Abstract
Amygdala structural and functional abnormalities have been associated to reactive aggression in previous studies. However, the possible linkage of these two types of anomalies has not been examined. We hypothesized that they would coincide in the same localizations, would be correlated in intensity and would be mediated by reactive aggression personality traits. Here violent (n = 25) and non-violent (n = 29) men were recruited on the basis of their reactive aggression. Callous-unemotional (CU) traits were also assessed. Gray matter concentration (gmC) and reactivity to fearful and neutral facial expressions were measured in dorsal and ventral amygdala partitions. The difference between responses to fearful and neutral facial expressions was calculated (F/N-difference). Violent individuals exhibited a smaller F/N-difference and gmC in the left dorsal amygdala, where a significant coincidence was found in a conjunction analysis. Moreover, the left amygdala F/N-difference and gmC were correlated to each other, an effect mediated by reactive aggression but not by CU. The F/N-difference was caused by increased reactivity to neutral faces. This suggests that anatomical anomalies within local circuitry (and not only altered input) may underlie the amygdala hyper-reactivity to social signals which is characteristic of reactive aggression.
Keywords: fMRI, VBM, amygdala, fear processing, violence, reactive-aggression
INTRODUCTION
The amygdala is part of the neural circuitry underlying emotion and social interactions (Davidson and Irwin, 1999; Davis and Whalen, 2001). In particular, it is implicated in launching and expressing aggressive behaviors (Blair et al., 2006, Coccaro et al., 2011). Concordantly, the amygdala plays an important role in several neurobiological models of violence, such as the ‘violence inhibition mechanism’ (Blair, 1995), the integrated emotion systems model (Blair, 2005) and the paralimbic system dysfunction model of psychopathy (Kiehl, 2006). Therefore, amygdala dysfunction has been a prime suspect when searching for neural correlates of personality traits leading to increased risk for aggression. These traits include anger, hostility and the callous-unemotional (CU) facet of psychopathy. Amygdala function has been examined with neuroimaging in forensic or psychiatric populations in which these traits are expressed within personality disorders, and it has also been linked to the more moderate variability of these traits present within the general population (Dolan, 2010; Koenigs et al., 2011; Anderson and Kiehl, 2012). Much of this work has used the fact that amygdala responses are typically larger for fearful and angry faces than for neutral faces (Morris et al., 1996; Adolphs, 2002; Yang et al., 2002; LaBar et al., 2003).These negative facial expressions can signal potential threats and thus help trigger aggressive behaviors (Coccaro et al., 2011).
Two (opposed) anomalies in amygdala function have been reported, each apparently related to a different cluster of personality traits (Coccaro, et al., 2011; Blair, 2012). The reactivity of the amygala to negatively valenced stimuli (including angry or fearful faces) is increased in psychiatric syndromes with elevated risk for reactive aggression (Blair, 2012), including intermittent explosive disorder (IED) (Coccaro et al., 2007), borderline personality disorder (Herpertz et al., 2001; Minzenberg et al., 2007; Silbersweig et al., 2007) and children with conduct problems (Sterzer et al., 2005). Trait anger is a reliable predictor of reactive aggression (Bettencourt et al., 2006). Congruently, amygdala reactivity is positively correlated with trait anger in normal individuals (Carlson et al., 2010; Carre et al., 2012). In chronically violent subjects, the amygdala hyper-reactivity extends to neutral faces (Pardini and Phillips, 2010), which highly aggressive individuals tend to perceive as negatively valence (Best et al., 2002). In contrast, individuals with the CU facet of psychopathy present an amygdala hypo-reactivity to faces with negative expressions or to pictures/words with negative emotional content. This has been found in both incarcerated and community-living psychopathic subjects (Kiehl et al., 2001; Muller et al., 2003; Birbaumer et al., 2005), children with conduct disorders (Marsh et al., 2008; Jones et al., 2009) and psychiatric patients (Dolan and Fullam, 2009), in all of which the CU facet of psychopathy was ascertained with validated instruments like the Psychopathy Checklist-Revised (PCL-R, Hare, 1991). Therefore the reviewed literature supports a dichotomy: increased reactive aggression associated with a hyper-reactive amygdala and CU traits associated with a hypo-reactive amygdala.
Nevertheless, this hypothesis requires further examination. First, it is important to confirm the specificity of the two proposed trait–dysfunction associations. This is difficult since reactive aggression and CU traits are positively correlated in most forensic samples (Porter and Woodworth, 2006). Individuals scoring high on the CU traits also present increased risk for reactive aggression as well as increased impulsivity and antisocial behavior (Frick et al., 2003). Moreover, the psychiatric conditions with altered emotional processing usually present high co-occurrence with other mental disorders (including alcohol and drug abuse), which could themselves influence amygdala function. One way to deal with these problems, which we adopt here, is to measure both reactive aggression and CU traits in a sample of individuals selected from the community and to examine the relationship of each trait with amygdala function when the other is controlled for.
A second issue to address relates to the sources of the purported amygdala dysfunctions. Anomalous amygdala reactivity can spring from very different causes. Just to mention some possibilities, it could arise from deficient processing in other brain areas that provide input to the amygdala, abnormal anatomical connectivity to the amygdala and local damage to the amygdala circuitry itself (Blair, 2010; Anderson and Kiehl, 2012). The last option would gain credence if the dysfunction could be linked to local anatomical changes in the amygdala. Many studies (revised in Yang et al., 2008; Wahlund and Kristiansson, 2009; Dolan, 2010; Koenigs et al., 2011) have examined amygdala morphology in relation to different types of aggressive behaviors. Morphometric analysis of brain regions has revealed reduced volumes or gray matter concentration (gmC) of the amygdala in psychopathic adults (Yang et al., 2009, 2010; Boccardi et al., 2011; Ermer et al., 2011) and adolescent with conduct problems (Sterzer et al., 2007). Deformations of the amygdala shape and inter-hemispheric asymmetries have also been described (Yang et al., 2009; Ermer et al., 2011). However, the association of amygdala structural characteristics with the CU trait is not clear, with some studies describing anomalies (Yang et al., 2009, 2010; Boccardi et al., 2011; Ermer et al., 2011) and others failing to find them (de Oliveira-Souza et al., 2008; Muller et al., 2008; De Brito et al., 2009).
