appraise). We found activity in the right visual cortex (Table 3). Finally, to explore unique brain activity during the preparation for the ERS, we compared cue-related preparatory activity for expressive suppression with preparatory activity for the alternative regulation strategy reappraisal (Cue Suppression > Cue Reappraisal). We found activity in the right IFG, bilateral putamen, supplementary motor area (SMA) and pre-SMA and right supramarginal gyrus to be more pronounced during the preparation for expressive suppression than for cue reappraisal (Figure 2; Table 4). The reverse contrast of Cue Reappraisal > Cue Suppression revealed no significant clusters of preparatory activity.
Table 4.
Areas showing significant activation during the cue-phase within the contrasts Cue Suppression > Cue Reappraisal and Cue Reappraisal > Cue Suppression (FWE, P < 0.05)
| Area | BA | Peak coordinates (MNI) | Z-score | Voxel extent |
|---|---|---|---|---|
| Cue Suppression > Cue Reappraisal | ||||
| Left putamen | –25, 7, 4 | 7.14 | 282 | |
| Right putamen | 28, 11, 7 | 7.10 | 422 | |
| Right inferior frontal gyrus | 44 | 56, 11, 18 | ||
| Right supramarginal gyrus | 40 | 63, –35, 35 | 6.15 | 85 |
| (Pre-)supplementary motor area (SMA) | 6/32 | 11, 7, 42 | 6.11 | 212 |
| Cue Reappraisal > Cue Suppression | ||||
| No significant voxels | ||||
Cue vs target emotion regulation
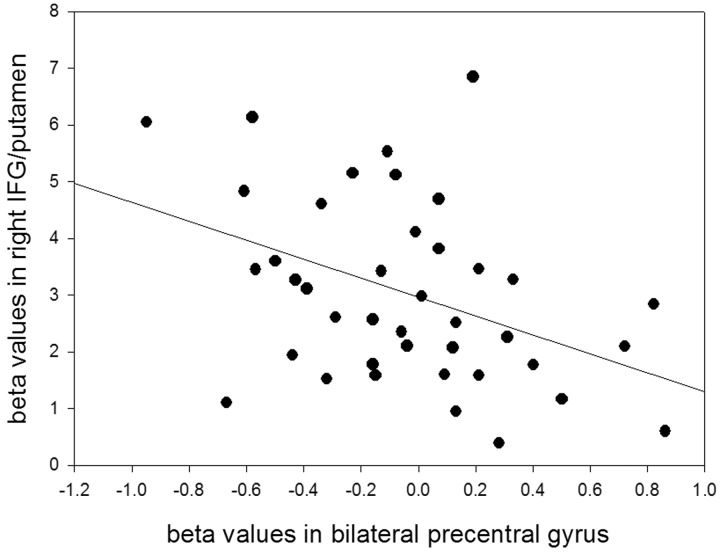
During anticipation for suppression, preparatory control was observed in the right IFG and putamen during Cue Suppression > Cue Reappraisal, areas that represent motoric inhibitory activation. To relate motoric preparatory activation with motor activation during the expressive suppression of emotions, activity in the bilateral precentral gyrus was isolated by means of an ROI analysis as a reflection of facial motoric activation. A negative association between activation in regions activated during anticipation for suppression, namely the right IFG and putamen during Cue Suppression > Cue Reappraisal and activity in bilateral precentral gyrus during Target Suppression > Target Reappraisal, was found [r(41) = –0.423, P < 0.01; Figure 3]. This suggests that activating the right IFG and putamen during the proactive preparation for suppression is supporting motoric processes involved in the suppression/inhibition of overt displays of emotion.
Fig. 3.
Scatter plot of beta values in right inferior frontal gyrus extending into right putamen during Cue Suppression > Cue Reappraisal (based on whole-brain analysis) and beta values in bilateral precentral gyrus during Target Suppression > Target Reappraisal (based on mask).
Rating of emotion regulation
The rating score of how successful the participants were in regulating/appraising their emotions was not significantly different between Suppression (M = 3.1, s.d. = 0. 53), Reappraisal (M = 3.05, s.d. = 0.52) and Appraisal (M = 3.10, s.d. = 0.50) trials, Ps > 0.43. The ratings of mood (unhappy—happy) and arousal (calm—aroused), using nine-point Likert scales, demonstrate that participants felt negative (M = 2.33, s.d. = 1.71) and aroused (M = 7.52, s.d. = 2.43) while looking at the pictures, with no outliers. Importantly, participants felt less negative and aroused when, at the end of the emotion-regulation paradigm, they viewed pictures that had been reappraised than when they viewed pictures in the suppression and appraisal trials. [t(41)s < 2.33, Ps < 0.05]. This indicates that participants felt less negative and calmer while looking at pictures that had been reappraised during the emotion-regulation paradigm.
