Abstract
In order to examine the role of the ICAM-1 C-terminal domain in mediating transendothelial T-lymphocyte migration and ICAM-1 mediated signal transduction. Mutant human ICAM-1 molecules were expressed in rat brain microvascular endothelial cells. Expression of human wild type ICAM-1 in rat brain endothelial cells (EC) resulted in a significant increase over basal levels in both adhesion and transendothelial migration of rat T-lymphocytes. EC expressing ICAM-1, in which the conserved tyrosine residue at codon 512 was substituted with phenylalanine (hICAM-1Y512F), also exhibited increased lymphocyte migration albeit less than with wild type ICAM-1. Conversely, expression of truncated human ICAM-1 proteins, in which either the intracellular domain was deleted (hICAM-1ΔC) or both the intracellular and transmembrane domains were deleted through construction of a glycophospholipid anchor (GPI-hICAM-1), did not result in an increase in lymphocyte adhesion and their ability to increase transendothelial migration was attenuated. Truncated ICAM-1 proteins were also unable to induce ICAM-1 mediated Rho GTPase activation. Rat EC treated with cell permeant penetratin-ICAM-1 peptides comprising human or rat ICAM-1 intracellular domain sequences inhibited transendothelial lymphocyte migration but not adhesion. Peptides containing a phosphotyrosine residue were equally potent at inhibiting lymphocyte migration. These data demonstrate that the intracellular domain of ICAM-1 is essential for transendothelial migration of lymphocytes, and that peptidomimetics of the ICAM-1 intracellular domain can also inhibit this process. Such competitive inhibition of transendothelial lymphocyte migration, in the absence of an affect on adhesion, further implicates ICAM-1-mediated signalling events in the facilitation of T-lymphocyte migration across brain EC. Thus agents which mimic the ICAM-1 intracellular domain may be attractive targets for novel anti-inflammatory therapeutics.
Keywords: Endothelial cells, Adhesion molecules, Cell trafficking, Neuroimmunology
Introduction
Endothelial cells (EC) of the blood-central nervous system (CNS) barriers display characteristics that differentiate them from endothelium of other organs. In particular these cells express well-developed intercellular tight junctions and are responsible for exceptionally low paracellular permeability. The migration of leukocytes across the specialised vascular barriers of the CNS also differs from that observed at other vascular beds and is dependent upon a variety of controlling factors (1-3). One of these factors is provided by the endothelia, which facilitates the passage of migratory cells across the vessel wall through the expression of cell adhesion molecules. However, these molecules do not appear to be simply points of attachment but are involved in transducing leukocyte adhesion-mediated signalling responses to EC (4-6)
The activation state of a circulating T-lymphocyte is important in determining its ability to migrate out of the circulation (3, 7-10). Nevertheless, using functional assays we have shown that brain EC are also intimately and actively involved in facilitating lymphocyte diapedesis (5). It has previously been demonstrated that the expression of the adhesion molecule ICAM-1 on EC is pivotal in supporting lymphocyte migration across vascular endothelium in both peripheral tissue (8,9) and the CNS (2,3,6,11). We have shown that following ICAM-1 cross-linking or co-culture with T-lymphocytes, brain EC ICAM-1 is capable of evoking signal transduction events, suggesting that EC actively respond to leukocyte adhesion (4,5,12). Mimicking leukocyte attachment through antibody cross-linking of EC ICAM-1 molecules in vitro, we have reported induction of EC actin stress-fibres (5) and an enhanced tyrosine phosphorylation of cortactin (12), focal adhesion kinase (FAK), paxillin (PAX) and p130cas (CAS) (4). Both ICAM-1 cross-linking and co-culture of EC with T-lymphocytes results in increased levels of GTP-loaded EC Rho proteins, indicating that EC Rho proteins are also involved in ICAM-1 mediated signalling events (5). Transendothelial lymphocyte migration is also inhibited following inactivation of EC Rho protein function (5,13). How ICAM-1 elicits these signals remains unclear. ICAM-1 has no catalytic activity associated with its short cytoplasmic domain, but in spite of this, it is known to bind a variety of molecules such as α-actinin (14), β-tubulin, glyceraldehyde-3-phosphate dehydrogenase (15) and ezrin (16,17). These proteins all have the potential to perform signalling functions. In addition, it has already been described that ICAM-1 can interact with F-actin (18), and that the avidity of this interaction increases following ICAM-1 cross-linking (19).
The cytoplasmic domain of ICAM-1 consists of 27 amino acids in rat and 28 amino acids in human. A striking feature of this sequence are the presence of a high number of positively charged amino acid residues, which appear to be important for ICAM-1 to bind ezrin (20), a property that is shared with other ezrin binding adhesion molecules such as CD44 (20). It is also interesting to note that the tyrosine residue within the cytoplasmic domain at position 512 in human ICAM-1 is conserved in both rat and mouse ICAM-1, and is also conserved in the shorter cytoplasmic domain of VCAM-1. It has already been demonstrated that ICAM-1 can be phosphorylated on the cytoplasmic tyrosine residue in response to the binding of immobilized fibrinogen to ICAM-1 (21) and that this results in the recruitment of the tyrosine phosphatase SHP-2.
In order to define the role of the ICAM-1 cytoplasmic domain in EC signalling and control of transendothelial lymphocyte migration within the CNS, we have expressed mutant human ICAM-1 molecules in rat brain EC and used competitive intracellular peptides mimicking the ICAM-1 cytoplasmic domain. We report here that the presence of the cytoplasmic domain of ICAM-1 is essential for transendothelial migration of T-lymphocytes as well as ICAM-1-mediated activation of Rho. Thus, deletion of the cytoplasmic domain of ICAM-1 results in an inhibition of lymphocyte adhesion and migration whereas treatment with competitive peptides resulted only in a reduction in migration.
Materials and Methods
Materials
Mouse mAbs to rat ICAM-1 (1A29 and 3H8) were generated from hybridomas kindly provided by Dr M. Miyasaka (Osaka University, Japan) and Dr W. Hickey, (Dartmouth Medical School, USA) respectively. Mouse mAbs to human ICAM-1 (clone BBA4) were obtained from R&D systems (Oxford, UK). Rabbit anti-mouse antibodies (RAM) were from Sigma (Poole, UK). Mouse anti-phosphotyrosine mAb clone 4G10 was obtained from Upstate Biotechnology (Lake Placid, NY, USA) and clone PY20 from Autogenbioclear, (Calne, UK). Mouse anti-rat focal adhesion kinase (FAK) was obtained from BD Transduction Laboratories (Heidelberg, Germany). HRP-conjugated anti mouse IgG was obtained from Pierce (Chester, UK). [32P]orthophosphate, [α-32P]NAD and ECL reagents were obtained from Amersham International (Bucks, UK). Fugene transfection reagent was from Boehringer (Mannheim, Germany). Streptavidin-FITC was obtained from Jackson Immunochemicals, (VA, USA). The reduction sensitive cross-linking reagent 3,3′-Dithiobis[sulphosuccininimidyl propionate] (DTSSP) was obtained from Pierce (Chester, UK). Unless otherwise stated all chemicals used were obtained from the Sigma Chemical Company (Dorset, UK). Streptavidin-sepharose was obtained from Pharmacia, UK
Endothelial cells
The immortalised Lewis rat brain microvascular EC line GP8/3.9 (22) was maintained in Ham’s F-10 medium supplemented with 10% FCS, 2mM glutamine, 100 U/ml penicillin and 100μg/ml streptomycin. GP8/3.9 cells transfected with RSVpuro human ICAM-1 constructs were maintained in GP8/3.9 medium containing puromycin (20μg/ml).
cDNA constructs
Wild type human ICAM-1 and mutant ICAM-1 molecules were generated using RT-PCR from CHO cells overexpressing human ICAM-1 (a gift from Dr. J. Pearson, Kings College London, UK). RNA was extracted from ICAM-1 expressing CHO cells using an RNeasy® kit (Qiagen, West Sussex UK) according to the manufacturer’s instructions. Reverse transcriptase was used to generate cDNA, from oligo(dT)15-18 primed RNA, which was used as a template for PCR. PCR reactions were performed using the forward primer 5′-GGAAGCTTCTAGAATGGCTCCCAGCAGCCCCC-3′ and one of three reverse primers 5′-GGGTCGACTCTAGAGTTATAGAGGTACGTGCTGAGG-3′, 5′-GGGTCGACTCTAGATCAGTTATAGAGGTACGTGCTGAG-3′ and 5′-GGGTCGACTCTAGATCAGGGAGGCGTGGCTTGTGTGTTCGGTTTCATGGGGGTCCCTTTTTGGGCCTGTTGTAGTCTGAATTTCTG-3′ to generate wild type, C-terminal truncation at amino acid 504 and Tyr512→Phe512 point mutations respectively. Hind III and SalI sites, which were engineered into the forward and reverse PCR primers respectively were used to clone the PCR fragment into a Hind III/Xho I restricted pcDNA3/RSVpuro plasmid. The identity of the PCR generated ICAM-1 inserts was confirmed by DNA sequencing. A GPI anchored human ICAM-1 construct (pCDM8 GPI-ICAM-1) in which both the C-terminal domain and transmembrane domain are deleted by truncating ICAM-1 at codon 480 and fusing this sequence to the GPI-anchor sequence of human LFA3 (14) was a gift from T. Springer (Centre for Blood Research, Harvard Med. School, MT, USA). The GPI-ICAM-1 sequence was excised from pCDM8 using HindIII and Xba1 and directionally cloned into HindIII/Xba1 digested RSVpuro.