Several studies have described associations between morphology and measures of aggressive behavior. In particular, a negative association between amygdala volume or gmC and reactive aggression has been found in most studies where these variables have been tested (Sterzer et al., 2007; Reuter et al., 2009; Matthies et al., 2012). Although structural anomalies in other brain areas have also been associated with violent behavior (Laakso et al., 2001, 2002; de Oliveira-Souza et al., 2008; Muller et al., 2008; Tiihonen et al., 2008; De Brito et al., 2009), here we focus on the amygdala. Note that despite many studies of brain function and structure in aggressive individuals, the relationship between anomalies in these two domains has not been evaluated. What is needed is a direct (within-voxel) comparison of functional and structural amygdala anomalies in subjects with personality traits predisposing to aggression and violence. Any such study should take into account the structural and functional heterogeneity of the amygdala (Davis and Whalen, 2001).
Here we study the possible linkage of structural and functional amygdala anomalies associated to reactive aggression in a combined functional magnetic resonance imaging (fMRI) and voxel based morphometry (VBM) performed in the same individuals. We recruited men living within their communities in Mexico City and created a violent and a non-violent control group according to their level of reactive aggression. The presence of the CU traits was also assessed in the two groups. The blood oxygen level dependent (BOLD) response amplitudes to fearful and neutral faces and their difference—hereafter dubbed fear/neutral response difference (F/N-difference)—as well as local gmC were measured in predefined inclusive amygdala regions of interest (ROIs). As a first step in addressing the heterogeneity of this area, ventral and dorsal amygdala ROIs were also defined following previous work (Carre et al., 2012; Lerner et al., 2012). We hypothesized that F/N-difference and gmC would differ between groups and would be selectively and negatively correlated with reactive aggression traits independently from the CU trait. Furthermore, we expected that F/N-difference and gmC would colocalize to the same voxels in the amygdala and would be positively correlated. Finally, we postulated that the relationship between F/N-difference and gmC would be mediated by reactive aggression, so that this association would disappear when reactive aggression was controlled for.
METHODS
Participants
A total of 230 male subjects from a community sample completed a screening questionnaire, the Spanish version of the Reactive and Proactive Aggression Questionnaire (RPQ) (Raine et al., 2006; Andreu et al., 2009). The 109 (47.4%) subjects with scores above eight points in the reactive aggression subscale were classified as violent, whereas the other 121 subjects (52.6%) were classified as non-violent (cutoff adjusted for Mexican population, Ostrosky et al., 2010). From the total sample of 230 male 60 volunteers were selected in order to obtain two samples matched in age, cultural level and socioeconomic status, excluding those subjects that refused to participate in research and those excluded for other reasons (ie, non availability for examination, inability to see small objects without the aid of spectacles, claustrophobia, pacemaker and metal implants and some chronic disease, etc). The final samples consisted of 60 subdivided into a violent (n= 30) and non-violent group (n= 30). Additional behavioral assessments included a neuropsychological evaluation using the Neuropsy battery (Ostrosky-Solís et al., 2007), and Spanish versions of Buss–Durkee Hostility Inventory (BDHI) (Buss and Durkee, 1957; Oquendo et al., 2001), Plutchik Impulsivity Scale (PIS) (Plutchik and Van, 1989; Páez et al., 1996), Novaco Anger Scale (NAS)-reactions to provocation (RP) (Novaco, 1994), Levenson Self-reported Psychopathy scale (LPSP) primary and secondary facets (Levenson et al., 1995) and the PCL-R Factor 1 (F1 emotional detachment) and Factor 2 (F2 antisocial behavior, Hare, 2003). Note that the BDHI was used as an independent measure of reactive aggression and anger, which permitted eschewing the reactive-RPQ (used to define the samples) as a dependent variable. The PCL-R/F1, proactive-RPQ and the secondary LPSP were used as different indexes of the UC trait. Because of movement artifacts, one non-violent and five violent participants were excluded from MRI analysis. All subjects provided written informed consent for this study and were guaranteed confidentiality of the information they provided. Subjects with a history of neurological conditions, mental retardation, psychotic symptoms, or drug abuse were also excluded from this study. The study was approved by Ethics Committees of the participant institutions.
Image acquisition
A 1.5 T system (Signa, GE Medical Systems, Milwaukee, WI, USA) was used to acquire the images. BOLD-contrast-weighted echoplanar (EPI) images for functional scans consisted of 21, interleaved, axial slices of 5-mm thickness that covered the whole brain. In-plane resolution was 4 × 4 mm, with the following parameters: field of view (FOV) = 256 × 256 mm; matrix = 64 × 64; echo time (TE) = 60 ms; TR = 2 s with no time gap; flip angle = 90°. Subsequently, a MPRAGE T1-weighted structural image (1 × 1 × 1 mm resolution) was acquired with the following parameters: echo time (TE) = 3930 ms, repetition time (TR) = 3000 ms, flip angle = 15° and FOV = 256 × 256 × 160 mm3. This yielded 160 contiguous 1-mm thick slices in a sagittal orientation.
fMRI procedure
Stimuli
Face pictures were taken from the Pictures of Facial Affect series (Ekman and Friesen, 1976) and included neutral and fear expressions from 10 different individuals (five males and five females). Twenty neutral and 20 fearful faces were randomly arranged in an event-related design and were presented for 1000 ms, with random inter-stimulus intervals varying between 4000 and 6000 ms. This set was repeated in two runs, each time in a different order, separated by 1-min breaks. Participants were asked to discriminate between neutral and fearful faces and their response was used as an online attention control.
FUNCTIONAL DATA ANALYSIS
Functional data were analyzed using SPM5 and related toolboxes (Wellcome Department of Imaging Neuroscience http://www.fil.ion.ucl.ac.uk/spm). After preprocessing, a first-level analysis was performed on each subject using the general linear model. See details in Supplementary Data. A t-statistic was obtained at each voxel for the contrast fearful > neutral faces. Consistent effects across subjects were tested employing the SPM5 random effects model, in which images for the contrast fearful > neutral (F/N-difference) for all the subjects in each particular group were included. For the group comparison, we tested the hypothesis that the contrast value F/N-difference was greater in non-violent controls than in violent subjects using a voxelwise two-sample, one-tailed t-test. The threshold for voxel activations was set at P < 0.005 (uncorrected). Additionally, we applied a small volume correction in a priori ROIs using the toolbox within SPM (Worsley et al., 1996), and significance of the contrast was estimated with random field theory (pFWE) and cluster level (cluster level corr.).