Interestingly, we observed a trend toward a significant correlation between the observed signal change in the IFG following the cue suppression and the participants’ success rating at the end of the trial [r(42) = 0.28, P = 0.08].
DISCUSSION
Using event-related fMRI, we investigated neural correlates while participants were anticipating reappraisal or suppression (proactive, cue phase), as well as while they were applying these ERS (reactive, target phase), all within the context of negative, high-arousing emotions.
Whole-brain imaging data of the target phase demonstrated that both the strategies of suppression and reappraisal elicited activation in overlapping prefrontal brain areas (compared with appraisal, FWE-corrected). More specifically, for suppression, we observed right frontal cortex, right inferior parietal lobe, lower precuneus and left dorsolateral PFC activation. For reappraisal, we observed activity in the left and right middle temporal gyrus, left and right IFG, middle frontal gyrus and right inferior parietal lobe. These areas of activation are in line with prior emotion-regulation studies (e.g. Beauregard et al., 2001; Ochsner et al., 2004; Goldin et al., 2008). These imaging data, together with high success ratings in applying the ERS (mean rating of >3 on a Likert scale from 1 to 4), suggest that participants were able to regulate their emotions elicited by the images presented.
These findings reveal differences in preparatory control for expressive suppression and cognitive reappraisal (cue phase). The findings show that the anticipation for suppression (Cue Suppression > Cue Reappraisal) is associated with activation in the right IFG, bilateral putamen, SMA, pre-SMA and right supramarginal gyrus (FWE-corrected). Moreover, the anticipation for suppression (Cue Suppression > Cue Appraisal) is associated with activity in the putamen, right visual cortex, left premotor cortex and the right IFG/precentral gyrus. These neural correlates are all related to motoric regulation. It is well known that the right IFG, putamen and pre-SMA have been implicated in processes of motor inhibition. Moreover, these neural correlates have been observed in stop-signal and go/no-go tasks associated with refraining from certain actions (Aron et al., 2004; Aron and Poldrack, 2006). The fact that these preparatory brain correlates are mainly associated with the motor inhibition system is not surprising because expressive suppression is an ERS that prohibits the outward display of emotions. In addition, as outlined in the introduction, neural correlates of inhibitory control seem to play a critical role in the expressive suppression of emotions (Garavan et al., 2006; Lee et al., 2008; Kunz et al., 2011). These findings propose that the intention to suppress the expression of negative emotions is already reflected in neural correlates associated with inhibitory control.
In addition, we were interested in how the preparation to inhibit facial expressions (cue phase) predicted the actual suppression of emotions (target phase). We observed that the more participants’ enhanced activity in brain areas involved in inhibitory control during the preparation for expressive suppression (namely right IFG and putamen), the less motor-related activity was observed in the precentral gyrus during the suppression of negative emotional expressions in the target phase. This precentral gyrus ROI was chosen because it comprises the primary motor cortex, which is involved in facial movements (Birn, et al., 1999; Alkadhi et al., 2002). Our findings, therefore, suggest that motor regulation during the preparation for expressive suppression (cue phase) acts on the primary motor cortex during the actual expressive suppression in the target phase; enhanced inhibitory proactive control reduces motoric activation when actually controlling facial expressions. Moreover, we observed a trend toward a significant positive correlation between the activity in the IFG during the preparation for expressive suppression and participants’ individual ratings of how successful they were in suppressing their emotions. This rating refers to how successful participants evaluated their ability of showing emotions on an outward level. Ultimately, our findings point to the importance of the preparation to expressively suppress emotions that are expected from negative, high-arousing events. This process might be what is captured in the well-known phrase ‘to put on a poker face’, and might refer to a facial expression that conceals current and upcoming emotions. Possibly, this anticipatory control mechanism that is activated when individuals prepare to suppress their emotions might facilitate the application of this behavioral ERS when needed. For example, when freshmen arrive at their new college, they use suppression more than usual (Srivastava et al., 2009). Interestingly, in contrast to expressive suppression, we did not observe neural correlates of anticipatory control for cognitive reappraisal (Cue Reappraisal > Cue Suppression), even though participants (i) knew that a high-arousing negative image was going to be presented and (ii) had a feeling of control over the ability to down-regulate upcoming negative feelings. The actual reappraisal of negative emotions, on the other hand, was associated with typical prefrontal brain activation (i.e. middle temporal gyrus, left and right IFG, middle frontal gyrus and right inferior parietal lobe). Moreover, participants felt less negative while viewing IAPS images at the end of the study that had been reappraised during the emotion-regulation paradigm, which suggests that participants were adequately reappraising the emotional picture being presented. Therefore, these findings suggest that even though participants were able to cognitively reappraise emotional images, preparatory control supporting cognitive reappraisal was harder to achieve compared with preparatory control for expressive suppression. Of note, we only observed neural correlates in the right visual cortex during the reappraisal preparatory phase (Cue Reappraisal > Cue Appraisal). This may be because participants were using visual mental imagery to visualize internally generated scenery to generate a subjective feeling of control, although this speculation warrants further research.
