Abstract
Aim
This study aimed to develop a 3D culture model to test the extent to which transplanted stem cells modulate astrocyte reactivity, where exacerbated glial cell activation could be detrimental to CNS repair success.
Materials & methods
The reactivity of rat astrocytes to bone marrow mesenchymal stem cells, neural crest stem cells (NCSCs) and differentiated adipose-derived stem cells was assessed after 5 days. Schwann cells were used as a positive control.
Results
NCSCs and differentiated Schwann cell-like adipose-derived stem cells did not increase astrocyte reactivity. Highly reactive responses to bone marrow mesenchymal stem cells and Schwann cells were equivalent.
Conclusion
This approach can screen therapeutic cells prior to in vivo testing, allowing cells likely to trigger a substantial astrocyte response to be identified at an early stage. NCSCs and differentiated Schwann cell-like adipose-derived stem cells may be useful in treating CNS damage without increasing astrogliosis.
Keywords: 3D cell culture, astrocytes, CNS, reactive gliosis, spinal cord, stem cell therapy
Stem cells have the potential to provide new approaches for the treatment of CNS disorders, either through replacing lost or malfunctioning cells directly or through other mechanisms including angiogenesis, remyelination, neuroprotection and modulation of inflammation [1]. Various stem cells have been shown to be beneficial in animal models of human CNS diseases, with adult stem cells derived from bone marrow, adipose and skin tissues being of particular interest owing to their potential as autologous sources. In order for stem cell therapies to be translated successfully into the clinic it is essential to understand more about their effects. This is often difficult to study in animal models owing to the complexity of the CNS microenvironment and the challenges involved in controlling variables and monitoring cell-level responses.
Cell culture systems provide a more reductionist tool to complement animal models, allowing specific cellular responses and interactions to be studied in a carefully defined environment, with greater reproducibility and more options for continuous monitoring than can be achieved in vivo. Therefore, it is common for researchers to use co-culture models during the development of cell therapies, for example to study their mechanism of action on specific neural cell types [2-6].
However, one important cellular response that could play a critical role in the effectiveness and safety of cell therapies for CNS repair, and has not yet been routinely tested in vitro, is the extent to which transplanted cells might potentiate reactive astrogliosis and glial scarring [7]. The glial scar forms following CNS injury, presenting a physical and chemical impediment to neuronal regeneration. It is comprised predominately of reactive astrocytes, which exhibit a characteristic hypertrophic ramified morph ology with increased expression of GFAP. The extent and persistence of the glial scar is a feature that is of great interest to researchers in regenerative medicine, since modifying the glial response following CNS damage has been associated with changes in functional outcome in animal models [8,9]. While glial scarring has some beneficial effects following CNS damage [10], researchers generally seek to minimize the extent of additional reactive gliosis associated with implanted materials and cells. Monitoring the extent of GFAP expression around a graft is a common output in experiments using animal models. However, such models offer only a snapshot view post-mortem and have numerous complex pathways that could cause changes in reactive gliosis, therefore limiting the ability of researchers to modify their approaches in response to observed changes. A model system in which the response of individual glial cells to engrafted cell therapies can be assessed in a controlled environment would provide useful information about how glia respond to cells from different sources or at different stages of differentiation. This would complement knowledge about efficacy that can be gained from other co-culture approaches (e.g., support of neuronal growth), and would allow greater refinement of cell therapies in vitro prior to testing in vivo, thus reducing the cost, time and reliance on animal models at early stages of therapy development.
Astrocytes are the principal cells responsible for mediating glial scar formation, but it is difficult to predict the extent of astrocyte response to a potential therapy using traditional cell culture approaches, where astrocytes exhibit a highly reactive phenotype. Previously we have developed a 3D culture system in which astrocytes can be maintained in a less reactive manner than in 2D culture and, when stimulated, undergo a classical reactive response reminiscent of their behavior in vivo [11,12]. Here we have adapted this approach to provide a co-culture system to assess the response of astrocytes to potential stem cell therapies. Having established robust protocols for generating and monitoring the model, it was validated using peripheral nervous system glia, which are known to elicit a substantial astrocyte response [13], then used to assess the response of astrocytes to three typical examples of cell therapies that are currently under investigation for CNS repair: neural crest stem cells (NCSCs) from hair follicles [14], differentiated Schwann cell-like adipose-derived stem cells (dADSCs) [15] and mesenchymal stem cells from bone marrow (BM-MSCs) [16].