VBM analysis
The VBM used the optimized procedure included in SPM5 applied to the T1 images. Each participant’s T1 scan was inhomogeneity bias corrected, spatially normalized to MNI-space and segmented into gray matter, white matter and cerebrospinal fluid, using the unified procedure in SPM5 (Ashburner and Friston, 2005). The segmented/normalized gray tissues were smoothed with an 8-mm (FWHM) isotropic Gaussian kernel. A voxelwise one-tailed t-test was performed searching for lower gmCs in the violent relative to the non-violent control group. A significance level of P < 0.005 (uncorrected) was selected.
The spatial co-localization of gmC and F/N-difference anomalies were examined in a conjunction analysis. This approach tests the conjunction null hypothesis that the k studied maps (k = 2: VBM and fMRI) show real effects at the same time. The suitable P-value for this hypothesis test is the maximum of the k P-values and the significance level is reduced to the more stringent level of  (Friston et al., 1999). The EPI images were resliced to the resolution of the T1 images.
(Friston et al., 1999). The EPI images were resliced to the resolution of the T1 images.
ROIs ANALYSIS
Since we had specific a priori hypotheses about discrete ROIs, the left and right amygdala were defined anatomically with the corresponding compartment from the AAL atlas (Tzourio-Mazoyer et al., 2002). See Supplementary Methods for the definition of dorsal and ventral partitions.
Functional and structural measures were extracted from these ROIs by averaging either the values of the gmC or the values of the fear > neutral contrast (F/N-difference) across the voxels in each ROI. Differences in both ROI measurements between the violent and the non-violent groups were tested in an analysis of variance (ANOVA) using age—and global gMCs (GGM) for gmC—as nuisance continuous predictors. Additional analysis also included the reactive aggression and CU scores as continuous predictors. Finally, the mean beta values for neutral and fearful faces in the ROIs were entered separately into an ANOVA with GROUP as a between-subject factor and EXPRESSION (neutral vs fearful) and SIDE (left vs right) as within-subject factors.
The inter-relationship between gmC and F/N-difference in the amygdala ROIs was examined in a multiple regression analysis, controlling for the effects of age and the GGM. The same analysis was repeated with the anger/hostility and psychopathy scales as additional predictors in order to perform a mediation analysis (Sobel, 1982). We also examined the two-sided associations between gmC and F/N-difference each with the behavioral scales scores.
RESULTS
All subjects discriminated the neutral and fearful expressions with >96% accuracy.
The between-group comparisons on the psychological scales are presented in Table 1. Age and neuropsychological performance did not differ between groups; although the violent group had slightly less years of education than controls, this variable was not associated with the imaging measures and will not be further considered. As intended, the violent group presented more reactive aggression on the RPQ scale (which was confirmed with the aggression subscales of the BDHI to avoid circularity), as well as presenting significantly larger scores on anger, hostility, impulsivity and proactive aggression. The violent group also had higher total psychopathy (LPSP and PCL-R) scores, which was true also for the subscales comprising these instruments. However, group differences were dominated by the antisocial facet in both scales (Supplementary Data). Scores on the reactive-RPQ and total PCL-R were strongly correlated (r = 0.54, P < 0.0002).
Table 1.
Demographic and clinical characteristics of non-violent and violent men
| Characteristic | Group; mean (SD) |
t | P-value | |
|---|---|---|---|---|
| Non-violent, n = 29 | Violent, n = 25 | |||
| Age, year | 28.93 (7.03) | 30.63 (8.54) | 0.84 | 0.4 |
| Years of education | 15.96 (1.26) | 14.9 (1.89) | 2.51 | 0.01 |
| Neuropsychological performance | ||||
| Total attention and memory | 106.43 (15.09) | 102.48 (14.2) | 0.93 | 0.35 |
| Total attention and executive functions | 114.34 (10.12) | 112.16 (10.43) | 0.73 | 0.46 |
| Total memory | 104.04 (19.45) | 96.68 (15.7) | 1.44 | 0.15 |
| Reactive aggression/anger traits | ||||
| RPQ reactive | 4.25 (1.51) | 12.04 (2.77) | 12.6 | 0.000001 |
| BDHI: total | 24.7 (9.41) | 36.9 (9.38) | 4.08 | 0.00018 |
| PIS: total | 10.12 (6.49) | 17.79 (6.28) | 4.16 | 0.0013 |
| NAS: total | 66.6 (10.19) | 85.1 (14.77) | 5.35 | 0.000002 |
| RP: total | 46.8 (14.28) | 60.25 (11.3) | 3.64 | 0.0007 |
| LPSP: secondary | 16.95(3.81) | 21.63(4.66) | 3.68 | 0.0001 |
| PCL-R F2 antisocial behavior | 1.71 (2.17) | 5.95 (3.42) | 5.11 | 0.000006 |
| CU traits | ||||
| RPQ: proactive | 0.51 (0.8) | 4.83 (2.56) | 8.3 | 0.000001 |
| LPSP: primary | 28.21(6.55)6 | 34.59(8.28) | 2.86 | 0.006 |
| PCL-R F1: emotional detachment | 1.45 (1.91) | 3.52 (3.4) | 2.58 | 0.013 |
| RPQ: total | 4.77(1.62) | 16.9(4.23) | 13.8 | 0.000001 |
| LPSP: total | 43.48 (7.98)6 | 54.76(8.78) | 4.47 | 0.00006 |
| PCL-R: total | 3.0 (3.78) | 10.59(6.62) | 4.97 | 0.00001 |
RPQ = reactive and proactive aggression questionnaire; BDHI = Buss-Durkee hostility inventory; PIS = Plutchik impulsivity scale; NAS = Novaco anger scale; RP = reactions to provocation LPSP = Levenson Self-reported Psychopathy scale. PCL-R. Psychopathy Checklist.
fMRI
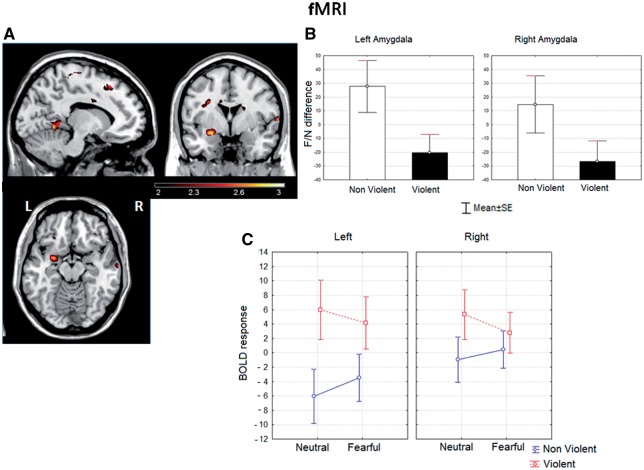
See Supplementary Data for within-group results. The F/N-difference was reduced in the violent group. Locations that exhibited significant between-group differences in the voxelwise analysis are shown in Figure 1A. In violent men, a significant reduction of the F/N-difference was present in the left dorsal amygdala (maxima at xyz = −24 0 −12). Significant between-group differences were also found in orbitofrontal cortex (OFC) bilaterally, in the right superior temporal and in the left precuneus, lingual and precentral areas (Table 2). The small volume analysis with the amygdala ROI evinced a significant group difference for the local maxima on the left side (t = 2.89, pFWE-corr. < 0.022, P-cluster level corr. < 0.047). No voxel was significant in this analysis on the right side.
