Abstract
Brief periods of unconscious thought (UT) have been shown to improve decision making compared with making an immediate decision (ID). We reveal a neural mechanism for UT in decision making using blood oxygen level-dependent (BOLD) functional magnetic resonance imaging. Participants (N = 33) encoded information on a set of consumer products (e.g. 48 attributes describing four different cars), and we manipulated whether participants (i) consciously thought about this information (conscious thought), (ii) completed a difficult 2-back working memory task (UT) or (iii) made an immediate decision about the consumer products (ID) in a within-subjects blocked design. To differentiate UT neural activity from 2-back working memory neural activity, participants completed an independent 2-back task and this neural activity was subtracted from neural activity occurring during the UT 2-back task. Consistent with a neural reactivation account, we found that the same regions activated during the encoding of complex decision information (right dorsolateral prefrontal cortex and left intermediate visual cortex) continued to be activated during a subsequent 2-min UT period. Moreover, neural reactivation in these regions was predictive of subsequent behavioral decision-making performance after the UT period. These results provide initial evidence for post-encoding unconscious neural reactivation in facilitating decision making.
Keywords: decision making, fMRI, neural reactivation, unconscious thought
It is now believed that a great deal of human behavior arises from unconscious processes (for reviews, Wilson, 2002; Dijksterhuis and Aarts, 2010; Baumeister et al., 2011; van Gaal et al., 2012). For example, unconscious mental processes have been shown to facilitate goal-directed behavior (Bargh et al., 2001), memory consolidation (Tamminen et al., 2010), creativity and insight (Wagner et al., 2004) and decision making (Dijksterhuis and Nordgren, 2006; Dijksterhuis et al., 2006; Soon et al., 2008; Strick et al., 2011). In decision making, Dijksterhuis et al. have recently shown that brief periods of unconscious thought (UT) facilitate decision making when decisions are complex, such as when selecting an apartment or buying a car (Dijksterhuis et al., 2006; Bos et al., 2008). In this work, participants were presented with complex decision information (e.g. 48 attributes describing four apartments), and then asked to complete a difficult distractor task that restricted their ability to think consciously about this decision information. Notably, participants who were asked to complete a distractor task made superior decisions compared with participants who consciously thought about the decision information and participants who made an immediate decision (ID) after encoding the information (Dijksterhuis and Nordgren, 2006; Dijksterhuis et al., 2006; Strick et al., 2010). The emerging work on UT suggests that the brain is processing decision information outside of conscious awareness during the distractor task (cf. Brooks et al., 2012). But how does the brain support UT?
This work was guided by the hypothesis that neural reactivation occurring in extrastriate and prefrontal regions explains how UT improves decision making. Specifically, extrastriate and prefrontal neural regions that are active during the encoding of decision information continue to process that information during a subsequent distractor task. Indeed, studies in human and animal models have demonstrated neural reactivation processes in an extrastriate–hippocampal–prefrontal network (Wilson and McNaughton, 1994; Miller et al., 1996; Peigneux et al., 2006; Gelbard-Sagiv et al., 2008; Mazoyer et al., 2009; Tambini et al., 2010; Poch et al., 2011), with some work linking reactivation processes to improved performance on memory and learning tasks (Peigneux et al., 2006; Gelbard-Sagiv et al., 2008; Tambini et al., 2010; Poch et al., 2011). One particularly noteworthy observation to come from this work is that neural reactivation processes can occur outside of conscious awareness (e.g. during sleep; Wilson and McNaughton, 1994), with some work suggesting that neural reactivation may continue even while the brain is distracted by new tasks (Fuster and Alexander, 1971). Previous studies highlight an important role for reactivation in extrastriate visual cortex (e.g. Miller et al., 1996), hippocampus (e.g. Carr et al., 2011) and areas of prefrontal cortex (PFC), including dorsolateral PFC (e.g. Fuster and Alexander, 1971) for improved memory and learning outcomes. No previous studies have tested for a neural reactivation process in the context of multi-attribute decision making, like tasks used in the UT literature (Strick et al., 2011). But it is possible that reactivation occurring in these extrastriate–hippocampal–dorsolateral prefrontal regions might support continued visual and semantic processing of decision information during an UT period.