Penetratin-ICAM-1 peptides
Penetratin peptides were N-terminally biotinylated and consisted of 16 residues of the penetratin sequence (RQIKIWFQNRRMKWKK). The 13 C-terminal amino acids of human (h) or rat ICAM-1(r) were synthesised distal to the penetratin sequence. Peptides were HPLC purified before use. Sequences used were hICAM-1 (QRKIKKYRLQQAQ), YP-hICAM-1 (QRKIKK(YP)RLQQAQ), rICAM-1 (QRKIRIYKLQKAQ) YP-rICAM-1 (QRKIRI(YP)KLQKAQ) and an irrelevant sequence from the soluble part of rat rod opsin (CKPMSNFRFGENH). Penetratin peptides were localised in cells using streptavidin-FITC (1:50, Jackson, USA) following fixation in 3.7% paraformaldehyde and permeabilisation with 0.2% triton-X100
Generation of stable GP8/3.9 rat brain EC lines overexpressing human ICAM-1
Pre-confluent GP8/3.9 EC (approx. 0.5×106 cells) were transfected with 3-6 μg of each of the ICAM-1 constructs or with the pCDNA3/RSVpuro vector (no insert) using Fugene transfection reagent according to the manufacturer’s instructions. After 24-48h, puromycin (20 μg/ml) was added to cultures to select for ICAM-1 expressing cells. In subsequent studies transfected cells were maintained in medium containing 20μg/ml puromycin and removed prior to co-culture with T-lymphocytes.
Flow cytometric analysis of ICAM-1 transfectants
Puromycin resistant GP8/3.9 brain EC clones were generated and assessed by flow cytometric analysis using a human specific anti-ICAM-1 antibody (clone BBA4) to demonstrate the presence of human ICAM-1 expression. After detachment with collagenase (1 mg/ml) for 20 min cells were washed and incubated with the BBA4 mAb (10μg/ml) for 1h on ice. After washing, cell pellets were resuspended in 100μl of FITC-conjugated goat-anti-mouse IgG (1/100 dilution) and incubated for a further 30 min prior to standard paraformaldehyde fixation and flow cytometric analysis. Data was quantified and rendered using Cellquest® software. A secondary isotype matched IgG control sample for each cell line was also acquired.
Metabolic labelling
GP8/3.9 brain EC (5 × 106) were incubated in phosphate free DMEM overnight in the presence of 0.2mCi/ml [32P]orthophosphate. Cells were washed in HBSS and were subsequently co-cultured with Concanavalin A stimulated lymphocytes (107) or cross-linked with ICAM-1 in complete DMEM.
Immunopreciptation of ICAM-1
1×106 brain EC were washed with ice cold HBSS containing 1 mM Na3VO4 prior to lysis in buffer containing 20 mM HEPES pH 7.5, 0.5% NP-40, 3 mM MgCl2, 100 mM NaCl, 1 mM EGTA, 50 mM β-glycerophosphate, 1 mM Na3VO4, 0.5 mM PMSF, 1 mM NaF, 10 μg/ml leupeptin, 10 μg/ml aprotinin for 30 min at 4°C. Cell nuclei were pelleted by centrifugation and discarded. Lysates (0.1-0.5 mg protein) were incubated with specific antibodies (1:100) for 2h at 4°C with end-over-end rotation. Immune complexes were captured by incubation with protein G agarose for a further 2h at 4°C before extensive washing in lysis buffer. The washed immune complexes were eluted with SDS sample buffer (100 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 1 mM orthovanadate, 100 mM DTT with bromophenol blue), heated to 95°C for 5 min, and proteins resolved on SDS-PAGE
Western blotting
Immunoprecipitated samples or cell lysates were heated in SDS sample buffer and subjected to SDS-PAGE before transfer to nitrocellulose membranes (Schleicher and Schuell, London UK) using a semi-dry blotter (Biorad, North Yorks.) at 10V (3.55mA/cm2) for 30 min. The membranes were blocked in 5% fat-free milk in PBS for 2h before incubation overnight at 4°C with 1:5 dilution of 3H8 hybridoma supernatant, 1μg/ml 1A29 or BBA4 at an appropriate dilution in PBS containing 5% BSA. Membranes were washed several times with PBS/0.1% Tween 20 before a 1h incubation with an anti-mouse HRP-conjugated IgG at a dilution of 1:15,000 (Pierce, Chester, UK). After several washes in PBS/0.1% Tween-20, blots were developed using the ECL system (Amersham International) according to the manufacturer’s instructions and exposed to X-ray film.
Dot-blot Analysis of Penetratin-hICAM-Peptides
Biotinylated peptides (100g/μml) were incubated with GP8/3.9 cells expressing WT-hICAM-1 for 2 h and cells subsequently lysed in buffer containing 20 mM HEPES pH 7.5, 0.5% NP-40, 3 mM MgCl2, 100 mM NaCl, 1 mM EGTA, 50 mM β-glycerophosphate, 1 mM Na3VO4, 0.5 mM PMSF, 1 mM NaF, 10 μg/ml leupeptin, 10 μg/ml aprotinin for 30 min at 4°C and incubated with 20μl of streptavidin-.sepharose and washed 3 times in the same buffer. Samples were subsequently boiled for 5 min and dot-blotted on nitrocellulose membranes prior to immunoblotting with anti-phosphotyrosine (4G10)mAb as described
Adhesion of peripheral lymph node lymphocytes to EC and T-lymphocyte transendothelial migration
Adhesion assays and transendothelial migration assays were carried out as previously described using cells harvested from Lewis rat peripheral lymph nodes and MBP-antigen specific T-lymphocyte lines (2,3,10). The results are expressed as the means ± SEM and significant differences between groups determined by Student’s t-test.
Fluorescence microscopic analysis of biotinylated ICAM-1 peptides, and F-actin localisation
Untransfected brain EC were treated with 100U/ml rat recombinant IFNγ for 48 h to up-regulate endogenous ICAM-1 expression. Cells were fixed with 3% paraformaldehyde for 20 min at room temperature, washed and permeabilised with 0.25% triton X-100 followed by blocking with 10% FCS in PBS. Streptavidin-Texas Red (1:100, Jackson immunochemicals) was used to localise C-terminal ICAM-1 biotinylated peptides or 0.1μg/ml Oregon-green phalloidin (Molecular Probes) used to visualise F-actin. Cells were viewed on a laser scanning confocal microscope (Leica or Ziess).
Rho Activation
GST-rhotekin was expressed from pGEX-2T-rhotekin in E. Coli for 5 h using 1 mM IPTG (Gibco/BRL, Paisley, UK) and used to precipitate activated rho as previously described (23,24). Rho proteins were labelled by ADP-ribosylation as previously described (5).
Analysis of ICAM-1 expression on GP8/3.9 brain EC
To evaluate whether manipulation of the endothelial cell lines resulted in alteration of ICAM expression, the surface expression of ICAM-1 and ICAM-2 was evaluated using ELISA as previously described (22).
Results
Co-culture of T-lymphocytes with brain EC does not result in phosphorylation of ICAM-1
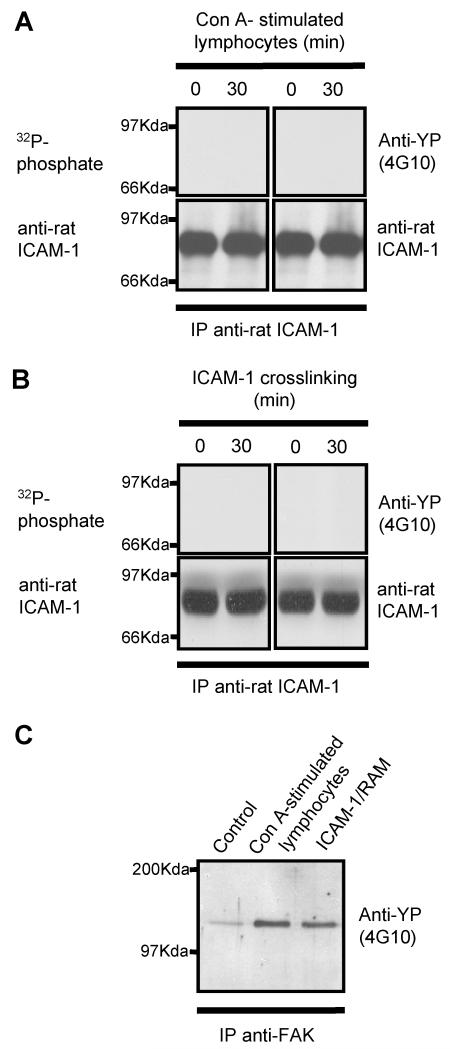
The rat brain microvascular EC line, GP8/3.9, was pre-labelled with [32P]phosphate and co-cultured with concanavalin A-stimulated peripheral lymph node lymphocytes, or cross-linked with anti-rat ICAM-1 mAb and rabbit anti-mouse (RAM) mAb. ICAM-1 immunoprecipitated from these EC was not phosphorylated (Figure 1, upper left panel). Similarly, ICAM-1 immunoprecipitates from EC treated in the same manner were immunoblotted with anti-phosphotyrosine mAb and found to be negative (Figure 1, upper right panel). However, it was noted that both ICAM-1 cross-linking and co-culture with lymphocytes led to an enhanced tyrosine phosphorylation of focal adhesion kinase (FAK) (Figure 1, lower panel), which has previously been shown to be phosphorylated in response to ICAM-1 cross-linking (4).