Fig. 1.
(A) Statistical parametric map of differences between the violent and the non-violent control groups in F/N-difference response. Voxels with significance ≤0.01 (uncorrected) are displayed. Note the local maximum in the left amygdala (z = 2.89, P < 0.003). Displayed slices are left x = −12; middle y = 0; right z = −14 (coordinates in MNI space). (B) Histogram of the mean F/N-difference from the anatomically based left and right amygdala ROIs in both groups. Whiskers represent standard error of the mean. (C) Mean ROI beta values for the response to faces as a function of group, emotional expression and cerebral hemisphere. Whiskers represent standard error of the mean.
Table 2.
Brain regions showing greater activation in non-violent than violent groups, in the response to fearful relative to neutral faces (F/N-difference) Whole-brain voxel-wise analysis
| Regions | Side | x y z (mm)a | z | P < 0.01 (uncorr.) |
|---|---|---|---|---|
| Precuneus | L | −20 −46 10 | 3.17 | 0.001 |
| Lingual | L | −10 −46 0 | 2.66 | 0.005 |
| Amygdala | L | −24 0 −12 | 2.89 | 0.003 |
| Frontal_Inf_Orb | L | −28 32 −18 | 2.86 | 0.003 |
| Frontal_Inf_Tri | L | −44 34 4 | 2.59 | 0.005 |
| Precentral | L | −48 −6 28 | 2.64 | 0.004 |
| Cerebelum | L | −6 −54 −4 | 3.05 | 0.002 |
| Vermis | L | 2 −52 −4 | 2.68 | 0.005 |
| Frontal_Inf_Tri | R | 50 40 2 | 2.83 | 0.002 |
| Frontal_Inf_Tri | R | 40 32 12 | 2.71 | 0.003 |
| Temporal_Sup | R | 68 −2 4 | 2.75 | 0.003 |
| Temporal_Sup | R | 62 −12 6 | 2.59 | 0.005 |
Side R: right hemisphere; L: left hemisphere.
aCoordinates x,y,z are given in MNI space (Montreal Neurological Institute, http://www.bic.mni.mcgill.ca).
P-values are uncorrected for multiple comparisons and only clusters showing a spatial extent of at least 30 contiguous voxels are reported to protect against Type I errors.
ROI-based analysis evinced a significant decrease of F/N-difference in both hemispheres for the violent group (Figure 1B). The main effects of group [F(1,51) = 4.24, P< 0.04] and side [F(1,51) = 5.43, P< 0.02], but not of age, were significant in the corresponding ANOVA. Planned comparisons showed significant differences only in the left amygdala [left F(1,51) = 4.67, P < 0.035, right F(1,51) = 3.03, ns]. A medium effect size (d = 0.52), (Cohen, 1988) was found for this contrast. The results of an ANOVA including partition as a factor revealed that it had a significant effect [F(1,51) = 6.99, P< 0.01]. Planned comparisons showed significant differences only in the dorsal partition in the left amygdala [F(1,51) = 4.47, P< 0.039], while in the right side no significant difference was found in any partition (see Supplementary Figure).
The ANOVA on the ROI beta values (Figure 1C) was used to identify the source of the reduced F/N-difference in the violent subjects. A significant interaction was found between expression and group [F(1,51) = 5.38, P < 0.02] in the absence of significant main effects. The main effect of expression was not significant, probably due to opposite trends in the two groups (response to fear larger in controls, but slightly larger to neutral in the violent group). Planned comparisons showed that the interaction between expression and group was present only on the left side [F(1,51) = 4.37, P < 0.04], where the violent group presented an elevated response for neutral faces relative to the controls [F(1,51) = 4.87, P < 0.03], and a small but non-significant increase in the response to fearful faces [F(1,51) = 2.32, ns]. Thus, the reduced F/N-difference basically resulted from an enhanced response in the violent group to neutral faces on the left side. The effect size for the latter contrast was strong (Cohen’s d = 0.72).
The BDHI and NAS scales were negatively correlated with mean F/N-difference in both amygdala ROIs (Table 3) and positively correlated with the mean beta values for neutral faces (Supplementary Data) albeit at lower significance levels. Also, secondary-LPSP and RP were negatively correlated with F/N-difference in the left amygdala. Proactive-RPQ, F1 from PCL-R and primary LPSP, the scales that index the CU facet of psychopathy, were not correlated with F/N-difference.
Table 3.
Pearson’s correlation between left amygdala structural and functional measurements and psychological scales
| Reactive aggression/anger traits |
CU traits |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| BDHI | PIS | NAS | RP | LPSP secondary | PCL-R F2 | RPQ-Proactive | LPSP primary | PCL-R F1 | |
| F/N dif | −0.47 | −0.37 | −0.36 | −0.27 | −0.38 | −0.03 | −0.23 | −0.21 | −0.05 |
| left | P = 0.001 | P = 0.009 | P = 0.01 | P = 0.05 | P = 0.009 | P = 0.81 | P = 0.1 | P = 0.15 | P = 0.69 |
| F/N dif | −0.34 | −0.26 | −0.31 | −0.27 | −0.26 | −0.02 | −0.2 | −0.25 | 0.03 |
| right | P = 0.02 | P = 0.08 | P = 0.01 | P = 0.06 | P = 0.08 | P = 0.87 | P = 0.16 | P = 0.10 | P = 0.85 |
| gmC | −0.36 | −0.10 | −0.24 | −0.24 | −0.23 | −0.13 | −0.2767 | 0.02 | −0.07 |
| left | P = 0.01 | P = 0.48 | P = 0.08 | P = 0.08 | P = 0.11 | P = 0.36 | P = 0.05 | P = 0.87 | P = 0.63 |
| gmC | −0.3 | −0.01 | −0.28 | −0.21 | −0.22 | −0.20 | −0.34 | −0.08 | 0.02 |
| right | P = 0.04 | P = 0.93 | P = 0.06 | P = 0.15 | P = 0.16 | P = 0.17 | P = 0.01 | P = 0.61 | P = 0.88 |
Significant level was set at P < 0.01. Values above the significance level of P < 0.01 are displayed in bold.