Materials & methods
Astrocyte cultures
Experiments were performed according to the UK Animals (Scientific Procedures) Act (1986) and approved by the Open University animal ethics advisory group. Sprague–Dawley rats (a β-actin–GFP reporter line or wild-type) were used from established in-house breeding colonies. Primary astrocyte cultures were prepared from postnatal 2-day-old rat cortices as described previously [11]. Cells were maintained in DMEM (Gibco, Life Technologies, CA, USA) supplemented with penicillin and streptomycin (100 U/ml and 100 mg/ml, respectively; Sigma-Aldrich, MO, USA) and 10% v/v fetal calf serum (standard culture medium) in 75 cm2 flasks (Greiner Bio-One Ltd, UK) coated with poly-d-lysine (Sigma-Aldrich). After 8 days, cells reached confluence and flasks were shaken at 150 rpm for 4 h to detach microglia and less adherent cells. Resulting cultures were 95% astrocytes and 5% microglia (as determined by immunoreactivity for GFAP and lectin IB4, respectively). Cells were trypsinized, washed and counted prior to seeding into collagen gels.
Stem cell cultures
BM-MSCs from adult female Fisher 344 rats were obtained from Merck Millipore (MA, USA) and cultured in Mesenchymal Stem Cell Expansion Media (Merck Millipore). Nutrient medium was replaced every 2–3 days until cells were approximately 80% confluent, as observed under phase-contrast microscopy. Cells were removed with 3 ml Accutase™ (Chemicon, Merck Millipore) for 5 min at 37°C and recovered by centrifugation at 300 × g for 5 min.
NCSCs were obtained from the whisker hair follicles of adult Sprague–Dawley rats (250–300 × g). They were cultured as described previously [17], except that the culture medium was supplemented with 10 ng/ml bFGF.
The dADSCs were prepared as described previously [18] and were maintained in DMEM supplemented with 10 μM forskolin (Sigma-Aldrich), 10 ng/ml bFGF (Invitrogen, Life Technologies) and 100 ng/ml neuregulin-1 (R&D Systems) until approximately 80% confluent. Cells were harvested for use before passage 8 with 0.25% Trypsin–EDTA solution for 7–10 min at 37°C. Cells were recovered by centrifugation at 400 × g for 5 min and the pellet was resuspended in standard culture medium.
3D cell cultures
Astrocytes were seeded at 2 × 106 cells/ml within type I collagen gels as described previously [11,12]. Gels were cast in 24-well plates (1.5 ml/well; resulting in gels approximately 4-mm thick) before placing at 37°C to set (~5 min). Wells were topped up with standard culture medium, after first adding a 100-μl suspension of the control (additional astrocytes or Schwann cells) or test stem cells to the surface of gels. Either the astrocytes within the gels or the cells seeded onto the surface were prepared from GFP animals, providing a means to distinguish between cell populations during subsequent analysis (Supplementary Figure 1). Cultures were maintained at 37°C, 5% CO2 for 5 days with media replaced daily. Initial experiments assessed the effect of adding additional astrocytes (negative control), Schwann cells (positive control) or no cells to the surface of the gels. Astrocytes were seeded onto the surface of gels at 1 × 105 cells in 100 μl. The rat Schwann cell line SCL 4.1/F7 (Health Protection Agency, UK) was maintained in standard culture medium and seeded onto the surface of gels at 2 × 104 cells in 100 μl.
Immunodetection of GFAP
After 5 days in culture, medium was removed and gels were fixed in 4% paraformaldehyde following a brief rinse in phosphate-buffered saline. A slice was removed from the center of each gel (Figure 1) and immunofluorescence staining to detect GFAP was conducted as described previously [11]. Hoechst 33258 (Sigma-Aldrich) was included with the secondary antibody to label cell nuclei.