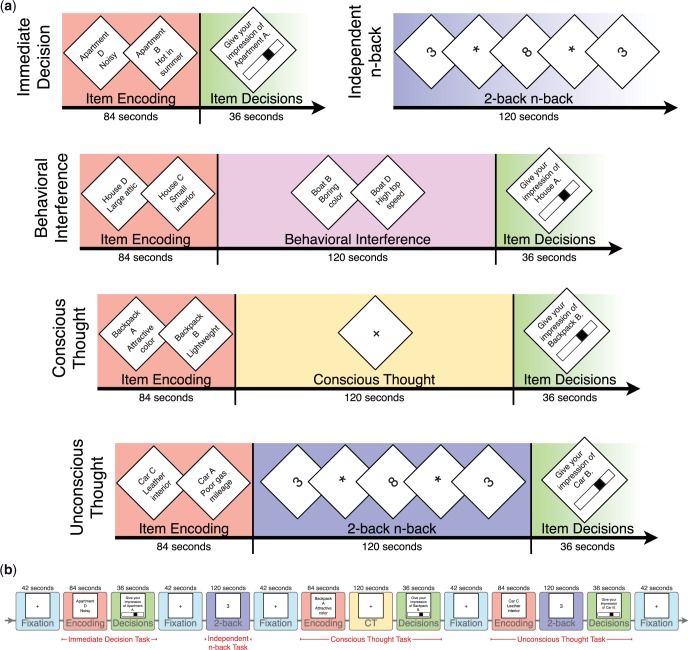
To evaluate the role of neural reactivation in decision making, we conducted a decision-making experiment while participants underwent functional magnetic resonance imaging (fMRI). Participants completed several decision-making tasks (Figure 1). In the UT task, participants first encoded decision information and then completed a 2-back distractor task. This 2-back task restricts one’s ability to consciously think about the initial decision information, thus any decision-related thought is unconscious. To control for 2-back neural activity occurring during this UT period, participants completed an independent 2-back task that was not embedded in any decision task during the experimental session. To differentiate neural activity specific to UT, this independent 2-back neural activity was subtracted from neural activity during the UT 2-back task period. We then tested whether neural reactivation occurring during the UT period accounts for UT effects on decision-making performance. Specifically, we predicted that (i) neural regions involved in the encoding of decision information continue to be active during an UT period in an extrastriate–prefrontal network and (ii) that this neural reactivation during UT predicts subsequent decision making after the UT period. It is possible that conscious thought (CT) may share a comparable neural reactivation mechanism for decision making (with the same or different neural regions), we thus tested for neural reactivation during CT.
Fig. 1.
(a) A visual depiction of the experimental conditions. Condition and decision task (e.g. apartments and cars) order was counterbalanced. (b) A sample chronological experimental session for a single subject.
METHODS
Participants
Thirty-three healthy volunteers (21 males) were recruited from the Pittsburgh, Pennsylvania community. Participants were excluded based on standard MRI safety guidelines (e.g. metal implants). Participants spoke English as their first language, were between 18 and 35 years of age, were right-handed, weighed <135 kg and were non-smokers. The Carnegie Mellon University Institutional Review Board approved this experiment and all participants provided informed consent. Six participants were excluded from analyses: five participants had excessive head motion during the scans (sudden head motion of ∼2 mm or 2°), and one participant was an outlier (>2.5 s.d.s from the mean) on his behavioral decision performance, resulting in a final sample of 27 participants. Participants were paid $25.
Procedure
Participants completed the experimental tasks while undergoing fMRI (Figure 1). All experimental stimuli were presented via E-Prime software (Psychology Software Tools, Pittsburgh, PA, USA), and participants made responses using a right-handed button glove.
Item encoding
During the initial encoding period, participants were serially shown 12 attributes, each for 1.75 s, describing each of four different items (e.g. for cars: ‘Car A has leather seats’ and ‘Car D has poor gas mileage’) in random order, for 48 attributes total. One item (e.g. Car A) had eight positive and four negative attributes, two items had six positive and six negative attributes and the remaining item had four positive and eight negative attributes, creating a hierarchy of item quality for use in determining decision-making performance (stimulus materials are available upon request).
Processing: conscious or unconscious thought
After encoding the attributes in each decision task, participants either (i) made an ID about the items without any further processing (ID control condition), (ii) consciously processed the decision information while viewing a fixation cross for 2 min (CT condition) or (iii) performed a 2-back distractor task for 2 min (UT condition) (Figure 1). The ID control condition was not functionally imaged: It was performed either immediately before or immediately after the other experimental conditions during structural scans, counterbalanced across participants. In the UT and CT conditions, participants were shown an instruction screen stating that they would soon rate the items according to their quality, but that they would first complete a 2-back memory task (UT condition) or that they were to consciously think about the items (CT condition). In the 2-back task, participants were shown single-digit numbers serially and asked to make a button press when a currently presented number matched the one presented two digits prior. Each digit was displayed for 0.5 s, followed by an asterisk displayed for 2.5 s. This 2-back task consumes conscious processing resources (and activates the PFC), such that subsequent processing of the decision information must rely on unconscious processes (Owen et al., 2005; Dijksterhuis and Nordgren, 2006).