Figure 1. Endogenous endothelial rat ICAM-1 is not phosphorylated following co-culture with T-lymphocytes or cross-linking of endothelial ICAM-1.
Unlabelled or [32P]-labelled rat brain EC were either (A) co-cultured for various times with concanavalin-A stimulated peripheral lymph node lymphocytes or (B) crosslinked with anti-rat ICAM-1 followed by rabbit anti-mouse IgG (RAM). EC were washed and ICAM-1 or FAK immunoprecipitated from EC lysates using anti-rat ICAM-1 (1A29) or anti-FAK. ICAM-1 immunoprecipitates from [32P]-labelled cells were resolved on 10% SDS-PAGE and exposed to autoradiographic film or immunoblotted with anti-phosphotyrosine mAb (4G10). Membranes were subsequently stripped and re-probed for rat ICAM-1 using 1A29 mAb. FAK immunoprecipitates (C) were immunoblotted with anti-phosphotyrosine mAb (4G10).
Expression of human ICAM-1 in rat brain EC
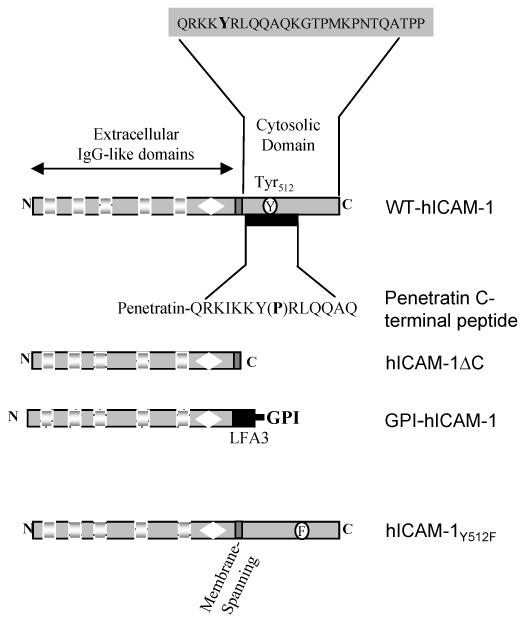
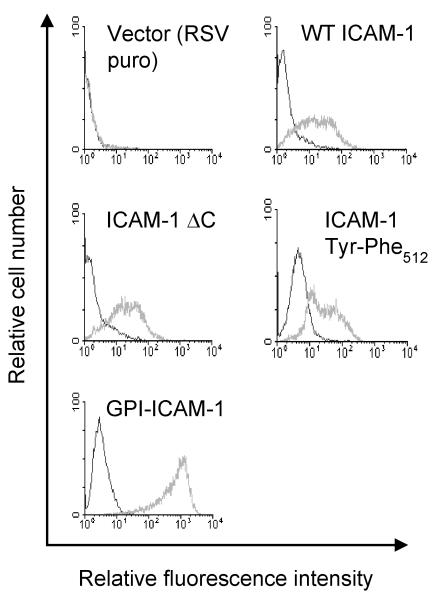
In order to determine whether the intracellular domain, membrane spanning domain, or C-terminal tyrosine residue are important in mediating EC signalling events induced by ICAM-1 and in supporting transendothelial migration of T-lymphocytes, a series of human ICAM-1 molecules were constructed. Human ICAM-1 molecules were generated that i) carried a C-terminal truncation in which the intracellular domain was removed (hICAM-1ΔC) ii) consisted of a C-terminal truncation at the membrane spanning domain which was fused to the GPI-anchor of LFA3 (GPI-hICAM-1), iii) a C-terminal domain carrying a single phenylalanine amino acid substitution at the conserved tyrosine residue codon 512 (hICAM-1Y512F) and iv) wild type ICAM-1 (WT-hICAM-1) (Figure 2). Each cDNA was cloned within the pRSVpuro expression vector and was used for the transfection of GP8/3.9 rat brain EC. Transfected brain EC colonies were selected following growth in media containing 5μg/ml puromycin. Clonal cell lines were generated and evaluated for the expression of human ICAM-1 by flow cytometry. Cell clones were selected expressing each of the human mutant ICAM-1 molecules at approximately equivalent levels to endogenous rat ICAM-1 expressed on GP8/3.9 cells following treatment with IFNγ for 48 hours (Figure 3), apart form the GPI-ICAM-1 positive clones which exhibited higher levels of expression. No cross-reactivity was observed between BBA4 anti-hICAM-1 mAb and 1A29 anti-rat ICAM-1 mAb in untransfected rat brain EC (not shown) or EC transfected with RSVpuro human ICAM-1 (Figure 3). Surface expression of all human ICAM-1 molecules was also detected by indirect immunofluorescence (data not shown).
Figure 2. Schematic illustration of wild type and mutant human ICAM-1 molecules and targeted peptide sequences within the cytoplasmic domain of ICAM-1.
Wild type human ICAM-1 (WT-hICAM-1); human ICAM-1 with 28 residue C-terminal truncation (hICAM-1ΔC); human ICAM-1 in which the cytoplasmic and transmembrane domains have been removed and which has subsequently been fused to the GPI-linker region of human LFA3 (GPI-hICAM-1); human ICAM-1 carrying a tyrosine to phenylalanine substitution at codon 512 (hICAM-1Y512F).
Figure 3. Flow cytometric analysis of rat brain EC expressing wild type and mutant human ICAM-1 molecules.
Cell lines transfected with human ICAM-1 constructs were stained with mouse anti-human ICAM-1 (BBA4; black histogram) or isotype matched control IgG (grey histogram) followed by anti-mouse-FITC (1:50). 10,000 events were acquired and histograms show analysis of human ICAM-1 expression of live cell population.
Human ICAM-1 expressed in brain EC is not phosphorylated following co-culture of endothelial cells with lymphocytes or following specific cross-linking of hICAM-1
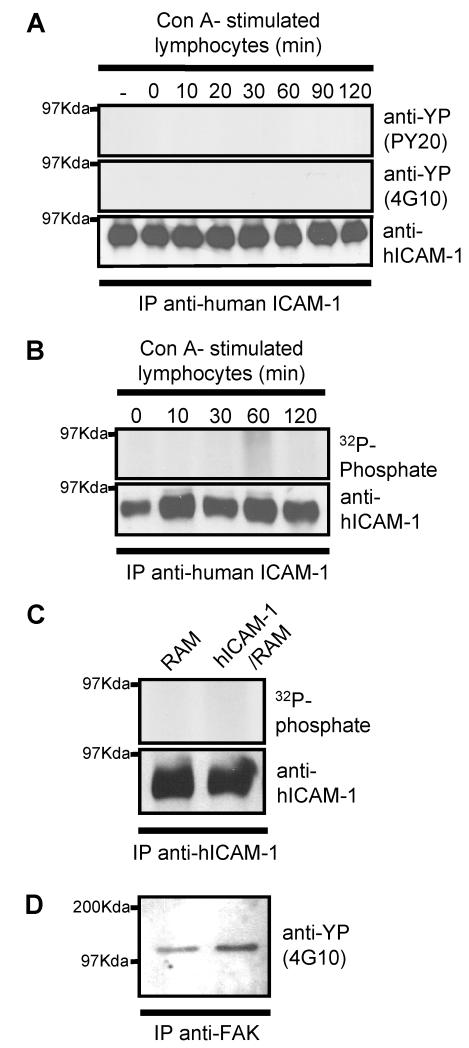
In a similar manner to endogenous ICAM-1, brain EC over expressing WT-hICAM-1 also showed no evidence of [32P]-phosphate labelling (Figure 4B) or enhanced tyrosine phosphorylation in human ICAM-1 immunoprecipitates following co-culture with lymphocytes (Figure 4A) or specific cross-linking of human ICAM-1 (with anti-human ICAM-1/RAM) (Figure 4C). Cross-linking of WT-hICAM-1 on rat brain EC with specific anti-human ICAM-1 mAb induced enhanced tyrosine phosphorylation of FAK in a manner identical to that observed following cross-linking of endogenous rat brain EC ICAM-1. This observation demonstrates that rat brain EC are able to respond to specific cross-linking of human ICAM-1 expressed on their surface (Figure 4C).