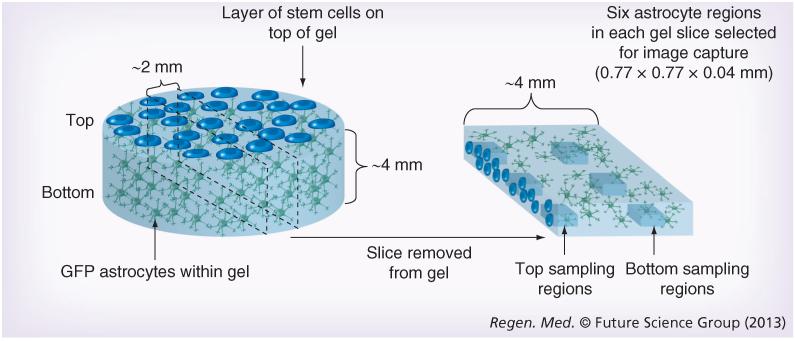
Figure 1. 3D cell culture system.
Astrocyte gels were set within 24-well culture plates before control or test cells were seeded onto the top surface. Following 5 days in culture, gels were fixed and a slice approximately 2-mm thick was removed from the center of each gel for confocal microscopy. Images were captured using standardized microscope settings from three positions at the top and three positions near the bottom of each gel slice.
Confocal microscopy following a predefined protocol was performed using a Leica TCS SP5 confocal microscope (Leica Microsystems, Germany) to sample designated fields within the gel slices (Figure 1). Fields for quantification were 0.77 × 0.77 × 0.04 mm (XYZ) with one image per 1 μm in the Z direction. Three fields were sampled at the top edge of the gel slice where the astrocytes were in contact with the test cells. Equivalent fields, 1 mm from the bottom edge of the gel, were also sampled for comparison with the top fields to assess whether cellular responses were contact- and/or distance-dependent. Volume of GFAP immunoreactivity was measured using automated ana lysis protocols (Volocity 6.1, Perkin-Elmer, MA, USA) as described previously [12]. Cell number was determined by counting Hoechst stained nuclei so GFAP volumes could be expressed per cell (Supplementary Table 1). GFP labeling was used to distinguish between astrocytes within the gel and test cells applied to the surface, to ensure that only astrocytic GFAP and astrocyte nuclei were included in the analyses. A minimum of five independent gels were analyzed from at least two separate cell preparations in each case.
Statistical analysis
Data were analyzed using GraphPad Prism® software (Prism 5, GraphPad Software, Inc., CA, USA). Normality and quality of variance tests were performed on all data to determine which test was appropriate. A paired t-test was used with significance level 95% for comparison between the top and bottom regions within gels. An analysis of variance with Tukey’s multiple comparison test was performed with significance level 95% to compare the top and bottom regions between gels that had received different test cell populations (or controls with Schwann cells or no added test cells). A Kruskal–Wallis test with Dunn’s multiple comparison test was used with significance level 95% to compare astrocyte numbers between groups. All values are indicated as mean ± standard error of the mean.
Results
Co-cultures with astrocytes in a 3D collagen gel and control or test cells seeded on top were set up and analyzed as demonstrated in Figure 1. Stem cell populations were prepared according to previously established protocols and characterized by detection of immunoreactivity to markers as follows: NCSCs (SOX10+), BM-MSCs (CD54+, integrin β1+, CD45− and CD14−) and dADSCs (S100+, GFAP+, p75+ and SOX10+).
Initial experiments (Figure 2) showed little difference in the GFAP volume per astrocyte after 5 days when 1 × 105 astrocytes were added to the surface of the gels compared with control gels with no additional cells seeded on top. This confirmed that any response of astrocytes within the gels observed in subsequent experiments was the result of the specific cell type under test, rather than the presence of a layer of cells per se. The number of astrocytes used in this initial test was five-times greater than the numbers of test cells used in subsequent experiments, providing assurance that any localized alterations in oxygen/nutrient levels that might occur owing to application of an additional cell layer would not affect astrocyte reactivity within the gels. Furthermore, there was no difference in GFAP volume per astrocyte between the cells in the top and bottom regions of these gels after 5 days, with both regions remaining equivalent to controls that received no additional cells.