Controlling for 2-back distractor task neural activity
To identify neural activity related to unconscious processing of the decision information and not neural activity related to the 2-back task, we also had each participant complete an additional 2-back task during the experiment, and this ‘independent 2-back’ occurred in the absence of any decision task. By subtracting neural activation observed during this independent 2-back task from activation observed during a 2-back task within an item decision task, neural activation related to UT is revealed.
Item decisions
At the end of each decision task, participants were asked to make decisions about the overall quality of each of the four items (e.g. Car A–D) using 21-point sliding scales anchored from ‘very negative’ to ‘neutral’ to ‘very positive’.
Behavioral data analysis
Following standard conventions (Dijksterhuis and Nordgren, 2006), decision-making performance was determined by creating a difference score of participants’ ratings between the best and worst items (higher scores indicate better discrimination of the best vs worst items). Consistent with previous studies, we expected decision-making performance in the UT condition to be superior to performance in the ID and CT conditions (Dijksterhuis et al., 2006; Bos et al., 2008), so we conducted two-tailed paired-samples t-tests comparing UT and ID and UT and CT. Paired-samples t-tests compared 2-back working memory performance differences (hits, misses and false alarms) between the UT 2-back and independent 2-back tasks. All behavioral analyses were conducted in SPSS 19.0 (IBM, Armonk, NY, USA).
MRI acquisition and data analysis
MRI scans were acquired using a Siemens 3-T Verio Scanner equipped with a 32-channel head coil (Siemens AG, Erlangen, Germany). An MP-RAGE sequence (44 sagittal slices, 256 × 256 in-plane matrix, TR = 1700 ms, TE = 2.48 ms, TI = 900 ms, 1.0 mm3 isotropic voxels, flip angle = 9°) was used to obtain a whole-brain volume. Functional images were acquired with a gradient-echo T2*-weighted echo-planar sequence (42 sagittal slices, 64 × 64 in-plane matrix, TR = 3000 ms, TE = 30 ms, 3.1 × 3.1 × 3.2 mm3 voxels, flip angle = 90°) in three functional runs. Preprocessing and statistical analysis of the fMRI data were carried out using SPM8 (Welcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (MathWorks, Inc., Natick, MA, USA).
Preprocessing
A standard preprocessing stream included motion correction and reslicing into 2 mm cubic voxels, coregistration of the structural image to the mean of the functional images, spatially normalization to the MNI template and spatial smoothing with an 8-mm Gaussian kernel. The ArtRepair toolbox (Center for Interdisciplinary Brain Science Research at Stanford, Palo Alto, California) was used to improve motion correction in included participants who had more than 1 mm or 1° of phasic head motion, following recommended procedures (P. Mazaika, S. Whitfield-Gabrieli and A. Reiss, submitted for publication). Specifically, their functional images were smoothed to 4 mm, corrected with ArtRepair (Center for Interdisciplinary Brain Science Research at Stanford, Palo Alto, California) and then smoothed again to 7 mm, resulting in an 8-mm smoothing (P. Mazaika, S. Whitfield-Gabrieli and A. Reiss, submitted for publication).
First- and second-level models
For first-level statistical analyses, a general linear model (GLM) was fitted to each subject’s imaging data. The main regressors modeled the study conditions as boxcar blocks convolved with the canonical hemodynamic response function. The unmodeled fixation period served as an implicit baseline in the GLM. Contrast maps generated from these first-level models were then submitted to a second-level random-effects group analysis. To first identify neural activity related to UT, we conducted a whole-brain analysis using a voxel (P < 0.001, uncorrected) and extent (k ≥ 20) threshold for the UT 2-back > independent 2-back contrast. Then, to test the unconscious neural reactivation hypothesis, we examined whether these regions engaged by UT were also active during encoding (information encoding > fixation contrast) in a conjunction analysis [P < 0.05, small volume corrected for family-wise error (FWE); voxel-wise P < 0.001, k ≥ 20]. We used the same approach in testing for conscious neural reactivation in the CT task (using the CT > fixation contrast, and a subsequent conjunction analysis also including the information encoding > fixation contrast).