F/N dif = Fear neutral difference gmC = Gray matter concentration (after controlling the effect of age and global gray matter).
BDHI = Buss-Durkee hostility inventory; PIS = Plutchik impulsivity scale; NAS = Novaco anger scale; RP = reactions to provocation LPSP = Levenson Self-reported Psychopathy scale. PCL-R. Psychopathy Checklist
Morphometry
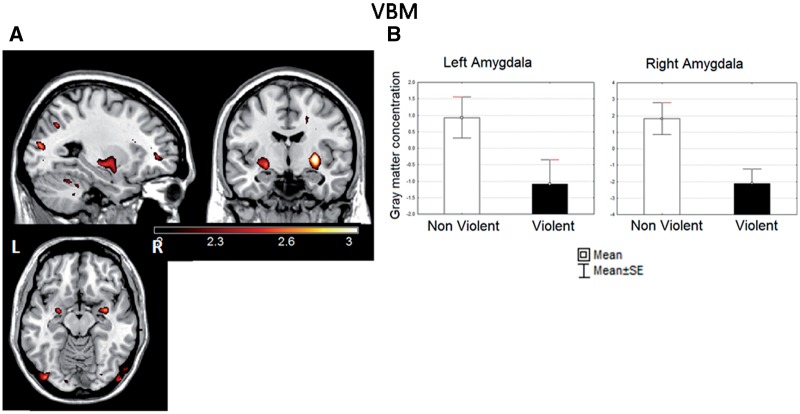
Figure 2A shows regions that exhibited decreased gmC in violent respect to non-violent subjects in the voxelwise analysis, which included local maxima in both amygdala (Table 4). The maxima in the left (xyz = −26 −2 −12)—but not in the right—anatomical amygdala ROI was significant in the small volume analysis (t = 2.65, pFWE-corr. < 0.05, P-cluster level corr. < 0.005). The mean gmC in the amygdala ROIs was lower in violent than in the non-violent subjects (Figure 2B). The results of the ANOVA on the inclusive amygdala ROIs revealed a significant main effects of group [F(1,50) = 11.6, P< 0.0039] and GGM [F(1,50) = 43.4, P< 0.00001], but age was not significant. Planned comparisons showed significant differences in the left [F(1,50) = 4.45, P< 0.039] and the right [F(1,50) = 8.7, P < 0.004] hemispheres. The effects sizes for these contrasts were respectively moderate and strong (left: Cohen’s d = 0.6; right: d = 0.76). On the left side we found (using η2) that that the between-group differences explained about 15.7% of the variability in gray matter density, after controlling for age and brain size.
Fig. 2.
(A) Statistical parametric map of differences in gmC between the violent and the non-violent control groups. Voxels with significance ≤0.01 (uncorrected) are displayed. Note the bilateral clusters including the amygdala (both with z > 2.55 and P < 0.005). Displayed slices are left x = −24; middle y = −3; right z = −13 (coordinates in MNI space). In this and subsequent figures, maps were plotted with MRIcron (http://www.sph.sc.edu/comd/rorden/mricron) over a prototypical brain. The left–right orientation used here is indicated in this figure. (B) Histogram of the mean gmC (arbitrary units) from the anatomically based left and right amygdala ROIs in both groups. Whiskers represent standard error of the mean.
Table 4.
Areas of decreased gray matter in violent group relative to non-violent control group
| Regions | Side | x y z (mm)a | z | P < 0.01 (uncorr.) |
|---|---|---|---|---|
| Occipital_Mid | L | −24 −86 16 | 2.73 | 0.003 |
| Amygdala | L | −26 −2 −12 | 2.55 | 0.005 |
| Cerebelum | L | −22 −44 −26 | 3.19 | 0.001 |
| Cerebelum | L | −24 −52 −24 | 2.96 | 0.002 |
| Cerebelum | L | −40 −36 −32 | 2.74 | 0.003 |
| Amygdala | R | 32 −2 −12 | 2.67 | 0.004 |
| Putamen | R | 30 −6 0 | 3.14 | 0.001 |
| Cerebelum | R | 34 −42 −34 | 3.14 | 0.001 |
Side: R: right hemisphere; L: left hemisphere. aCoordinates x,y,z are given in MNI space (Montreal Neurological Institute, http://www.bic.mni.mcgill.ca). P-values are uncorrected for multiple comparisons and only clusters showing a spatial extent of at least 30 contiguous voxels are reported to protect against Type I errors.
When the analysis was performed including amygdala partition as a factor, significant main effects of group [F(1,50) = 9.1, P< 0.0039], partition [F(1,50) = 181.7, P< 0.00001] and GGM [F(1,50) = 43.5, P< 0.00001], but not age, were found. Planned comparisons showed significant differences in the left dorsal amygdala [F(1,50) = 8.6, P< 0.004] and in both dorsal [F(1,50) = 5.7, P< 0.02] and ventral [F(1,50) = 6.47, P < 0.014] partitions on the right side.
Both amygdala gmC were negatively correlated with BDHI, but only on the left side this effect was significant (Table 3). With the exception of proactive-RPQ, the other scales (including those indexing the unemotional-callous facet of psychopathy: F1 from PCL-R, primary-LPSP) were not correlated with gmC. Only the proactive-RPQ was negatively correlated with gmC (significantly only in the right), but controlling the contribution of the BDHI render this effect no significant. In contrast, the correlation of BDHI and gmC in the left amygdala was remained significant when the effect of the proactive-RPQ was controlled for [partial r = −0.31, t(44) = −2.11, P< 0.041].