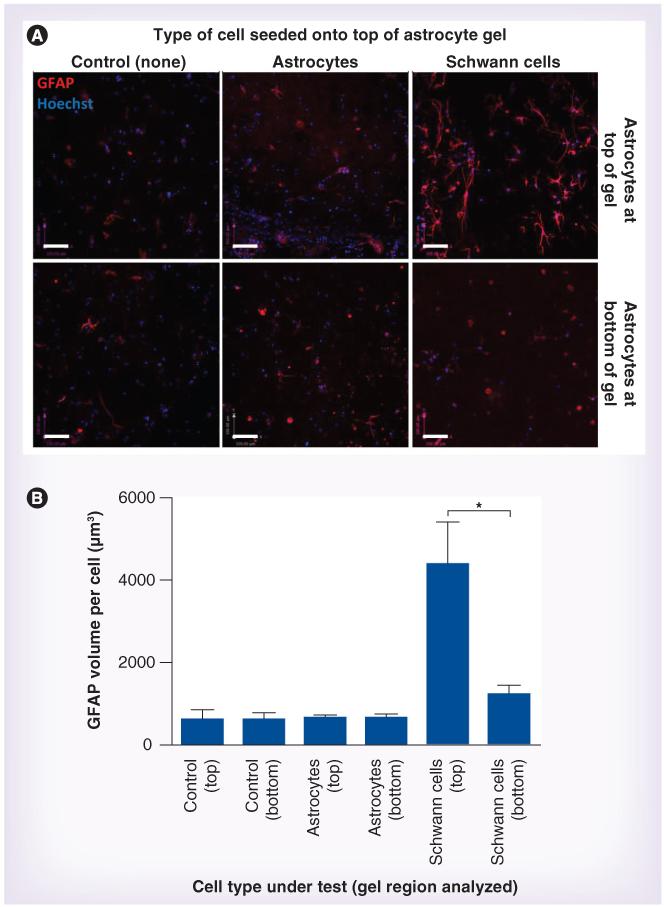
Figure 2. Astrocyte reactivity in response to control cells.
(A) Astrocytic GFAP immunoreactivity (red) was detected after 5 days in the absence of cells (control), or in response to astrocytes (negative control) or Schwann cells (positive control) seeded onto the top of the gels. Scale bars = 100 μm. (B) Astrocytes within the gels were distinguished from cells seeded on the surface using GFP, and nuclei were stained using Hoechst (blue). Confocal image series were analyzed to quantify the volume of GFAP immunoreactivity per astrocyte within the gel regions. Three replicate series were analyzed within each region (top and bottom) of each gel and data are means ± standard error of the mean from at least five independent gels. Top and bottom regions were compared using a paired t-test. *p < 0.05.
To investigate whether the parameters used in this model system would allow quantification of the response of astrocytes to a cell type known to trigger reactive gliosis, a Schwann cell line was used as a positive control. Five days after 2 × 104 Schwann cells were seeded onto the astrocyte gels, there was an increase in GFAP volume per astrocyte compared with control gels, with a 3.5-fold difference between the GFAP volume per astrocyte at the top of the Schwann cell-seeded gels compared with the bottom (Figure 2). There was a slight increase in GFAP volume per astrocyte in the bottom region compared with the control condition shown in Figure 2, but this was not significant (Table 1). Pilot experiments (data not shown) also tested 1 × 105 Schwann cells in this experiment; a greater increase in GFAP volume was observed but due to the adequate distinction between Schwann cell and control conditions (Figure 2), and in order to minimize the numbers of test cells required in subsequent experiments, the density of 2 × 104 cells per 100 μl was used for the experiments described hereafter.
Table 1. Statistical comparison of the astrocyte response using Tukey’s multiple comparison test.
| Schwann cells | NCSCs | dADSCs | BM-MSCs | |
|---|---|---|---|---|
| Top | ||||
| Control | ** | NS | NS | ** |
| BM-MSC | NS | ** | * | – |
| dADSC | ** | NS | – | – |
| NCSC | *** | – | – | – |
| Bottom | ||||
| Control | NS | NS | NS | * |
| BM-MSC | NS | ** | NS | – |
| dADSC | NS | NS | – | – |
| NCSC | NS | – | – | – |
GFAP volume per astrocyte was compared between groups for both the top and the bottom regions of the gels using one-way analysis of variance. Tukey’s multiple comparison test was used to compare astrocyte reactivity between all test groups as well as the controls with no cells or Schwann cells on top.
p < 0.05;
p < 0.01;
p < 0.001.
BM-MSC: Mesenchymal stem cell from bone marrow; dADSC: Differentiated Schwann cell-like adipose-derived stem cell; NCSC: Neural crest stem cell; NS: Nonsignificant.