Figure 4. Human ICAM-1 is not phosphorylated following co-culture of rat brain EC with T-lymphocytes or cross-linking with human ICAM-1.
Unlabelled or [32P]-labelled brat rain EC expressing human wild type ICAM-1 were co-cultured for various times with concanavalin-A stimulated peripheral lymph node lymphocytes. Cells were washed and human ICAM-1 immunoprecipitated from EC lysates using anti-human ICAM-1 mAb (BBA4). Immunoprecipitates were resolved on SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with (A) anti-phosphotyrosine clone PY20 (upper panel) and clone 4G10 (middle panel) or immunoblotted with anti-human ICAM-1 (lower panel.) (B) Exposed to autoradiographic film (upper panel) or immunoblotted with anti-human ICAM-1 (lower panel.). (C) [32P]-labelled rat brain EC, expressing wild type human ICAM-1, were cross-linked with anti-human ICAM-1 mAb (BBA4) followed by rabbit anti-mouse IgG (RAM). Human ICAM-1 or rat FAK was immunoprecipitated with BBA4 or anti-FAK. ICAM-1 immunoprecipitates were resolved on SDS-PAGE and exposed to autoradiographic film (upper panel) or immunoblotted with anti-human ICAM-1 (lower panel.). (D) FAK immunoprecipitates from human ICAM-1 cross-linked cells immunoblotted with anti-phosphotyrosine mAb (4G10)(lower panel).
The cytoplasmic domain of EC ICAM-1 is essential for both lymphocyte-endothelial adhesion and subsequent transendothelial migration
Both control brain EC and cells transfected with RSVpuro (vector control cell line) showed similar levels of transendothelial migration of myelin basic protein antigen specific T-lymphocytes following co-culture with endothelial cells for 4 h (RSV puro cells 107% ± 6.6% of control value, mean ± SEM, n=26). Brain EC expressing WT-hICAM-1 significantly augmented the transendothelial migration of T-lymphocytes to 204% ± 14.2% of control value (p<0.001 verses RSVpuro control, n=24) (Figure 5A). A doubling in transendothelial migration of rat T-lymphocytes across EC expressing WT-hICAM-1 is not surprising since we have already determined that rat T-lymphocytes efficiently transmigrate across monolayers of human umbilical vein EC (data not shown). In addition, human antigen specific T-lymphocytes are also capable of transendothelial migration when co-cultured with rat brain EC (data not shown).
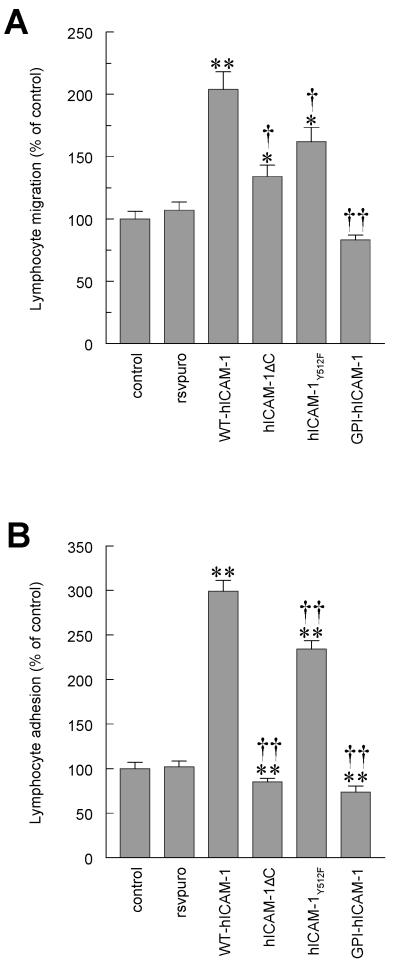
Figure 5. Lymphocyte adhesion to and migration through rat brain EC monolayers expressing human ICAM-1.
(A) Migration of antigen specific (MBP) T cells through or (B) adhesion of [3H]-labelled activated rat peripheral lymph node lymphocytes to control EC monolayers, EC transfected with RSVpuro empty vector (rsvpuro), EC expressing human wild type ICAM-1 (WT-hICAM-1), EC expressing ICAM-1 truncated at codon 504 (hICAM-1ΔC), EC expressing human ICAM-1 with tyrosine to phenylalanine substitution at codon 512 (hICAM-1Y512F) or EC expressing human ICAM-1 truncated at amino acid 480 and fused to the glycophosphatidyl inositol linker of LFA3 (GPI-hICAM-1). Data are expressed as Mean ± SEM percent of control of a minimum of three independent experiments (n= >6 per experiment). Significant differences were determined by Student’s t-test. Compared to control (RSVpuro vector) *p<0.01, **p<0.001 or compared to Wild Type human ICAM-1 † <0.02, † † p<0.001, Student t-test.
Contrary to the results obtained with EC expressing WT-hICAM-1, expression of human GPI-linked ICAM-1 (GPI-hICAM-1) lacking the intracellular and transmembrane domains, failed to augment lymphocyte migration at all (Figure 5A). Thus, transendothelial lymphocyte migration when compared with that observed on EC transfected with RSVpuro was 83.1% ± 4.04% (NS verses RSVpuro, n=15), and was significantly less than transendothelial lymphocyte migration observed on cells expressing WT-hICAM-1 (p<0.001, n=15) despite being expressed at higher levels. Expression of hICAM-1ΔC resulted in a modest increase in migration when compared to RSVpuro control (134% ± 9.2%, p<0.01 verses RSVpuro control, n=22) but was significantly lower than that observed with WT-hICAM-1 (p<0.02). Lymphocyte migration across rat brain EC expressing hICAM-1Y512F was significantly greater than that observed in RSVpuro transfected cells (161.9% ± 11.4%, p<0.01, n=20), but was marginally less than the migration seen with EC expressing WT-hICAM-1 (p<0.02, n=20) (Figure 5A).
As with migration, there was no difference in lymphocyte adhesion between the controls and RSVpuro transfected rat brain EC (102% ± 6.9% of control value). As expected, adhesion of lymphocytes to EC expressing WT-hICAM-1 was significantly increased to 299.0% ± 12.4% of that recorded with RSVpuro transfected EC (p<0.001, n=32). Rat brain EC expressing hICAM-1Y512F also resulted in a significant increase in adhesion of lymphocytes when compared to RSVpuro controls (234.0% ± 9.6%, p<0.001, n=32) but was significantly less than in cells expressing WT-hICAM-1 (p<0.001). In contrast, EC expressing hICAM-1ΔC or GPI-hICAM-1 failed to increase adhesion above control levels, remaining significantly below the level observed with EC expressing WT-hICAM-1 (p<0.001 for both). Indeed, both mutant ICAM-1 proteins resulted in a decrease in the basal adhesion of lymphocytes to rat brain EC expressing RSVpuro alone (85.0% ± 4.3%; p<0.001, n=32 and 73.8% ± 6.8%; p<0.001, n=32 respectively). Thus, human ICAM-1 proteins lacking the intracellular domain are unable to mediate efficient adhesion of lymphocytes or their subsequent transendothelial migration. Although the tyrosine substitution mutant reduced the ability to mediate these events the effects are not as striking as those carrying C-terminal truncations.