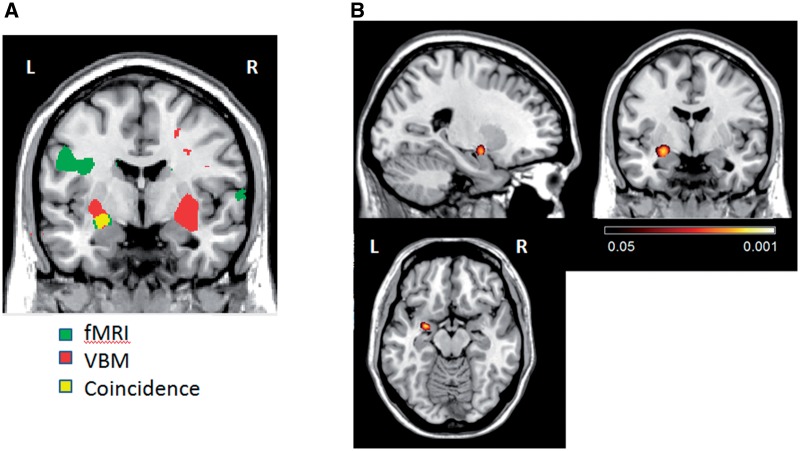
Co-localization of structural and functional damage
The coincidence of areas with both decreased F/N-difference and decreased gmC was examined by plotting together the results of fMRI and VBM group comparisons (Figure 3A). An area in the left amygdala showed significant differences in both tests. The conjunction analysis (Figure 3B) only evinced one cluster with a significant coincidence (peak voxel P< 0.006). It comprised 116 voxels and was centered at xyz = −26 −3 −11, which is within the left dorsal amygdala compartment.
Fig. 3.
Co-localization of F/N-difference and gmC anomalies. (A) Overlapped plots of areas with significant differences between violent and non-violent control groups for both measures (voxelwise P < 0.01). Significant group differences: in green (F/N-difference) and in red (gmC). In yellow, voxels that were significant in both analysis. y = 0. Coordinates in MNI space. (B) Statistical parametric map of conjunction between fMRI and VBM results. Voxels with significance ≤0.05 (uncorrected) are showed. Displayed slices are left x = −24; middle y = 0; right z = −14 (coordinates in MNI space).
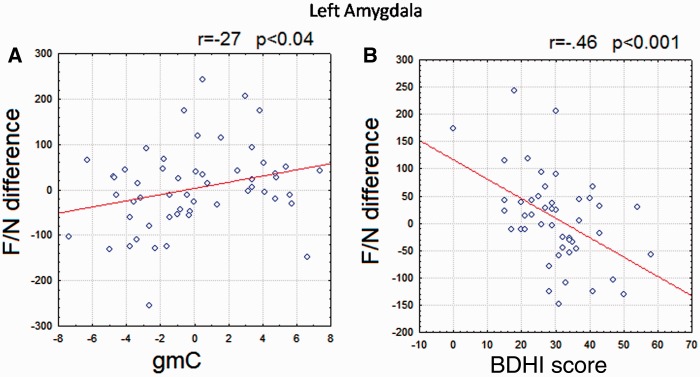
We also examined the association between F/N-difference and gmC across subjects within both amygdale ROIs in a multiple regression analysis, while controlling the effect of age and GGM. In line with our original hypothesis, a significant positive association was found in the left amygdala [r = 0.38, t(50) = 2.03, P < 0.04]. However, this was not so for the right amygdala (r = −0.16, t(50) = −0.89, P = 0.38]. The scatter plots for F/N-difference and gmC (after correcting for age and GGM) are plotted in Figure 4A.
Fig. 4.
Scatter plot of measures in the left amygdala ROI. Pearson correlation coefficient between variables is shown in each figure. (A) Scatter plot between F/N-difference and gmCs values after controlling for age and total gmC. (B) Scatter plot between F/N-difference and BDHI score (total).
The relationship between gmC and F/N-difference was re-evaluated controlling the effects of psychometric scores (together with age and GGM), but excluding the reactive-RPQ to avoid circularity since it was used to define the samples. The association between gmC and F/N-difference was not significant when the effects of either BDHI or the secondary LPSP were controlled. The association between gmC and F/N-difference remained significant when the effects of primary LPSP, PCL-R (and subscales), PIS, NAS, RP were successively controlled for. Mediation analysis (Sobel, 1982) evinced that the association between F/N-difference and gmC was mediated by the BDHI scores (z = 2.01 P< 0.045), but not by any of the other scales (Figure 4B)
DISCUSSION
Our two groups were chosen to differ on the RPQ reactive aggression scale. The violent group also scored higher than controls subjects on independent measures of aggression and trait anger. Although none of the violent subjects reached the PCL-R cutoff for psychopathy, they scored higher than controls on CU traits. The violent group had smaller F/N-difference than controls in the left dorsal amygdala, which was due to a greater increase in reactivity to neutral than to fearful faces. The violent group also had lower gmC in the dorsal bilateral amygdala and in the right ventral amygdala. Both gmC and F/N-difference were negatively correlated with measures of reactive aggression traits in the left amygdala, but not with measures of CU traits. A significant within-voxel coincidence of F/N-difference and gmC anomalies was found in small subregion within the left dorsal amygdala. Interestingly, F/N-difference was positively correlated with gmC in the left amygdala, an association mediated by reactive aggression but not by the CU traits.
The F/N-difference was smaller for our violent subjects than for the controls in the left amygdala. At first glance this seems analogous to findings in previous studies that have described similar reductions in F/N-difference in aggressive subjects with CU traits (Marsh et al., 2008; Dolan and Fullam, 2009; Jones et al., 2009). Negative correlations of F/N-difference with CU traits (Dolan and Fullam, 2009) have also been reported. However, the F/N-difference can be smaller because of a reduced response to fear or an enhanced response to neutral faces, or both. In our case, the F/N-difference decreased in the violent subjects because of a larger reactivity to neutral faces together with a less marked (and non-significant) decrease in the responses to fearful faces. Unfortunately, studies of subjects with CU traits have only reported the F/N-difference (perhaps over confidently described as a fear response) without isolating the reactivity to different facial expressions. Given the emotional deficit typical of psychopathy, reduced amygdala reactivity to both neutral and negative facial expressions should be expected (respect to a low-level baseline), but this requires explicit testing.
In the few studies of reactive aggressive individuals that have measured amygdala reactivity to each facial expression separately, there is only partial agreement with our results. In contrast to our findings, larger responses to angry or fearful faces (Coccaro et al., 2007; Minzenberg et al., 2007), as well as to negative pictures (Sterzer et al., 2005) or words (ia-Klein et al., 2009), have been described in high reactive aggressive subjects. On the other hand, a trend for larger reactivity to neutral faces was described in one study (Coccaro et al., 2007) and is evident in figure of another (Minzenberg et al., 2007). In a study (Pardini and Phillips, 2010) very similar to ours, chronically violent subjects exhibited increased left amygdala responses to neutral faces (in the context of happy faces), but not to negatively valenced expressions, an effect independent from CU traits. Elevated amygdala responses to neutral but not angry faces have also been described in adolescents with early-onset conduct disorders (Passamonti et al., 2010), and amygdala hyper-reactivity to neutral faces has been described in pediatric bipolar disorder (Hall et al., 2008) and schizophrenia (Rich et al.,2006). Furthermore, trait anger in healthy subjects is positively correlated with left amygdala responses to angry, although not to fearful, faces (Carre et al., 2012). It is not clear why reactive aggressive subjects in some studies exhibit increased amygdala reactivity to only neutral faces, and also increased reactivity to negative expressions in others. This may be due to differences in the face stimuli, task or subject characteristics. In line with previous reports (Passamonti et al., 2010), we believe that our results are congruent with the fact that neutral faces tend to be perceived as negative by reactive aggression individuals (Best et al., 2002). This would imply increased amygdala response to neutral stimuli that are interpreted as aversive, consistent with more easy provocation leading to negative social interactions and conflicts. This hypothesis could be tested in future studies of aggressive individuals by looking at the association between valence ratings for the face stimuli and the amplitude of the amygdala response to different facial expressions.