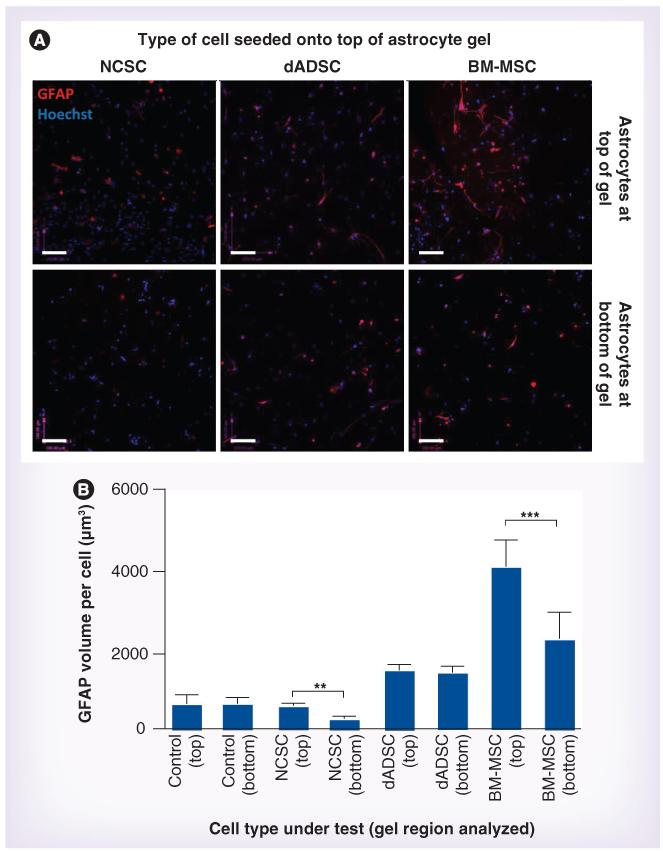
The effect of adding NCSCs, dADSCs or BM-MSCs (2 × 104 cells in 100 μl) to the surface of the astrocyte gels was determined (Figure 3) and Table 1 summarizes the results of comparing both the top and the bottom regions between the groups (and controls). BM-MSCs caused a significant twofold increase in GFAP volume per astrocyte at the top of the gels compared with the bottom of the same gels and also a sixfold increase compared with the top of the control gels (no added cells). There was a significant increase in astrocytic GFAP volume in fields at the bottom of the BM-MSCs compared with the bottom of control gels and the relative responses of the top and bottom fields may indicate a gradient of response to some secreted factor originating from the BM-MSCs or proximal astrocytes responding to these cells. The level of GFAP per astrocyte in the top fields in cocultures with BM-MSCs was equivalent to that in Schwann cell-positive controls (Figure 2 & Table 1). Neither the NCSCs nor the dADSCs caused a significant increase in GFAP per astrocyte at the top of the gels compared with controls (Table 1), although there was a slight increase in GFAP per astrocyte in the dADSC condition (not significant). Interestingly, there was a difference in GFAP per astrocyte between the top and bottom regions of the gels that received NCSCs, although this appears to be due to a slightly reduced GFAP volume per astrocyte in the bottom region (which was not significant when compared with the bottom regions of the other conditions; Table 1).
Figure 3. Astrocyte reactivity in response to test cell populations.
(A) Astrocytic GFAP immunoreactivity (red) was detected in defined locations within gels after 5 days in response to NCSCs, dADSCs or BM-MSCs seeded onto the top of gels. Astrocytes within the gels were distinguished from cells seeded on the surface using GFP, and nuclei were stained using Hoechst (blue). Scale bars = 100 μm. (B) Confocal image series were analyzed to quantify the volume of GFAP immunoreactivity per astrocyte within the gel regions. Three replicate series were analyzed within each region (top and bottom) of each gel and data are means ± standard error of the mean from at least six independent gels. Top and bottom regions were compared using a paired t-test. **p < 0.01; ***p < 0.001. BM-MSC: Mesenchymal stem cell from bone marrow; dADSC: Differentiated Schwann cell-like adipose-derived stem cell; NCSC: Neural crest stem cell.