Regression analyses relating neural reactivation to decision-making performance
To test the degree to which neural activity during UT predicts behavioral performance on the decision task, parameter estimates from spheres within the overlapping clusters from the information encoding > fixation/UT 2-back > independent 2-back conjunction analysis were extracted and regressed onto behavioral decision performance in the UT condition. These parameter estimates were extracted by (i) identifying cluster peak voxel in the conjunction image, (ii) extracting the parameter estimates (eigenvariates) from 8 mm spheres around these peak voxels in the UT 2-back > independent 2-back activation map and (iii) regressing these parameter estimates onto behavioral decision performance scores in the UT and CT conditions.
Connectivity analyses
We hypothesized that the unconscious neural reactivation regions (during the UT task period) would also be coupled as a network. To test this, we conducted a psychophysiologic interaction (PPI) analysis to assess connectivity between neural regions during the UT 2-back task compared with the independent 2-back task (our psychological variable was task: UT 2-back vs independent 2-back task). In each subject, we masked and extracted a volume of interest in the UT 2-back > independent 2-back condition by the 8-mm sphere in left intermediate visual cortex identified around the cluster’s peak voxel in the conjunction analysis. The intermediate visual cortex sphere was selected as the PPI seed for each subject because reactivation in visual cortex may reflect an unconscious visual representation that is passed forward to right dorsolateral PFC during the UT period. Single-subject PPI maps were used in a second-level one-sample t-test to identify regions exhibiting connectivity with the seed region in a group analysis (Friston et al., 1997, 2005). The unconscious neural reactivation conjunction map was used as a mask in the PPI analysis to determine whether the neural reactivation regions (left intermediate visual cortex and right dorsolateral PFC) exhibited connectivity with each other. The significance level for the PPI analysis was set at P < 0.05, small volume corrected for FWE; voxel-wise P < 0.001, k ≥ 5.
RESULTS
A brief period of UT resulted in better behavioral decisions compared to both the ID and CT comparison conditions (Figure 2). Paired t-tests indicated that UT produced better decisions compared with the ID [t(26) = 2.15, P = 0.04] and CT [t(26) = 2.10, P = 0.04] conditions [the overall one-way ANOVA was marginally significant, F(2,52) = 2.48, P = 0.09]. To examine the neural basis of this effect, a whole-brain analysis comparing neural activity during the UT 2-back task to neural activity during the independent 2-back task (UT 2-back > independent 2-back contrast) revealed activity related to unconscious processing of decision information in bilateral intermediate visual cortex, right dorsolateral and right ventrolateral PFC, right thalamus and left frontal operculum (Figure 3; Table 1). It is also notable that we did not observe 2-back performance differences between the UT 2-back compared with the independent 2-back task [paired t-tests; hits: t(26) = 1.00, P = 0.33; misses: t(26) = 0.61, P = 0.55; false alarms: t(26) = 1.44, P = 0.16], suggesting that UT does not interfere with working memory performance on a 2-back task.
Fig. 2.
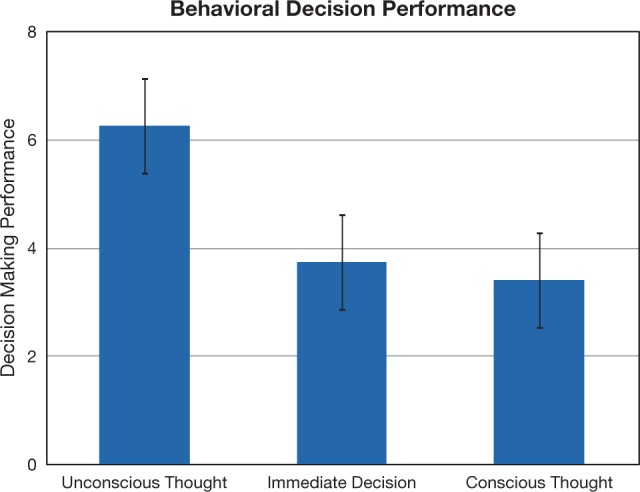
Behavioral decision performance on decision tasks. Participants made decisions immediately or after a period of UT or CT (condition order counterbalanced). Performance was scored by subtracting a participant’s rating of the worst item from her rating of the best item in each set. Error bars reflect ±1 standard error of the mean.