The violent group, when compared with non-violent controls, also exhibited significant reductions in the F/N-difference in other brain areas in addition to the amygdala. These areas were the left OFC, the inferior frontal gyrus bilaterally as well as at the right superior temporal gyrus. Our results are in line with other neuroimaging studies that have found reduced activations to different kinds of affective stimuli within the brain emotional systems in violent adult subjects and in adolescents with conduct disorder. Specifically, reduced OFC cortex activations in psychopaths have been demonstrated using affective stimuli (Kiehl et al., 2001) and also in adolescent with conduct problems (Sterzer et al., 2005) viewing of pictures with neutral or strong negative affective valence (Gordon et al., 2004) and more specifically during fear processing in psychopath adults (Veit et al., 2002; Birbaumer et al., 2005).
The reduction of gmC in the amygdala of our violent group and the negative correlation of this variable with reactive aggression traits in the left amygdala are consistent with previous reports focusing on reactive aggression. Negative correlations have been reported between bilateral amygdala volume and life history of aggression (Matthies et al., 2012) and between trait anger and local gray matter volume in the left amygdala (Reuter et al., 2009) in healthy subjects. A negative correlation between aggressiveness and local gray matter volume in the left amygdala has been reported for adolescents with conduct disorder (Sterzer et al., 2007). Most studies have not found a correlation between CU traits and VBM measures of the amygdala (de Oliveira-Souza et al., 2008; De Brito et al., 2009), although in larger samples (Yang et al., 2009; Ermer et al., 2011) a negative correlation was found between the total PCL-R score [although not with the F1 subscale (Ermer et al., 2011)]. This is in line with the lack of association of CU traits and amygdala VBM measures reported here.
Our conjunction analysis pinpointed the overlap of gmC and F/N-difference anomalies to a small subregion of the dorsal left amygdala. Furthermore, we found a significant correlation between gmC and F/N-difference in the left amygdala, mediated by BDHI and secondary LPSP scores. To our knowledge, the present study is the first to test the linkage between the structural and functional abnormalities in violent subjects. The results rule militate against a purely functional explanation, based on deficient input from other brain regions to a dysfunctional—but structurally intact—amygdala (see below for other brain areas which could fit into this scheme). This local damage could be compounded by impairment of fiber connections to this area. A recent DTI study (Craig et al., 2009) found significantly that fractional anisotropy in the uncinate fasciculus, which links the amygdala with middle OFC, was negatively correlated with the F2, antisocial facet of the PCL-R and not with F1 facet. Concordantly, deficient functional connectivity between amygdala and medial OFC has been reported in subjects with IED (Coccaro et al., 2007). In fact due to their tight anatomical and functional connections, the anomalies described in the present study for the amygdala probably extend to other limbic and para-limbic structures implicated in violent behavior (Kiehl, 2006).
This study has several limitations that have to be considered. The sample size for the study was relatively small, and the results must be replicated in larger samples with more extreme cases of violence (i.e. IED). Moreover, with the exception of PCL-R, which is not optimal for community-based samples, personality traits were measured with self-reports. Instruments based on external informants (Vazire and Carlson, 2011) (to confirm the self-reports) would be useful on a socially sensitive topic like aggression and to counteract possible manipulation by individuals with psychopathic traits. The correlation between morphometric and functional amygdala measurements needs to be performed in more detail, at the level of its component nuclei, a strategy that has been successful in the study of psychopaths (Yang et al., 2009; Boccardi et al., 2011). This would require a higher voxel resolution than used in our fMRI protocol. Our assessment of structural/functional linkage hinged on the outcome of the EPI and T1 image coregistration and normalization procedure. We may have underestimated the area of conjunction and the degree of correlation of these two types of measure because of residual intra-subject misalignment between the EPI and the T1 images, and residual between-subject misalignment. Furthermore, amygdala ROI definition and its subdivision into partitions were dependent on the accuracy of the normalization procedures. Also, the magnetic susceptibility artifact could have affected amygdala partitions differently, with possibly more in the ventral part. It is necessary to replicate our findings using fMRI protocols that minimize these artifacts. Given that the amygdala forms part of a larger system of limbic and paralimbic structures (Kiehl, 2006), it is necessary to explore the linkage of structural and functional anomalies within each of these areas and also as a network in violent subjects
Despite these limitations, this is the first study to verify a within-voxel coincidence of structural and functional anomalies amygdala of individuals with high reactive aggression, specifically in its left dorsal partition. The intensity of these anomalies was correlated across subjects and specifically mediated by reactive aggression traits. Further work is needed to establish why our subjects with elevated reactive aggression had larger responses to faces with neutral, but not fearful, expressions. The results indicate that anomalies in the local circuitry of the left dorsal amygdala participate in dysfunctional reactions to social signals which in turn are related to increased reactive aggression. These anomalies are largely independent from the CU traits in psychopathy and could contribute to variations in personality in the general population, and when more severe to various forms of antisocial and violent behavior.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
This research was partially supported by the Institute of Science and Technology of Mexico City ICYTDF 422.01 PICDS08-19.