Discussion
The results show that astrocytes in 3D collagen gels respond to cell populations seeded onto their surface, providing a useful model for comparing the extent to which different cell types can trigger reactive gliosis. Astrocytes in control gels showed a relatively unreactive phenotype after 5 days, which is consistent with previous reports [11], but when stimulated by the presence of Schwann cells the adjacent astrocytes adopted a classical ramified reactive phenotype with increased GFAP volume per cell. This is similar to the response of astrocytes adjacent to a dissociated dorsal root ganglion (DRG) culture demonstrated previously in another 3D culture system [12], and reminiscent of their behavior following CNS damage in vivo [19].
When other cell types were tested as examples of potential cell therapies it was possible to identify differences in their ability to modulate astrocyte reactivity. Of the cells tested, BM-MSCs elicited the greatest astrocyte response, with dADSCs and NCSCs being equivalent to unstimulated controls. In those groups where an increase in astrocyte reactivity was observed, the response was limited to the astrocytes adjacent to the cells under test. This suggests that the effect may be mediated by direct physical contact between cells rather than through the action of diffusible molecules, although the latter cannot be ruled out. The model described here could be used to investigate the mechanisms underpinning the astrocyte response; for example, through the application of conditioned media from cultures of the cells under test. In a previous study we showed that conditioned media from DRG cultures failed to elicit the astrocyte response that was observed when DRG cells and astrocytes were co-cultured in direct contact [12].
BM-MSC populations have been associated with both increased and reduced glial scarring in vivo [20-25] and in vitro [26]. It is possible that the observation here that direct contact between BM-MSCs and astrocytes in 3D culture for 5 days increases reactive gliosis may help to explain some of the diverse effects observed in other models. Schwann cells have been extensively tested for cellular therapy of spinal cord injury (reviewed in [27]); they can remyelinate damaged axons and provide a permissive environment for axon growth. However, axons regenerating into Schwann cell grafts fail to reenter the host spinal cord, most likely as a result of the Schwann cells potentiating the glial scar reaction, consistent with the results shown here. Autologous Schwann cell transplants necessitate the sacrifice of a peripheral nerve; therefore, alternative methods to generate Schwann cells have been investigated, such as dADSCs. It is interesting that while these resemble Schwann cells morphologically and in terms of expression of characteristic marker proteins [18], they differed considerably from the Schwann cells used in this study in terms of their ability to elicit reactive astrogliosis in 3D culture. A previous study, in which similar cells were tested in a rat contusion brain injury model, showed a reduction in reactive gliosis [15]. The model described here could potentially be used to explore whether dADSCs or other cell types are able to directly reduce the response of reactive astrocytes in vitro (e.g., following stimulation with TGF-β1) [11]. As these cells potentially mimic the neuroregenerative properties of Schwann cells without triggering the astrocyte responses associated with them, it would be interesting to explore their use in treating other CNS injuries. Another study showed that Schwann cells differentiated from nestin-positive spheroid structures derived from adipose stem cells could myelinate spinal cord axons [28]. Neurally induced adipose stem cells also have some beneficial effects on spinal cord repair [29], but how these cells influenced the glial scar was not determined. Given the abundance of adipose tissue that can be harvested with minimal morbidity and the ease of stem cell extraction for differentiation to a Schwann cell-like phenotype, we believe that dADSCs are a potentially useful cell source for treatment of CNS injuries.
NCSCs from skin offer another exciting possibility for CNS regeneration owing to their capacity to give rise to both neurons and glia [30], but despite having been used in experimental models for treating spinal cord trauma [14,31], little is known about their interaction with host astrocytes. Our findings indicate that they do not increase astrocyte reactivity during 5 days of contact in vitro. In this context it is interesting to note that specialized neural crest cells transiently populate the peripheral nervous system–CNS interface of the spinal cord, without gliosis, during a developmental window when DRG neurites grow into the CNS. It is only once these cells have disappeared and Schwann cells abut the CNS that neurite regeneration into the CNS is prohibited and is associated with gliosis [32].
While it is interesting to note the differences in astrocyte response elicited by the cell types tested here, it is important not to overinterpret these observations, since they were based on a single time point and a specific protocol for test cell preparation, and cells remained in vitro. Furthermore, although it is useful to understand the direct effect of a potential cell therapy on astrocytes in culture, it is important to consider the following: other cell types such as microglia are critical mediators of the glial response in vivo; the behavior and differentiation of the test cells in vivo may differ from in vitro; and the starting cell populations used here may be different to those used in other studies. Notwithstanding these limitations, the culture system developed here provides a novel tool for assessing an important aspect of CNS cell therapy and could be used for understanding fundamental cell biology involved in reactive gliosis.