Cell permeant peptides comprising the C-terminal domain of ICAM-1 attenuate transendothelial migration of lymphocytes but not adhesion
Next, we determined whether ICAM-1 mediated support of transendothelial lymphocyte migration could be inhibited with cell permeant peptide mimicking the intracellular domain of ICAM-1. Peptides comprising the membrane proximal part of the rat or human ICAM-1 sequence within the intracellular domain were used to antagonise the binding potential of ICAM-1 binding/signalling partners. Such ICAM-1 C-terminal sequences were fused to the penetratin sequence to facilitate uptake of peptides into cells (25,26). A penetratin peptide fused to the 13 amino acids of the intracellular domain of rat ICAM-1 immediately adjacent to the plasma membrane (rICAM-1: QRKIRIYKLQKAQ) or an identical peptide in which the conserved tyrosine residue corresponding to codon 512 of the rat ICAM-1 sequence was phosphorylated (YP-rICAM-1: QRKIRI(YP)KLQKAQ) were both effective in attenuating transendothelial migration of T-lymphocytes through untransfected rat brain EC following a pre-incubation with 100μg/ml peptides for 2 h followed by removal and addition of lymphocytes. The rICAM-1 C-terminal peptide reduced transendothelial migration to 45.9% ± 4.3% of control value (p<0.0001, n=29). Moreover, the phosphopeptide (YP-rICAM-1) was also effective in significantly inhibiting transendothelial migration of T-lymphocytes to 50% ± 4.6% of the control (p<0.0001, n=29) (Figure 6A). Significantly, both the phosphopeptide and the non-phosphorylated peptide were equally effective. However, treatment of untransfected brain EC with these peptides did not reduce lymphocyte adhesion, which was contrary to our findings with EC expressing human ICAM-1 lacking the intracellular domain. Thus, treatment with either the rICAM-1 peptide or the YP-rICAM-1 peptide resulted in adhesion values of 96.1% ± 1.8% (n=32) and 100.3% ± 2.2% (n=32) of control values respectively (Figure 6B). None of the rat penetratin-ICAM-1 peptides affected either the basal or IFNγ-induced cell surface expression of ICAM-1 as determined by ELISA (data not shown),
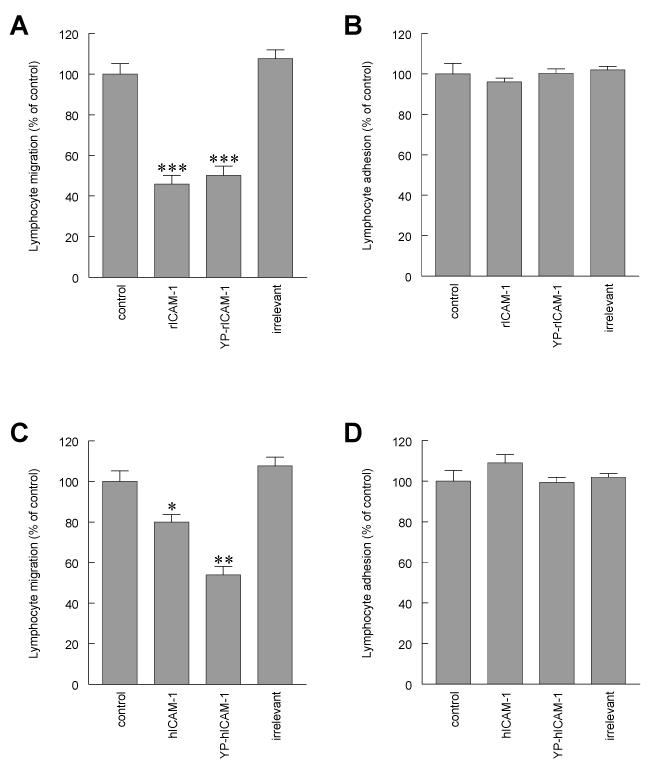
Figure 6. Lymphocyte adhesion to and migration through rat brain EC monolayers in the presence of human and rat penetratin-ICAM-1 peptides.
(A and C) Migration of antigen specific (MBP) T cells through or (B and D) adhesion of [3H]-labelled activated rat peripheral lymph node lymphocytes to control rat brain EC monolayers or EC pretreated with rat (A and B) or human (C and D) penetratin peptides. Penetratin peptide containing C-terminal 16 amino acids of rat ICAM-1 (rICAM-1), penetratin peptide containing C-terminal 16 amino acids of rat ICAM-1 in which the tyrosine residue is phosphorylated (YP-rICAM-1), penetratin peptide containing C-terminal 16 amino acids of human ICAM-1 (hICAM-1), penetratin peptide containing C-terminal 16 amino acids of human ICAM-1 in which the tyrosine residue is phosphorylated (YP-hICAM-1) and penetratin peptide containing 16 amino acids or rat opsin (irrelevant). Observations are a minimum of three independent experiments (n=6/experiment). Data are expressed as Mean ± SEM percent of control of a minimum of three independent experiments (n= >6 per experiment). Significant differences compared to control were determined by Student’s t-test *p<0.01, **p<0.001, ***p<0.0001
When identical experiments were conducted in untransfected rat brain EC using penetratin ICAM-1 peptides comprising an identical region of the human ICAM-1 molecule, both phosphorylated and non-phosphorylated human peptides were also effective in inhibiting transendothelial lymphocyte migration. Incubation of EC with hICAM-1 (QRKIKKYRLQQAQ) or YP-hICAM-1 (QRKIKK(YP)RLQQAQ) peptides caused a reduction of transendothelial migration to 79.9% ± 3.8% (p<0.01, n=27) and 53.9% ± 4.2% (p<0.001, n=28) of control values respectively (Figure 6C). In a similar fashion to the penetratin rat ICAM-1 peptides, human ICAM-1 peptides were also without effect on lymphocyte adhesion being 109.0% ± 1.1% (n=32) and 99.5% ± 2.4% (n=32) for hICAM-1 and YP-hICAM-1 respectively (Figure 6D). An irrelevant control peptide sequence corresponding to the soluble part of rat rod opsin (CKPMSNFRFGENH) had no significant effect on either lymphocyte adhesion or transendothelial migration. N-terminal biotinylation of penetratin peptides was employed to evaluate the entry of penetratin-peptides into rat brain EC. Following fixation and visualisation of biotin peptides with streptavidin-TRITC it was confirmed that in all cases there was efficient uptake of peptide. Thus, treatment with intracellular peptides comprising a sequence from the intracellular domain of either rat or human ICAM-1 appears to be effective in inhibiting transendothelial lymphocyte migration through rat brain EC without affecting lymphocyte adhesion.
Human ICAM-1 intracellular domain peptides abolish the enhanced transendothelial migration of lymphocytes mediated through ectopic expression of hICAM-1 in rat brain EC without affecting lymphocyte adhesion
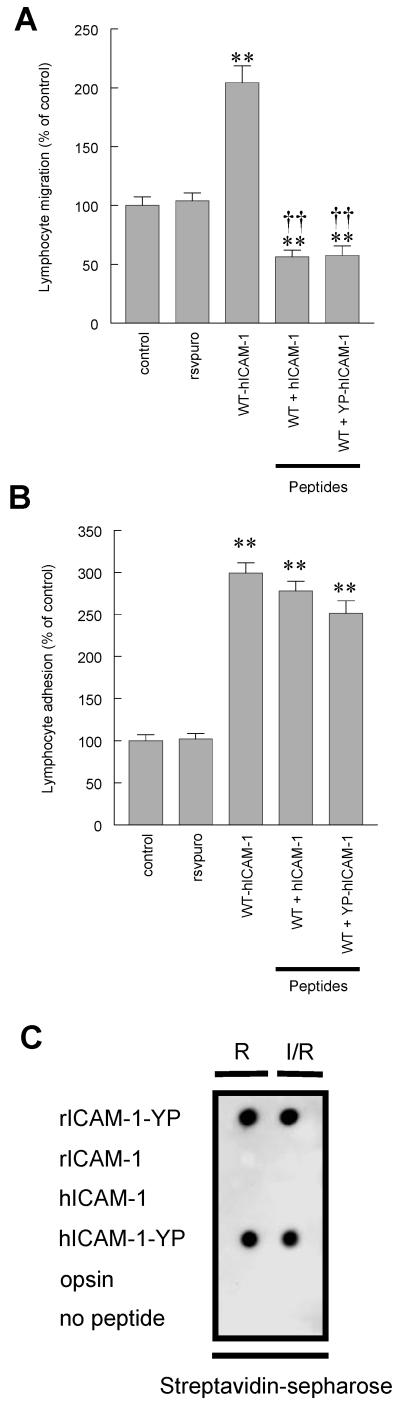
When penetratin peptides containing the human ICAM-1 sequence were used to treat rat brain EC expressing WT-hICAM-1, both non-phosphorylated and tyrosyl-phosphopeptides were able to abolish the increase in transendothelial lymphocyte migration associated with the ectopic expression of WT-hICAM-1. Lymphocyte migration was reduced to 56.3% ± 5.7% (p<0.001 verses cells expressing WT-hICAM-1, n=22) and 57.5% ± 8.2% of control value (p<0.001 verses cells expressing WT-hICAM-1, n=32) following treatment with hICAM-1 C-terminal peptide and YP-hICAM-1 peptide respectively (Figure 7A). It is noteworthy that both peptides were effective in significantly reducing migration to values significantly below that observed with control rat brain EC (p<0.001 verses control brain EC), which is consistent with the effects of these peptides on EC expressing only endogenous rat ICAM-1 (Figure 6C). Rat brain EC expressing WT-hICAM-1 treated with penetratin peptides containing the human ICAM-1 sequence were unable to significantly reduce lymphocyte adhesion which again is consistent with those experiments conducted on rat brain EC expressing only endogenous ICAM-1 (Figure 7B). Thus, adhesion of lymphocytes to brain EC expressing WT-hICAM-1 was 299.0% ± 12.4% and following treatment of EC with hICAM-1 and YP-hICAM-1 peptides was 278.0% ± 11.5% (n=32, NS) and 251.3% ± 14.9% (n=32, NS) respectively. In order to determine that penetratin-hICAM-1 peptides did not undergo phosphorylation or dephosphorylation both penetratin-hICAM-1 and the corresponding phosphopeptide were incubated with GP8/3.9 cells expressing WT-hICAM-1. Isolation of penetratin peptides from cell lysates, from RAM treated cells or cells which had been cross-linked with ICAM-1 mAb followed by anti-phosphotyrosine immunoblotting, demonstrated that these peptides did not show altered phosphorylation status (Figure 7C).