REFERENCES
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002;1:21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Anderson NE, Kiehl KA. The psychopath magnetized: insights from brain imaging. Trends in Cognitive Sciences. 2012;16:52–60. doi: 10.1016/j.tics.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu JM, Peña ME, Ramírez JM. Cuestionario de agresión reactiva y proactiva: un instrumento de medida de la agresión en adolescentes. Revista de Psicopatología y Psicología Clínica. 2009;14:37–49. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8448–53. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt BA, Talley A, Benjamin AJ, Valentine J. Personality and aggressive behavior under provoking and neutral conditions: a meta-analytic review. Psychological Bulletin. 2006;132:751–77. doi: 10.1037/0033-2909.132.5.751. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJ. A cognitive developmental approach to mortality: investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology. 2005;17:865–91. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. British Journal of Psychology. 2010;101:383–99. doi: 10.1348/000712609X418480. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Considering anger from a cognitive neuroscience perspective. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3:65–74. doi: 10.1002/wcs.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. The Journal of Child Psychology and Psychiatry. 2006;47:262–76. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Frisoni GB, Hare RD, et al. Cortex and amygdala morphology in psychopathy. Psychiatry Research. 2011;193:85–92. doi: 10.1016/j.pscychresns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of Consulting Psychology. 1957;21:343–9. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Mujica-Parodi LR. Blind rage? Heightened anger is associated with altered amygdala responses to masked and unmasked fearful faces. Psychiatry Research. 2010;182:281–3. doi: 10.1016/j.pscychresns.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Carre JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience. 2012;7:213–21. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biological Psychiatry. 2011;69:1153–9. doi: 10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Craig MC, Catani M, Deeley Q, et al. Altered connections on the road to psychopathy. Molecular Psychiatry. 2009;14:946–53, 907. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–52. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–13. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Dolan MC. What imaging tells us about violence in anti-social men. Criminal Behaviour and Mental Health. 2010;20:199–214. doi: 10.1002/cbm.771. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Fullam RS. Psychopathy and functional magnetic resonance imaging blood oxygenation level-dependent responses to emotional faces in violent patients with schizophrenia. Biological Psychiatry. 2009;66:570–7. doi: 10.1016/j.biopsych.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologist Press; 1976. [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. Journal of Abnormal Psychology. 2011;121(3):649–58. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. Journal of Abnormal Child Psychology. 2003;31:457–70. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–96. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56:516–21. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, McKirdy JW, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biological Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist—Revised. Toronto, Ontario: Multi-Health Systems; 1991. [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist—Revised. 2nd edn. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50:292–8. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- ia-Klein N, Goldstein RZ, Tomasi D, et al. Neural mechanisms of anger regulation as a function of genetic risk for violence. Emotion. 2009;9:385–96. doi: 10.1037/a0015904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callousunemotional traits. The American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–28. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Molecular Psychiatry. 2011;16:792–9. doi: 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Research. 2002;114:95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Koivisto E, et al. Psychopathy and the posterior hippocampus. Behavioural Brain Research. 2001;118:187–93. doi: 10.1016/s0166-4328(00)00324-7. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cerebral Cortex. 2003;13:1023–33. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Singer N, Gonen T, et al. Feeling without seeing? Engagement of ventral, but not dorsal, amygdala during unaware exposure to emotional faces. Journal of Cognitive Neuroscience. 2012;24:531–42. doi: 10.1162/jocn_a_00165. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. Journal of Personality and Social Psychology. 1995;68:151–8. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Matthies S, Rusch N, Weber M, et al. Small amygdala-high aggression? The role of the amygdala in modulating aggression in healthy subjects. World Journal of Biological Psychiatry. 2012;13:75–81. doi: 10.3109/15622975.2010.541282. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Research. 2007;155:231–43. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Muller JL, Ganssbauer S, Sommer M, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Research. 2008;163:213–22. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Wagner V, et al. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54:152–62. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Novaco RW. Anger as a risk factor for violence amongst the mentally disordered. In: Monahan J, Steadman HJ, editors. Violence and Mental Disorders: Developments in Risk Assessment. Chicago, IL: Chicago University Press; 1994. pp. 21–59. [Google Scholar]
- Oquendo MA, Baca-Garcia E, Graver R, Morales M, Montalvan V, Mann JJ. Spanish adaptation of the Barratt impulsiveness scale (BIS-11) European Journal of Psychiatry. 2001;15:147–55. [Google Scholar]
- Ostrosky F, Díaz K, Romero C, et al. Agresión reactiva y proactiva en generadores de violencia doméstica Unpublished manuscript. 2010 Laboratory of Neuropsychology and Psychophysiology, National University of Mexico, Mexico City, Mexico. [Google Scholar]
- Ostrosky-Solís F, Gómez-Pérez E, Matute E, et al. Neuropsi attention and memory: A neuropsychological test battery in Spanish with norms bye age and educational level. Applied Neuropsychology. 2007;14(3):156–170. doi: 10.1080/09084280701508655. [DOI] [PubMed] [Google Scholar]
- Páez F, Jiménez A, López A, Raull Ariza J, Ortega Soto H, Nicolini H. Estudio de validez de la traducción al castellano de la Escala de Impulsividad de Plutchik. Salud Mental. 1996;19:10–2. [Google Scholar]
- Pardini DA, Phillips M. Neural responses to emotional and neutral facial expressions in chronically violent men. Journal of Psychiatry and Neuroscience. 2010;35:390–8. doi: 10.1503/jpn.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutchik R, Van PH. The measurement of suicidality, aggressivity and impulsivity. Prog. Neuropsychopharmacol. Biological Psychiatry. 1989;13(Suppl):S23–34. doi: 10.1016/0278-5846(89)90107-3. [DOI] [PubMed] [Google Scholar]
- Porter S, Woodworth M. Psychopathy and aggression. In: Patrick CJ, editor. Handbook of Psychopathy. New York: The Guilford Press; 2006. pp. 481–94. [Google Scholar]
- Raine A, Dodge K, Loeber R, et al. The reactive-proactive aggression questionnaire: differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior. 2006;32:159–71. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Weber B, Fiebach CJ, Elger C, Montag C. The biological basis of anger: associations with the gene coding for DARPP-32 (PPP1R1B) and with amygdala volume. Behavioural Brain Research. 2009;202:179–83. doi: 10.1016/j.bbr.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. American Journal of Psychiatry. 2007;164:1832–41. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. Washington DC: American Sociological Association; 1982. pp. 290–312. [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–42. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Research. 2008;163:201–12. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vazire S, Carlson EN. Others sometimes know us better than we know ourselves. Current Directions in Psychological Science. 2011;20:104–8. [Google Scholar]
- Veit R, Flor H, Erb M, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328:233–36. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Wahlund K, Kristiansson M. Aggression, psychopathy and brain imaging - review and future recommendations. International Journal of Law and Psychiatry. 2009;32:266–71. doi: 10.1016/j.ijlp.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, et al. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13:1737–41. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behavioral Sciences & the Law. 2008;26:65–83. doi: 10.1002/bsl.788. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. Journal of Abnormal Psychology. 2010;119:546–54. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66:986–94. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]