Fig. 3.
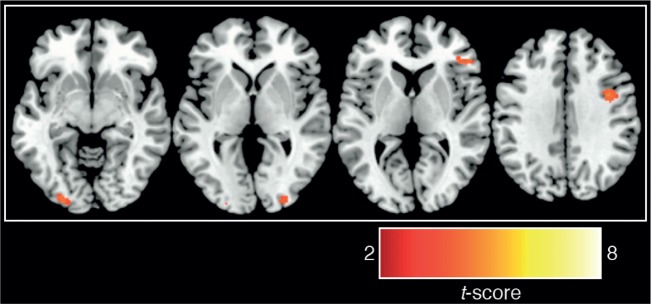
Neural activations observed in UT 2-back > independent 2-back [P < 0.001 (uncorrected), k ≥ 20; see Table 1 for cluster sizes, locations and statistics].
Table 1.
Peak voxels, cluster sizes (k) and t-values for neural activity during UT (UT 2-back > independent 2-back)
| UT n-back > independent n-back | |||||
|---|---|---|---|---|---|
| Region | Cluster peak (x, y, z) |
Cluster size (k) | t-value | ||
| Right dorsolateral PFC | 38 | 2 | 34 | 73 | 4.4 |
| Left intermediate visual cortex | −30 | −90 | −8 | 64 | 4.21 |
| Right ventrolateral PFC | 52 | 34 | 6 | 54 | 4.08 |
| Left inferior frontal operculum | −34 | 6 | 30 | 25 | 3.96 |
| Right thalamus | 14 | −20 | 12 | 20 | 3.94 |
| Right intermediate visual cortex | 28 | −92 | 0 | 22 | 3.86 |
P < 0.001 (uncorrected), k ≥ 20.
Consistent with the neural reactivation hypothesis, conjunct neural activation was observed in left intermediate visual cortex and right dorsolateral PFC during both the encoding and UT contrasts (Figure 4; Table 1). When using a sphere in the reactivated left intermediate visual cortex as a seed in the PPI analysis, we observed greater connectivity between this area of left intermediate visual cortex and a cluster in the right dorsolateral PFC reactivation region during the UT period compared with the independent 2-back period (46, 4, 32; t = 3.66, P < 0.05, FWE corrected; k = 7 voxels).
Fig. 4.

Unconscious neural reactivation as assessed by a conjunction of two contrasts: (i) encoding > fixation and (ii) UT 2-back > independent 2-back (P < 0.05, FWE corrected, k ≥ 20; see Table 2 for cluster sizes, locations and statistics).
We next tested if neural reactivations observed during UT predicted subsequent decision-making performance when participants were asked to make ratings of the items (e.g. cars). We observed that neural reactivation occurring in right dorsolateral PFC [β = 0.39, t(26) = 2.13, P = 0.04] and left intermediate visual cortex [β = 0.40, t(26) = 2.20, P = 0.04] predicted subsequent decision-making performance, such that more neural reactivation in these regions was associated with greater discrimination between the best and worst items on the decision-making task.
In this study, we did not find that a 2-min period of CT produced better decision making compared with the ID condition [paired samples t(26) = 0.20, P = 0.84] (cf. Payne et al., 2008). Nonetheless, we considered whether CT recruits distinct neural regions compared with UT. As shown in Table 3, CT recruited a distinct prefrontal cortical network that was non-overlapping with regions active during UT, suggesting dissociable neural mechanisms for conscious and UT. However, this result does not preclude the possibility that CT may share a common neural reactivation mechanism for decision-making effects, just with different neural regions. But we did not find evidence for a neural reactivation account of CT effects. Although we observed clusters of neural reactivation during CT (Figure 5; Table 4), none of these clusters significantly predicted decision-making performance. Specifically, CT reactivation clusters observed in right cerebellum [β = 0.27, t(26) = 1.39, P = 0.18], left supplementary motor area [β = 0.21, t(26) = 1.07, P = 0.29], right ventrolateral PFC [β = 0.18, t(26) = 0.90, P = 0.38] and right intraparietal lobule [β = −0.13, t(26) = −0.67, P = 0.51] did not predict decision performance after CT.
Table 2.