Figure 7. Lymphocyte adhesion to and migration through rat brain EC monolayers expressing human ICAM-1: Effect of penetratin-human ICAM-1 peptides.
(A) Migration of antigen specific (MBP) T cells through or (B) adhesion of [3H]-labelled activated rat peripheral lymph node lymphocytes to control EC monolayers, EC transfected with RSVpuro empty vector (rsvpuro) or EC expressing human wild type ICAM-1 (WT-hICAM-1). EC expressing WT-hICAM-1 were also pretreated with either penetratin peptide containing C-terminal 16 amino acids of human ICAM-1 (hICAM-1) or penetratin peptide containing C-terminal 16 amino acids of ICAM-1 in which the tyrosine residue is phosphorylated (YP-hICAM-1). Observations are a minimum of three independent experiments (n=6/experiment). Data are expressed as Mean ± SEM percent of control of a minimum of three independent experiments (n= >6 per experiment). Significant differences were determined by Student’s t-test. **p<0.001 compared to wild type human ICAM-1. † † p<0.001 compared to RSVpuro control. (C) Anti-phosphotyrosine dot-blot analysis of biotinylated penetratin-hICAM-1 peptides extracted with streptavidin-spepharose from RAM (R) only and ICAM-1/RAM (I/R) treated GP8/3.9 cells expressing WT-hICAM-1.
The intracellular domain of ICAM-1 is essential for ICAM-1 mediated signal transduction
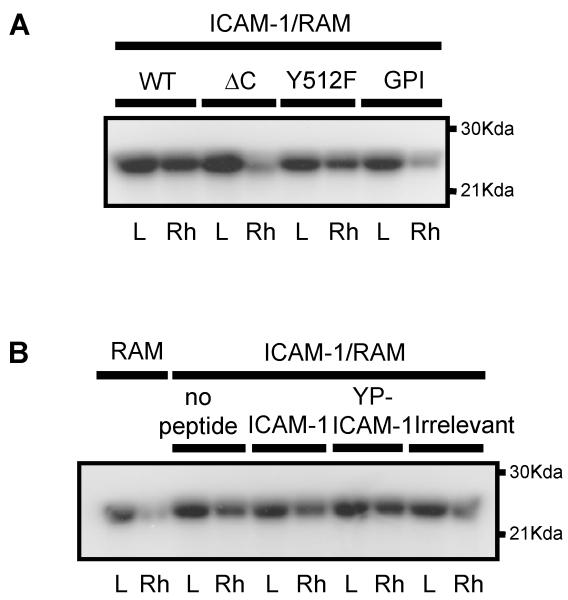
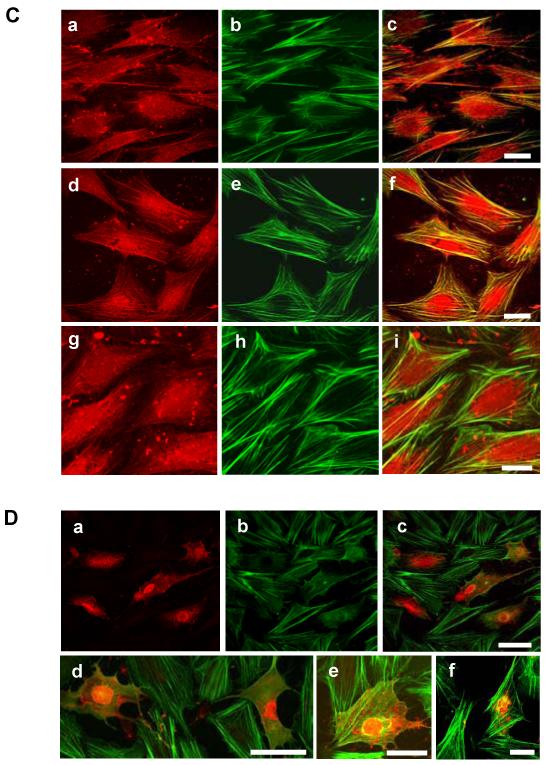
Cross-linking of WT-hICAM-1 expressed in rat brain EC with a specific anti-human ICAM-1 mAb resulted in activation of endothelial Rho proteins as previously described for endogenous ICAM-1 (5). The expression of human ICAM-1 carrying the tyrosine to phenylalanine substitution at codon 512 (hICAM-1Y512F) was also capable of inducing Rho activation. However, cross-linking of either hICAM-1ΔC or GPI-hICAM-1 failed to induce Rho activation (Figure 8A). In contrast to this, pre-incubation of brain EC expressing WT-hICAM-1 with penetratin-hICAM-1 peptides did not result in the inhibition of hICAM-1 stimulated Rho activation (Figure 8B). These findings were supported by the observation that treatment of cells with these peptides also failed to suppress ICAM-1 stimulated stress fibre formation (Figure 8C, panels a-c and panels d-f). It was noted, however, that the human ICAM-1 C-terminal domain peptides showed a significant co-localisation with F-actin stress fibres, which was not the case for the irrelevant penetratin peptide (Figure 8C, panels g-i). When the concentration of penetratin human ICAM-1 peptides was increased, following cytosolic microinjection of cells, both the hICAM-1 peptide (Figure 8D, panels d and e) and the corresponding phosphopeptide (Figure 8D, panels a-c), but not an irrelevant sequence (Figure 8D, panel f) were able to inhibit ICAM-1 stimulated stress fibre formation
Figure 8.
(A) Rat brain EC expressing human ICAM-1 constructs or (B) rat brain EC expressing WT human ICAM-1 in the presence of C-terminal ICAM-1 penetratin peptides were cross linked with anti-human ICAM-1 followed by rabbit anti-mouse IgG (RAM) or RAM only for 15 min, lysed and activated Rho proteins precipitated with GST-rhotekin. Total Rho proteins and GST-rhotekin precipitated Rho proteins were subsequently [32P]ADP-ribosylated, resolved on 15%SDS-PAGE and exposed to autoradiographic film. L = lysate, Rh = precipitated GST-rhotekin. (C) Rat brain EC treated with anti-rat penetratin ICAM-1 peptides (100μg/ml, 2 h) and subsequently cross-linked with anti-rat ICAM-1 for 30 min followed by RAM for 15 min. Cells were fixed and F-actin and biotinylated peptides detected by direct immunofluoresence. (a) penetratin-hICAM-1, (b) F-actin and (c) merge of a and b. (d) penetratin-YP-hICAM-1, (e) F-actin and (f) merge of d and e. (g) penetratin-opsin, (h) F-actin and (i) merge of g and h. Scale bars = 25 μm. (D) Brain EC microinjected in the cytosol with biotinylated penetratin-peptides (10 mg/ml). (a) Cells stained for biotiylated-YP-hICAM-1 peptide. (b) identical field to (a) showing F-actin. (c) merge of (a) and (b). (d and e) merged images of cells microinjected with biotinylated hICAM-1 peptide and (f) biotinylated penetratin-opsin. Scale bars: c and d = 50 μm; e and f = 20 μm
Expression human ICAM-1 in rat brain EC leads to the formation of human-rat heterodimers
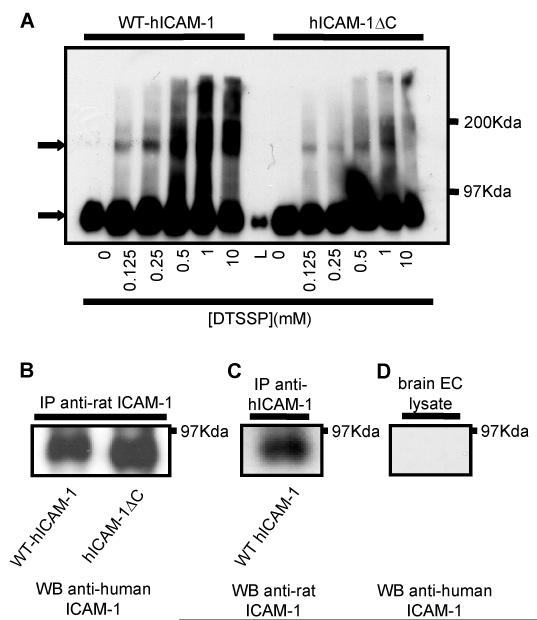
Brain EC monolayers were treated with various concentrations of the reduction sensitive chemical cross-linker 3,3′-Dithiobis[sulphosuccininimidyl propionate] (DTSSP) and lysates from EC expressing WT-hICAM-1 or hICAM-1ΔC analysed by western blot analysis following resolution of proteins on non-reducing SDS-PAGE. As the concentration of DTSSP was increased, proteins of larger molecular weight were detected following immunoblotting with anti-human ICAM-1. These higher molecular weight immunoreactive proteins suggest the presence of ICAM-1 dimers, given the apparent molecular weight of approximately 180Kd (Figure 9A). Higher molecular weight bands were not present when proteins were resolved by reducing SDS-PAGE (data not shown). Endogenous rat ICAM-1 immunoprecipitated from rat brain EC cells expressing WT-hICAM-1 with the specific anti-rat ICAM-1 mAb 1A29, was subsequently immunoblotted with anti-human ICAM-1 mAb (BBA4). Positive bands demonstrated that human ICAM-1 was effectively co-immunoprecipitated with endogenous rat ICAM-1 (Figure 9B) since the anti-ICAM-1 mAb is species specific (Figure 9D). Indeed, small differences in molecular weight in the human ICAM-1 molecules co-immunoprecipitated with rat ICAM-1 can be observed from cells expressing WT-hICAM-1 or hICAM-1ΔC. This demonstrates that human and rat ICAM-1 can exist as heterodimers. In keeping with this suggestion, immunoprecipitation of human ICAM-1 from WT-hICAM-1 expressing cells was also found to co-precipitate endogenous ICAM-1 (Figure 9C).