The difference in cognitive reappraisal and expressive suppression is of particular interest for research into the ability to control and modify emotions. As outlined previously, reappraisal is a cognitive strategy to change the emotional impact of an emotion-provoking stimulus, whereas suppression is a behavioral strategy that directly targets outward displays of emotion. This distinction between both ERS might not only be reflected in temporal differences in neural activity between the ERS (e.g. Goldin et al., 2008) but also in the amount of information that is needed to generate both ERS. Reappraisal might depend on the knowledge of the context of the emotional image, whereas suppression might depend on a global mindset to inhibit facial expressions. As a consequence, this difference might entail that a general awareness of an upcoming negative stimulus is insufficient to engage in cognitive reappraisal, which might then explain absent preparatory control supporting this strategy. However, a study from Herwig et al. (2007) demonstrated that a specific reappraisal strategy associated with upcoming (but still absent) unpleasant events activated similar prefrontal areas (e.g. medial and left dorsolateral PFC) to those typically associated with cognitive reappraisal. In this latter study (Herwig et al., 2007), participants knew that an unpleasant event was going to appear without knowing the exact content of this event. During the preparatory period, participants were asked to use a cognitive strategy of self-regulation to reduce anticipatory emotional arousal (i.e. reality checking: ‘repeatedly evaluate the realistic context of their actual situation by, e.g. thinking: I am lying in a scanner’ Herwig et al., pp. 654). Based on the results of their study, neural correlates of reappraisal seem to be measurable prior to the presentation of the emotional stimulus, but might be dependent on the instructions for the specific cognitive reappraisal strategy. Various forms of reappraisal strategies have been defined in the literature (e.g. self-focused and situation-focused reappraisal), each associated with distinct neural correlates (Ochsner et al., 2004). The reappraisal instructions in the study from Herwig et al. (2007) are most closely related to the self-focused instructions outlined by Ochsner et al. (2004). In this study, situation-focused instructions for reappraisal were chosen (i.e. give a different meaning to the situation to make it better) over the self-focused approach (i.e. distancing) to make the difference with expressive suppression as large as possible. However, both self-focused reappraisal and suppression might depend on a global mindset to control emotions, in contrast to situation-focused reappraisal, which might depend on the contextual information of the image. Future research is therefore needed to compare the anticipation for cognitive reappraisal using self-focused and situation-focused instructions, especially when contrasting suppression and reappraisal in the same study. Moreover, future studies could investigate a condition where participants know the context of the upcoming stimulus (for example, ‘you will see a woman crying outside the church’) and are asked to prepare for reappraising this upcoming negative and arousing picture. This way, in accordance with the process model of Gross (1998), participants would be able to cognitively reinterpret early in the emotion-generation process by preparing for specific emotion-eliciting features of the upcoming stimulus (and manipulating the input of the system).