Peak voxels, cluster sizes (k) and t-values for conjunction analysis of UT 2-back > 2-back and encoding > fixation
| Conjunction of UT n-back > independent n-back and encoding > fixation | |||||
|---|---|---|---|---|---|
| Region | Cluster peak (x, y, z) |
Cluster size (k) | t-value | ||
| Right dorsolateral PFC | 44 | 2 | 34 | 59 | 3.99 |
| Left intermediate visual cortex | −24 | −92 | −8 | 43 | 3.76 |
Active voxels are those exhibiting above-threshold activation in both contrasts when tested against the conjunction null hypothesis.
Table 3.
Peak voxels, cluster sizes (k) and t-values for neural activity during CT (conscious thought > fixation)
| Conscious thought > fixation | |||||
|---|---|---|---|---|---|
| Region | Cluster peak (x, y, z) |
Cluster size (k) | t-value | ||
| Left supplementary motor area | −8 | 14 | 56 | 331 | 5.96 |
| Bilateral cerebellum | 28 | −58 | −32 | 120 | 4.4 |
| Left inferior temporal lobe | −40 | 2 | −34 | 20 | 4.22 |
| Left ventrolateral PFC | 32 | −64 | 36 | 85 | 4.16 |
| Left ventrolateral PFC | −46 | 20 | 12 | 30 | 3.97 |
| Left middle occipital gyrus | −28 | −56 | 32 | 28 | 3.92 |
| Right ventrolateral PFC | 44 | 32 | 28 | 47 | 3.89 |
| Medial cerebellum | 2 | −58 | −36 | 21 | 3.62 |
P < 0.001 (uncorrected), k ≥ 20.
Fig. 5.
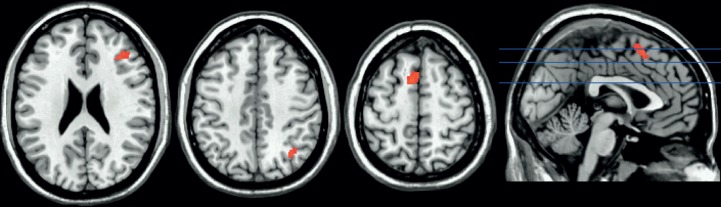
Conscious neural reactivation as assessed by a conjunction of two contrasts: (i) encoding > fixation and (ii) CT > fixation (P < 0.05, FWE corrected, k ≥ 20; see Table 3 for cluster sizes, locations and statistics).
Table 4.
Peak voxels, cluster sizes (k) and t-values for the conjunction analysis of CT > fixation and encoding > fixation
| Conjunction of CT > fixation and encoding > fixation | |||||
|---|---|---|---|---|---|
| Region | Cluster peak (x, y, z) |
Cluster size (k) | t-value | ||
| Right cerebellum | 32 | −54 | −32 | 78 | 4.15 |
| Left supplementary motor area | −8 | 10 | 58 | 192 | 4.14 |
| Right ventrolateral PFC | 36 | 32 | 24 | 33 | 3.86 |
| Right inferior parietal lobule | 34 | −62 | 46 | 44 | 3.85 |
Active voxels are those exhibiting above-threshold activation in both contrasts when tested against the conjunction null hypothesis. P < 0.05, FEW corrected, k ≥ 20.
DISCUSSION
The present findings shed light on an initially puzzling body of work on UT effects, which has shown that periods of conscious distraction can facilitate decision making when a decision is complex (for a review, see Dijksterhuis and Nordgren, 2006). Here, we make three novel contributions. First, previous behavioral studies have had to infer indirectly that an unconscious cognitive process was occurring, which has resulted in significant debate about the presence and role of unconscious processes in decision making (Dijksterhuis et al., 2006; Acker, 2008). Using BOLD contrast fMRI, we observed neural activity during an UT period, which challenges existing accounts that have claimed that deliberation without attention (UT) does not occur during periods of distraction (Acker, 2008). Our fMRI result is the first to show that a decision-related neural process is occurring during the UT period, which supports the view that processing of decision information occurs ‘offline’ during distractor periods (Strick et al., 2010, 2011).
Second, our work highlights a role for unconscious neural reactivation as a mechanism for UT effects. This unconscious neural reactivation perspective posits that neural regions involved in encoding decision information continue to process this information outside of conscious awareness. Our work shows the information encoding regions of left intermediate visual cortex and right dorsolateral PFC continue to be active even while the brain is engaged in performing other complex cognitive operations (such as a 2-back working-memory task), and that the neural reactivation occurring in these regions predicts subsequent decision-making performance after the distractor period.