Figure 9. Both WT-hICAM-1 and hICAM-1ΔC form rat:human heterodimers.
(A) Rat brain EC cells expressing human ICAM-1 or hICAM-1ΔC ere treated with varying concentrations of the reversible cross-linker DTSSP. Cells were lysed and proteins resolved on 6% non-reducing SDS-PAGE prior to immunoblotting with anti-human ICAM mAb (BBA4). Arrows denote ICAM-1 and ICAM-1 dimers. L = lysate of cells with no cross-linker. (B) Cells expressing WT human ICAM-1 or hICAM-1ΔC were immunoprecipitated using anti-rat ICAM-1 mAb (1A29) and immunoprecipitates immunoblotted with anti-human ICAM-1 mAb (BBA4). (C) Cells expressing WT human ICAM-1 were immunoprecipitated with anti-human ICAM-1 mAb (BBA4) and immunoblotted with anti-rat ICAM-1 mAb (1A29). (D) brain EC lysate immunoblotted with anti-human ICAM-1 mAb (BBA4) demonstrating no cross-reactivity with rat ICAM-1.
Discussion
It has previously been shown that ICAM-1 is required for efficient adhesion and migration of lymphocytes across monolayers of both peripheral (8,9) and CNS EC (1-3, 11). Studies from our laboratories, and those of others, have shown that ICAM-1 plays a pivotal role in transducing signals in EC following adhesion of lymphocytes, an essential step in the EC signal cascade responsible for facilitating lymphocyte transendothelial migration. The precise mechanism by which this EC ICAM-1 mediated signalling occurs has yet to be fully defined but is known to be dependent on both an intact endothelial actin cytoskeleton and functional endothelial Rho proteins (4,5,13). ICAM-1 is found to associate with F-actin (18), possibly through an intermediate actin-binding protein such as α-actinin (14), and shows increased avidity following cross-linking of ICAM-1 or co-culture of EC with T-lymphocytes (19). Although it is clear that both the endothelial actin cytoskeleton and activation of Rho proteins are important, it is not known how the ICAM-1 molecule itself mediates intracellular signalling responses in EC since the intracellular domain of ICAM-1 has no obvious catalytic activity.
Ectopic expression of human WT ICAM-1 in the rat brain endothelial cell line GP8/3.9 mediated an increase in both the adhesion and subsequent migration of rat lymphocytes across the endothelial monolayer. The inability of either C-terminally truncated hICAM-1 or a glycophospholipid anchored hICAM-1 protein to induce a similar enhancement of lymphocyte adhesion or transendothelial migration, strongly implies that the intracellular domain is a vital upstream component of the signaling cascade initiated through adhesion of lymphocytes, which subsequently controls lymphocyte transendothelial migration. In keeping with this suggestion, ICAM-1 proteins lacking the intracellular domain are incapable of activating Rho proteins following ICAM-1 cross-linking. These observations further support previous findings that efficient transendothelial migration of lymphocytes is dependent on ICAM-1 mediated Rho signaling in EC (5). This data are consistent with a report showing that transmigration of polymorphonuclear cells across monolayers of ICAM-1 transfected Chinese Hamster Ovary (CHO) cells was also abolished following deletion of the ICAM-1 intracellular domain (27). However, contrary to our data leukocyte adhesion was not affected by cells expressing the truncated ICAM-1 protein (27). Studies in mouse ICAM-1-deficient EC, which have been re-transfected with WT mouse ICAM-1 or C-terminally truncated ICAM-1, also show that both these molecules are able to mediate adhesion of lymphocytes to EC, but truncated ICAM-1 is unable to mediate transendothelial migration (unpublished observations). Why human ICAM-1 constructs lacking the intracellular domain, when expressed in rat brain EC, are capable of reducing adhesion of lymphocytes to levels below that observed in untransfected EC is currently unknown. This may reflect the fact that these mutant ICAM-1 proteins may no longer be able to associate with the actin cytoskeleton. Indeed, treatment of EC with cytochalasin D, which inhibits lymphocyte transendothelial migration, does not result in reduced lymphocyte adhesion (5). It is interesting to note that under these conditions, ICAM-1 is still able to associate with depolymerised actin (28).
Alternatively, ICAM-1 molecules are known to exist as dimers (29) and the formation of cross-species ICAM-1 heterodimers in which one ICAM-1 molecule is C-terminally truncated, may prevent efficient clustering of ICAM-1 or may result in a change in the conformation of ICAM-1 dimers, thus reducing lymphocyte adhesion. Such heterodimers would not have occurred in other studies employing ICAM-1 transfected CHO cells or ICAM-1-deficient mice EC re-transfected with mouse ICAM-1.
Expression of ICAM-1 containing a tyrosine to phenylalanine substitution at codon 512, which is a highly conserved residue in ICAM-1 molecules from different species, also resulted in a significant enhancement of lymphocyte adhesion and migration, albeit without quite reaching the values achieved in the WT-hICAM-1 expressing EC. ICAM-1 deficient mouse brain EC transfected with mouse ICAM-1 carrying an identical mutation does not result in an inhibition of transendothelial lymphocyte migration when compared with wild type ICAM-1 controls (unpublished observations). Together, these observations suggest that with respect to EC mediated lymphocyte adhesion and migration this amino acid is not a critical residue within the ICAM-1 intracellular domain, and is consistent with the observation that ICAM-1 is not tyrosine-phosphorylated following ICAM-1 cross-linking or co-culture with T-lymphocytes. Other studies have suggested that ICAM-1 can be tyrosine phosphorylated following adhesion of cells to immobilised fibrinogen, however it was notable that in these studies an ICAM-1 mutant lacking the conserved intracellular tyrosine residue was still able to bind SHP-2 and that both this ICAM-1 protein and a full C-terminally truncated version of ICAM-1 was still capable of being tyrosine-phosphorylated in response to binding of fibrinogen (21). It is therefore interesting to note that in our exhaustive studies we have been unable to show phosphorylation of either endogenous or overexpressed ICAM-1 in response to either ICAM-1 cross-linking or co-culture of EC with T-lymphocytes. These studies were performed under conditions which induced ICAM-1 dependent signalling responses as assessed by the hyperphosphorylation of FAK.
Cell permeant ICAM-1 C-terminal peptides mimicking the ICAM-1 intracellular domain were shown to be effective in inhibiting transendothelial migration of lymphocytes. In contrast to studies with truncated ICAM-1 proteins, these peptides were unable to moderate lymphocyte adhesion to EC. Furthermore, inclusion of a phosphorylated tyrosine residue at a position in the peptide corresponding to codon 512 of ICAM-1 was no more potent an inhibitor of transendothelial migration than the non-phosphorylated peptide. This supports the view that phosphorylation of the conserved tyrosine residue is not required for ICAM-1 mediated signal transduction enabling transendothelial migration of T-cells. It is interesting to note that although the amino acid sequence of human and rat ICAM-1 are different, they are similar in the juxta-membrane region to which the peptides were targeted. Thus peptides comprising the human ICAM-1 were effective in inhibiting transendothelial migration of lymphocytes across monolayers of rat brain EC expressing only endogenous ICAM-1 and were almost as effective as rat specific sequences. This suggests that common effector molecules bind both the rat and human ICAM-1 C-terminal sequence. Such studies further implicate the intracellular domain of ICAM-1 in T cell mediated signalling functions and the subsequent EC support of lymphocyte migration. However, treatment of EC with penetratin ICAM-1 peptides in the culture medium at levels which attenuated lymphocyte migration did not result in an inhibition of ICAM-1 mediated Rho activation or subsequent stress fibre formation. Although it was apparent the ICAM-1 peptides showed a significant co-localisation with F-actin stress-fibres suggesting that the ICAM-1 C-terminal domain interacts with the actin cytoskeleton as previously suggested (18,19,28). Conversely, when peptides were directly introduced into cells stress fibre formation was ablated (Figure 8D, panel b). This suggests that higher concentrations of peptides are required to block Rho-dependent signalling but that other ICAM-1 mediated events, which are essential for support of transendothelial migration, are inhibited at lower levels. Similar studies using mouse ICAM-1 peptides also lead to attenuation of transendothelial migration of lymphocytes without affecting lymphocyte adhesion to mouse brain EC (unpublished observations). Overall, these data support our previous findings that transendothelial migration of T-lymphocytes across monolayers of CNS EC can be inhibited by interfering with EC ICAM-1 mediated intracellular signalling responses without altering lymphocyte adhesion (5,13,30). Although ICAM-1 has been reported to bind a number of intracellular proteins such as α-actinin (14), β-tubulin, glyceraldehyde-3-phosphate dehydrogenase (15) and ezrin (16,17) it is still not clear which, if any of these molecules, are responsible for transducing signals from the intracellular domain of ICAM-1. Ezrin is known to link ICAM-1 to the actin cytoskeleton and it is clear that the actin cytoskeleton is important for both ICAM-1 mediated signalling and transendothelial lymphocyte migration. Coupled with the fact that proteins of the ezrin/radixin/moesin family are also effectors of Rho signalling pathways (31), ezrin is perhaps the leading candidate. It has recently been shown that ERM proteins may play a role in T-lymphocyte adhesion to immunoglobulin superfamily molecules (32). However, we have so far been unable to show direct interaction between ICAM-1 and ezrin in vitro, under conditions where ICAM-2/ezrin interactions can be demonstrated (28).