Some limitations of this study should be emphasized. Immediately after the presentation of the image, participants rated how successful they were in regulating/appraising their negative emotions to reduce/feel their negative affect. The idea was to investigate whether proactive preparation would help to successfully regulate emotions. Future research might include both an online rating of success (as in this study) and an online rating of current mood (as in standard emotion-regulation studies) at the end of each trial. This latter rating of current negative affect refers to ‘how are you feeling at this moment?’ and was only presented offline at the end of this study, and not online as in standard emotion-regulation paradigms. Second, in this study, we observed no difference between both ERS and the appraisal condition in amygdala activation. Although our neuroimaging of data acquisition and analyses were in accordance with the literature, this absence of amygdala effects is not in line with results of many fMRI studies on emotion regulation (e.g. Goldin et al., 2008). Notwithstanding the proactive anticipation for suppression or reappraisal, we observed no association between how participants prepared for ERS and amygdala activity during the actual regulation. This could be because participants were well aware that the upcoming picture would be negative and high arousing, and this combination of ‘knowing’ and ‘preparing’ led to an absence of amygdala effects. Nevertheless, this might also be due to signal drop out since slice position was not optimized for amygdala acquisition.
In conclusion, the present neural data demonstrate that participants increased inhibitory motor control when anticipating the expressive suppression of negative emotions, referring to a strategy to not show their emotions on an outward level. Moreover, participants seemed to benefit from this proactive anticipation to suppress emotional expressions because this cue activation was, on the one hand, negatively related to motoric activation during actual suppression and, on other hand, positively related to subjective ratings of success (trend toward significant correlation). The intention to cognitively reappraise emotions, in contrast, seems be related to anticipatory control processes. This suggests that a cognitive strategy to down-regulate negative feelings (by changing the meaning of the situation) is activated when the emotional stimulus is presented to the participant, or when contextual cues reveal emotional features of the upcoming negative stimulus.
FUNDING
M.A.V. (FWO08/PDO/168) and S.K. are postdoctoral fellows of the Research Foundation Flanders (FWO). Preparation of this paper was also supported by Grant BOF10/GOA/014 for a Concerted Research Action of Ghent University (awarded to R.D.R.).
Conflict of Interest
None declared.
Acknowledgments
The authors are grateful for the help of Sofie De Waele with collecting the data. M.-A.V. (FWO08/PDO/168) and S.K. are postdoctoral fellows of the Research Foundation Flanders (FWO).
REFERENCES
- Alkadhi H, Crelier GR, Boendermaker SH, Golay X, Hepp-Reymond MC, Kollias SS. Reproducibility of primary motor cortex somatotopy under controlled conditions. American Journal of Neuroradiology. 2002;23(9):1524–32. [PMC free article] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69:55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience. 2006;26(9):2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Human Brain Mapping. 1999;7(2):106–14. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. [abstract] presented at the 8th International Conference on Functional Mapping of the Human Brain; June; 2002. pp. 2–6. 2002, Sendai, Japan. Available on CD-ROM in NeuroImage 16(2) [Google Scholar]
- Carter CS. The ups and downs of emotion regulation. Biological Psychiatry. 2009;65(5):359–60. doi: 10.1016/j.biopsych.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Research. 2006;1105:130–42. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, et al. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007;37:652–62. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience Biobehavioral Review. 2009;33:1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kunz M, Chen JI, Lautenbacher S, Vachon-Presseau E, Rainville P. Cerebral regulation of facial expressions of pain. Journal of Neuroscience. 2011;31(24):8730–8. doi: 10.1523/JNEUROSCI.0217-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN International Affective Picture System (IAPS) Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention. Gainesville, FL: University of Florida; 1997. [Google Scholar]
- Lee TW, Dolan RJ, Critchley HD. Controlling emotional expression: behavioral and neural correlates of nonimitative emotional responses. Cerebral Cortex. 2008;18(1):104–13. doi: 10.1093/cercor/bhm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Morris WN, Reilly NP. Toward the self-regulafion of mood: theory and research. Motivation and Emotion. 1987;11:215–49. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16(10):1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecubrier Y, Sheehan KH, et al. The validity of the Mini International Neuropsychiatric Interviews (MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12:232–41. Dutch translation by Van Vliet, Leroy & Van Megen, 2000. [Google Scholar]
- Srivastava S, Tamir M, McGonigal KM, John OP, Gross JJ. The social costs of emotional suppression: a prospective study of the transition to college. Journal of Personality and Social Psychology. 2009;96:883–97. doi: 10.1037/a0014755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets A, Nyklicek I, Denollet J. Emotion Regulation: Conceptual and Clinical Issues. New York, NY: Springer Science; 2008. [Google Scholar]