Third, this experiment provided an opportunity to test for common or dissociable neural mechanisms of CT and UT. We provide preliminary evidence that CT and UT recruit distinct non-overlapping neural regions, and that only UT effects on decision making can be explained by a neural reactivation mechanism. It is important to note that our study was optimized for revealing UT effects, and more research is needed which carefully considers neural mechanisms of CT in decision making. We find that a 2-min period of CT did not improve decision making compared with our ID control condition, which is a result that is consistent with previous work indicating that CT can improve decision-making performance as long as it is self-paced (and lasting ∼30 s) (Payne et al., 2008). Future studies using self-paced decision-making procedures will help elucidate the neural mechanisms of CT.
One important question raised by work on UT is whether UT (and neural reactivation) is truly unconscious, or whether the processing of decision information during the distractor task is in fact conscious. Our findings support the former view. No participant in our experiment reported consciously thinking about the decision attributes during the 2-back distractor task when they were probed during debriefing. (We note that one subject reported thinking consciously about the item attributes during the UT 2-back task, but this subject was excluded from analyses due to excessive head motion during scans.) Although we cannot definitively rule out surreptitious CT that might be occurring during the 2-back distractor task, one might expect that if surreptitious CT were occurring during the UT period, this would disrupt 2-back performance. Notably, we observed no 2-back performance differences between the independent and UT 2-back tasks.
Our results can be thought to suggest necessary, but not sufficient, conditions for a neural reactivation account of UT effects in decision making. If the neural reactivation hypothesis explains how UT improves decision-making performance, it is necessary that (i) neural regions engaged during encoding maintain activity during the UT period and (ii) that activity in these neural regions during the distractor task is associated with behavioral decision-making performance effects. One exciting possibility is that these observed neural reactivation effects operate via similar cellular replay mechanisms as those observed in the sleep replay literature (Wilson and McNaughton, 1994), such that decision-related information is replayed during UT periods (cf. Carr et al., 2011). Future research might also consider whether unconscious neural reactivation comprises a more general neural mechanism for learning, incubation effects (Sio and Ormerod, 2009) and insight (Wagner et al., 2004).
One limitation of this study is that there was no strong incentive for making decisions. Participants were making ratings about fictitious consumer products (e.g. cars) that they would not receive. However, previous studies have shown that periods of UT improve real-world decision making (Dijksterhuis and van Olden, 2006; de Vries et al., 2010; Messner et al., 2011), and it is noteworthy that a period of UT still improved decision making in this study without a clear incentive to encode and unconsciously process decision related information.
Previous studies reveal neural activation patterns related to the automatic evaluation of decision information (Lebreton et al., 2009; Tusche et al., 2010). This study extends this work to multi-attribute decision making, showing that unconscious neural reactivation may be a neural mechanism for sustained UT related to decision attributes. Indeed, our work suggests the possibility that coordinated neural reactivation occurring in intermediate visual cortex and right dorsolateral PFC reflects unconscious visual and semantic processing of decision information (Bowden and Beeman, 1998). Specifically, visual cortex may maintain an unconscious visual representation of decision information while dorsolateral PFC semantically processes and consolidates that information, resulting in an unconscious decision preference that can be called up to consciousness when one is prompted to act or decide (Blumenfeld and Ranganath, 2007; Soon et al., 2008).
The nature of the unconscious mind has long challenged philosophers and scientists (Schooler, 2002; Wilson, 2002), but the present work offers a new perspective on this topic by way of examining the brain. We find that brain regions that are active during encoding new decision information reactivate while the brain coordinates responses to other unrelated tasks, and that this unconscious neural reactivation is associated with decision-making performance when participants are prompted to make decisions.
Conflict of Interest
None declared.
Acknowledgments
We would like to thank the research assistants in the Health and Human Performance Laboratory, L. Uddin, J. Pyles, M. Tarr, P. Gianaros and M. Behrmann for help and feedback. We are also grateful to the staff and facilities at the Scientific Imaging and Brain Research (SIBR) Center at Carnegie Mellon University. This research was supported by funding from the Pittsburgh Life Sciences Greenhouse Opportunity Fund.