Finally, our data raises the interesting possibility that agents based on peptide sequences within the C-terminal domain of ICAM-1 may provide the basis for a novel class of anti-inflammatory agent that can antagonise ICAM-1 mediated intracellular signal transduction and hence subsequent transendothelial lymphocyte migration.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council, The Wellcome Trust, The Multiple Sclerosis Society of Great Britain and Northern Ireland and Association de Recherche sur la Sclerose en Plaques and Fight for Sight.
References
- 1.Male D, Pryce G, Hughes C, Lantos P. Lymphocyte migration into brain modeled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell. Immunol. 1990;127:1. doi: 10.1016/0008-8749(90)90109-5. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood J, Wang Y, Calder VL. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. Immunology. 1995;86:408. [PMC free article] [PubMed] [Google Scholar]
- 3.Pryce G, Male DK, Campbell I, Greenwood J. Factors controlling T-cell migration across rat cerebral endothelium in vitro. J. Neuroimmunol. 1997;75:84. doi: 10.1016/s0165-5728(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 4.Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud P-O. Rho-dependent signaling pathways coupled to ICAM-1 in microvascular brain endothelial cells. J. Immunol. 1998;161:5755. [PubMed] [Google Scholar]
- 5.Adamson P, Etienne S, Couraud P-O, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signalling through endothelial ICAM-1 via a Rho-dependent pathway. J. Immunol. 1999;162:2964. [PubMed] [Google Scholar]
- 6.Etienne-Manneville S, Adamson P, Manneville JB, Wilbourn B, Greenwood J, Couraud P-O. ICAM-1 coupled cytoskeletal rearrangements and lymphocyte migration across brain endothelium involves intracellular calcium signalling. J. Immunol. 2000;165:3375. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 7.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 1991;28:254. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 8.Oppenheimer-Marks N, Davis LS, Lipsky PE. Human T-lymphocyte adhesion to endothelial cells and transendothelial cell migration. Alteration of receptor use relates to the activation status of both the T-cell and the endothelial cell. J. Immunol. 1990;145:140. [PubMed] [Google Scholar]
- 9.Oppenheimer-Marks N, Davis LS, Bogue DT, Ramberg J, Lipsky PE. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J. Immunol. 1991;147:2913. [PubMed] [Google Scholar]
- 10.Greenwood J, Calder VL. Lymphocyte migration through cultured endothelial cell monolayers derived from the blood-retinal barrier. Immunology. 1993;80:401. [PMC free article] [PubMed] [Google Scholar]
- 11.Reiss Y, Hoch G, Deutsch U, Engelhardt B. T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur. J. Immunol. 1998;28:3086. doi: 10.1002/(SICI)1521-4141(199810)28:10<3086::AID-IMMU3086>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud P-O. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J. Biol. Chem. 1994;269:12536. [PubMed] [Google Scholar]
- 13.Walters CE, Pryce G, Hankey DJ, Sebti SM, Hamilton AD, Baker D, Greenwood J, Adamson P. Inhibition of Rho GTPases with protein prenyltransferase inhibitors prevents leukocyte recruitment to the central nervous system and attenuates clinical signs of disease in an animal model of multiple sclerosis. J. Immunol. 2002;168:4087. doi: 10.4049/jimmunol.168.8.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with the actin cytoskeleton and α-actinin. J. Cell Biol. 1992;118:1223. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federici C, Camoin L, Hattab M, Strosberg AD, Couraud P-O. Association of the cytoplasmic domain of intercellular adhesion molecule-1 with glyceraldehyde-3-phosphate dehydrogenase and β-tubulin. Eur. J. Biochem. 1996;238:173. doi: 10.1111/j.1432-1033.1996.0173q.x. [DOI] [PubMed] [Google Scholar]
- 16.Helander TS, Carpen O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for natural killer cells. Nature. 1996;382:265. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- 17.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2) J. Biol. Chem. 1998;273:21893. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 18.Vogetseder W, Dierich MP. Intercellular adhesion molecule-1 (ICAM-1, CD 54) is associated with actin-filaments. Immunobiol. 1991;182:143. doi: 10.1016/S0171-2985(11)80198-1. [DOI] [PubMed] [Google Scholar]
- 19.Amos CL, Romero IA, Schulze C, Rousell J, Pearson JD, Greenwood J, Adamson P. Cross-linking of brain endothelial intercellular adhesion molecule (ICAM)-1 induces an association of ICAM-1 with a detergent insoluble cytoskeletal fraction. Arterioscler. Thromb. Vasc. Biol. 2001;21:810. doi: 10.1161/01.atv.21.5.810. [DOI] [PubMed] [Google Scholar]
- 20.Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43 and ICAM-2. J. Cell Biol. 1998;140:855. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pluskota E, Chen Y, D’Souza S. Src homology domain 2-containing tyrosine phosphatase 2 associates with intercellular adhesion molecule 1 to regulate cell survival. J. Biol. Chem. 2000;275:23371. doi: 10.1074/jbc.M000240200. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood J, Pryce G, Devine L, Male DK, dos Santos WL, Calder VL, Adamson P. SV40 large T immortalised cell lines of the rat blood-brain and blood-retinal barriers retain their phenotypic and immunological characteristics. J. Neuroimmunol. 1996;71:51. doi: 10.1016/s0165-5728(96)00130-0. [DOI] [PubMed] [Google Scholar]
- 23.Reid T, Bathoorn A, Ahmadian MR, Collard JG. Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. J. Biol. Chem. 1999;274:33587. doi: 10.1074/jbc.274.47.33587. [DOI] [PubMed] [Google Scholar]
- 24.Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 1998;143:1385. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall H, Williams EJ, Moore SE, Walsh FS, Prochiantz A, Doherty P. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr. Biol. 1996;6:580. doi: 10.1016/s0960-9822(02)00544-4. [DOI] [PubMed] [Google Scholar]
- 26.Peck D, Isacke CM. Hyaluronan-dependent cell migration can be blocked by a CD44 cytoplasmic domain peptide containing a phosphoserine at position 325. J. Cell Sci. 1988;111:595. doi: 10.1242/jcs.111.11.1595. [DOI] [PubMed] [Google Scholar]
- 27.Sans E, Delachanal E, Duperray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signalling pathway. J. Immunol. 2001;166:544. doi: 10.4049/jimmunol.166.1.544. [DOI] [PubMed] [Google Scholar]
- 28.Romero I, Amos C, Greenwood J, Adamson P. Ezrin and moesin co-localise with ICAM-1 in brain endothelial cells but are not directly associated. Mol. Brain Res. 2002;105:47. doi: 10.1016/s0169-328x(02)00392-3. [DOI] [PubMed] [Google Scholar]
- 29.Reilly PL, Woska JR, Jr., Jeanfavre DD, McNally E, Rothlein R, Bormann BJ. The native structure of intercellular adhesion molecule-1 (ICAM-1) is a dimer. Correlation with binding to LFA-1. J. Immunol. 155:529. [PubMed] [Google Scholar]
- 30.Greenwood J, Walters CE, Pryce G, Kanuga N, Beraud E, Baker D, Adamson P. Lovastatin inhibits brain endothelial Rho-dependent lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J. 2003 doi: 10.1096/fj.02-1014fje. On line. DOI 10.1096/fj.02-1014fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay DJG, Esch F, Furthmayr H, Hall A. Rho and rac dependent assembly of focal adhesion complexes and actin fillaments: an essential role for ezrin/radixin.moesin protein. J. Cell. Biol. 1997;138:927. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002;157:1233. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]