Conclusion
The principal finding is that this 3D culture system is suitable for screening potential therapeutic cells prior to in vivo testing, allowing cell populations likely to trigger a substantial astrocyte response to be identified at an early stage. The model system presented here is simple enough to encourage widespread adoption, requiring standard cell culture equipment and simple protocols for analyzing astrocyte responses. It should be of interest to researchers wishing to develop cell therapies for CNS damage and disease, or those involved in understanding astrocyte biology.
Future perspective
Given the growing interest in the use of therapeutic cells to treat chronic neurological injuries/diseases, there is increasing demand for experimental models that can be used to screen for the most promising candidates. It is likely that researchers will continue to seek to reduce reliance on animal models for ethical and practical reasons, so it is predicted that noninvasive in vitro models will form the initial test system of choice. 3D culture models permit continuous monitoring of specific cellular responses and interactions in a more physiologically relevant spatial arrangement than simpler cultures. In the future, the 3D model described here will lend itself to the inclusion of other CNS cell types, and to building up levels of complexity by investigating how co-cultures of CNS cells can respond to different therapeutic cell types. New technologies that yield cultures of human neural cells for research into CNS disorders will provide an opportunity to avoid limitations associated with using animal cells to model human conditions. Scaling, automation and commercial supply of 3D culture systems will facilitate their widespread adoption for routine use. We expect that increased utilization of these types of models will help to ensure that only suitable candidate therapeutic cells will be taken forward into preclinical in vivo testing.
Supplementary Material
Executive summary
Background
-
■
Astrocytes in 3D collagen gels can respond to cell therapy populations seeded onto their surface.
Methods
-
■
Changes in astrocyte reactivity were monitored 5 days after addition of neural crest stem cells, differentiated Schwann cell-like adipose-derived stem cells, bone marrow stem cells or control Schwann cells.
Results
-
■
Neural crest stem cells did not cause any increase in the levels of the astrocyte activation marker.
-
■
Differentiated Schwann cell-like adipose-derived stem cells did not increase astrogliosis.
-
■
Bone marrow stem cells elicited substantial reactive gliosis, with a twofold increase in levels of the astrocyte activation marker.
-
■
Schwann cells triggered a 3.5-fold increase in reactive gliosis as a positive control for system validation.
Discussion
-
■
Neural crest stem cells and differentiated Schwann cell-like adipose-derived stem cells may be useful therapeutic cells for treating CNS injuries without exacerbation of reactive astrogliosis.
-
■
This system provides a useful model for comparing the extent to which different cell types can elicit reactive astrogliosis.
-
■
This model is suitable for screening potential therapeutic cells prior to in vivo testing, allowing cell populations likely to trigger a substantial astrocyte response to be identified at an early stage.
-
■
The results presented should encourage widespread adoption of such models.
Acknowledgements
The authors are grateful to the The Open University Biomedical Research Unit for technical support.
Footnotes
Financial & competing interests disclosure
This work was funded by the Wellcome Trust (080309) and the Swedish Research Council. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Lindvall O. Why is it taking so long to develop clinically competitive stem cell therapies for CNS disorders? Cell Stem Cell. 2012;10(6):660–662. doi: 10.1016/j.stem.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Mauri M, Lentini D, Gravati M, et al. Mesenchymal stem cells enhance GABAergic transmission in co-cultured hippocampal neurons. Mol. Cell. Neurosci. 2012;49(4):395–405. doi: 10.1016/j.mcn.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro CA, Fraga JS, Graos M, et al. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012;3(3):18. doi: 10.1186/scrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens CL, Toda H, Palmer TD, Demarse TB, Ormerod BK. Adult neural progenitor cells reactivate superbursting in mature neural networks. Exp. Neurol. 2012;234(1):20–30. doi: 10.1016/j.expneurol.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Takayama Y, Moriguchi H, Kotani K, Suzuki T, Mabuchi K, Jimbo Y. Network-wide integration of stem cell-derived neurons and mouse cortical neurons using microfabricated co-culture devices. Biosystems. 2012;107(1):1–8. doi: 10.1016/j.biosystems.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Voulgari-Kokota A, Fairless R, Karamita M, et al. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp. Neurol. 