REFERENCES
- Acker F. New findings on unconscious versus conscious thought in decision making: additional empirical data and meta-analysis. Judgment and Decision Making. 2008;3:292–303. [Google Scholar]
- Bargh JA, Gollwitzer PM, Lee-Chai A, Barndollar K, Trotschel R. The automated will: nonconscious activation and pursuit of behavioral goals. Journal of Personality and Social Psychology. 2001;81:1014–27. [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Masicampo EJ, Vohs KD. Do conscious thoughts cause behavior? Annual Review of Psychology. 2011;62:331–61. doi: 10.1146/annurev.psych.093008.131126. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13:280–91. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Bos MW, Dijksterhuis A, Baaren RB. On the goal-dependency of unconscious thought. Journal of Experimental Social Psychology. 2008;44:1114–20. [Google Scholar]
- Bowden E, Beeman MJ. Getting the right idea: semantic activation in the right hemisphere may help solve insight problems. Psychological Science. 1998;9:435–40. [Google Scholar]
- Brooks SJ, Savov V, Allzén E, Benedict C, Fredriksson R, Schiöth HB. Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. NeuroImage. 2012;59:2962–73. doi: 10.1016/j.neuroimage.2011.09.077. [DOI] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nature Neuroscience. 2011;14:147–53. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M, Witteman CLM, Holland RW, Dijksterhuis A. The unconscious thought effect in clinical decision making: an example in diagnosis. Medical Decision Making. 2010;30:578–81. doi: 10.1177/0272989X09360820. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Aarts H. Goals, attention, and (un)consciousness. Annual Review of Psychology. 2010;61:467–90. doi: 10.1146/annurev.psych.093008.100445. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Bos MW, Nordgren LF, van Baaren RB. On making the right choice: the deliberation-without-attention effect. Science. 2006;311:1005–7. doi: 10.1126/science.1121629. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Nordgren LF. A theory of unconscious thought. Perspectives on Psychological Science. 2006;1:95–109. doi: 10.1111/j.1745-6916.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, van Olden Z. On the benefits of thinking unconsciously: unconscious thought can increase post-choice satisfaction. Journal of Experimental Social Psychology. 2006;42:627–31. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging* 1. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage. 2005;25:661–7. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–4. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64:431–9. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Houde O, Joliot M, Mellet E, Tzourio-Mazoyer N. Regional cerebral blood flow increases during wakeful rest following cognitive training. Brain Research Bulletin. 2009;80:133–8. doi: 10.1016/j.brainresbull.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Messner C, Wänke M, Weibel C. Unconscious personnel selection. Social Cognition. 2011;29:699–710. [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. Journal of Neuroscience. 1996;16:5154–67. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JW, Samper A, Bettman JR, Luce MF. Boundary conditions on unconscious thought in complex decision making. Psychological Science. 2008;19:1118–23. doi: 10.1111/j.1467-9280.2008.02212.x. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Orban P, Balteau E, et al. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biology. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch C, Fuentemilla L, Barnes GR, Duzel E. Hippocampal theta-phase modulation of replay correlates with configural-relational short-term memory performance. Journal of Neuroscience. 2011;31:7038–42. doi: 10.1523/JNEUROSCI.6305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler JW. Re-representing consciousness: dissociations between experience and meta-consciousness. Trends in Cognitive Sciences. 2002;6:339–44. doi: 10.1016/s1364-6613(02)01949-6. [DOI] [PubMed] [Google Scholar]
- Sio UN, Ormerod TC. Does incubation enhance problem solving? A meta-analytic review. Psychological Bulletin. 2009;135:94–120. doi: 10.1037/a0014212. [DOI] [PubMed] [Google Scholar]
- Soon CS, Brass M, Heinze HJ, Haynes JD. Unconscious determinants of free decisions in the human brain. Nature Neuroscience. 2008;11:543–5. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- Strick M, Dijksterhuis A, Bos MW, Sjoerdsma A, van Baaren RB, Nordgren LF. A meta-analysis on unconscious thought effects. Social Cognition. 2011;29:738–62. [Google Scholar]
- Strick M, Dijksterhuis A, van Baaren RB. Unconscious-thought effects take place off-line, not on-line. Psychological Science. 2010;21:484–8. doi: 10.1177/0956797610363555. [DOI] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–90. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. Journal of Neuroscience. 2010;30:14356–60. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A, Bode S, Haynes JD. Neural responses to unattended products predict later consumer choices. Journal of Neuroscience. 2010;30:8024–31. doi: 10.1523/JNEUROSCI.0064-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaal S, de Lange F, Cohen MX. The role consciousness in cognitive control and decision making. Frontiers in Human Neuroscience. 2012;6:1–15. doi: 10.3389/fnhum.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–5. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wilson TD. Strangers to Ourselves: Discovering the Adaptive Unconscious. Cambridge, MA: Harvard University Press; 2002. [Google Scholar]