2012;236(1):161–170. doi: 10.1016/j.expneurol.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Toft A, Tome M, Barnett SC, Riddell JS. A comparative study of glial and neural cell properties for transplant-mediated repair of the injured spinal cord. Glia. 2013;61(4):513–528. doi: 10.1002/glia.22452. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 2011;84(4-5):306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7(4):494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol. Neurobiol. 2012;46(2):251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- 11.East E, Golding JP, Phillips JB. A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J. Tissue Eng. Regen. Med. 2009;3(8):634–646. doi: 10.1002/term.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.East E, Golding JP, Phillips JB. Engineering an integrated cellular interface in threedimensional hydrogel cultures permits monitoring of reciprocal astrocyte and neuronal responses. Tissue Eng. Part C Methods. 2012;18(7):526–536. doi: 10.1089/ten.tec.2011.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakatos A, Barnett SC, Franklin RJ. Olfactory ensheathing cells induce less host astrocyte response and chondroitin sulphate proteoglycan expression than Schwann cells following transplantation into adult CNS white matter. Exp. Neurol. 2003;184(1):237–246. doi: 10.1016/s0014-4886(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 14.Sieber-Blum M. Epidermal neural crest stem cells and their use in mouse models of spinal cord injury. Brain Res. Bull. 2010;83(5):189–193. doi: 10.1016/j.brainresbull.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Fang JS, Wang W, Chen RK, Shen CF. Transplantation of Schwann cells differentiated from adipose-derived stem cells modifies reactive gliosis after contusion brain injury in rats. J. Int. Med. Res. 2011;39(4):1344–1357. doi: 10.1177/147323001103900421. [DOI] [PubMed] [Google Scholar]
- 16.Huang B, Tabata Y, Gao JQ. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J. Control. Release. 2012;162(2):464–473. doi: 10.1016/j.jconrel.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Jackson JS, Golding JP, Chapon C, Jones WA, Bhakoo KK. Homing of stem cells to sites of inflammatory brain injury after intracerebral and intravenous administration: a longitudinal imaging study. Stem Cell Res. Ther. 2010;1(2):17. doi: 10.1186/scrt17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007;207(2):267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Silver J, Miller JH. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 20.Abrams MB, Dominguez C, Pernold K, et al. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor. Neurol. Neurosci. 2009;27(4):307–321. doi: 10.3233/RNN-2009-0480. [DOI] [PubMed] [Google Scholar]
- 21.Ban DX, Ning GZ, Feng SQ, et al. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen. Med. 2011;6(6):707–720. doi: 10.2217/rme.11.32. [DOI] [PubMed] [Google Scholar]
- 22.Grigorian AS, Gilerovich EG, Pavlichenko NN, Kruglyakov PV, Sokolova IB, Polyntsev DG. Effect of transplantation of mesenchymal stem cells on neuronal survival and formation of a glial scar in the brain of rats with severe traumatic brain injury. Bull. Exp. Biol. Med. 2011;150(4):551–555. doi: 10.1007/s10517-011-1187-1. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 24.Pavlichenko N, Sokolova I, Vijde S, et al. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;1233:203–213. doi: 10.1016/j.brainres.2008.06.123. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Wei X, Li J, Heine LA, Rosenwasser R, Iacovitti L. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 2010;19(9):1073–1084. doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q, Li Y, Shen L, et al. Bone marrow stromal cells reduce ischemia-induced astrocytic activation in vitro. Neuroscience. 2008;152(3):646–655. doi: 10.1016/j.neuroscience.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma. 2011;28(8):1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi GF, Kim MR, Kim DW, Jiang MH, Son Y. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp. Neurol. 2010;222(2):304–317. doi: 10.1016/j.expneurol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Arboleda D, Forostyak S, Jendelova P, et al. Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cell. Mol. Neurobiol. 2011;31(7):1113–1122. doi: 10.1007/s10571-011-9712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achilleos A, Trainor PA. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22(2):288–304. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieber-Blum M, Schnell L, Grim M, Hu YF, Schneider R, Schwab ME. Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Mol. Cell. Neurosci. 2006;32(1-2):67–81. doi: 10.1016/j.mcn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Golding JP, Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Mol. Cell. Neurosci. 1997;9(5-6):381–396. doi: 10.1006/mcne.1997.0647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.